Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Encapsulation of C–N-decorated metal sub-nanoclusters/single atoms into a metal–organic framework for highly efficient catalysis†
Xuan
Qiu‡
,
Jianmin
Chen‡
,
Xinwei
Zou
,
Ruiqi
Fang
,
Liyu
Chen
,
Zhijie
Chen
,
Kui
Shen
 * and
Yingwei
Li
* and
Yingwei
Li
 *
*
State Key Laboratory of Pulp and Paper Engineering, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, China. E-mail: cekshen@scut.edu.cn; liyw@scut.edu.cn
First published on 26th September 2018
Abstract
Fabrication and modification of few-atom metal clusters and even single atoms in the pores of porous materials for catalysis are highly desired from an atom-efficiency aspect but remain a great challenge. Herein, we propose a facile and efficient strategy for the encapsulation of C–N-decorated Pd sub-nanoclusters (MSNCs)/single atoms (SAs) into MOFs by the confined thermolysis of Pd-based metal–organic polyhedra (MOPs) in MOF pores. The obtained hybrids contained both Pd MSNCs (∼0.8 nm) and Pd SAs, which were stabilized by the in situ formed C–N fragments and the confinement effect of MOF pores. Benefiting from the highly exposed Pd atoms and synergistic effect between Pd and C–N fragments, these catalysts exhibited extremely high catalytic activity and stability in various important chemical processes, making them comparable to the most active Pd-based catalysts reported in the literature even under milder reaction conditions. Considering the high tunability of MOPs, this proposed strategy might provide a new toolbox for enriching the family of decorated MSNC/SA catalysts.
Introduction
Owing to sustainable development in energy and environment areas, metal sub-nanoclusters (MSNCs) have attracted extensive attention due to their fascinating catalytic properties stemming from their high atom efficiencies.1,2 In particular, isolated single atoms (SAs) as an exceptional case feature atomically dispersed metal atoms and maximum atom utilization, which have resulted in superior catalytic performance in a number of reactions.3 Moreover, it has also been demonstrated that the catalytic performance of metal sub-nanoclusters (MSNCs) or/and even single atoms (SAs) could be further improved significantly upon functionalization (e.g., C–N decoration) because such modifications are believed to be able to affect the electron distribution, size and stability of the catalysts.4 However, the traditional synthetic approaches for C–N decorated MSNCs/SAs always caused serious aggregation in high-temperature pyrolysis processes due to the ultra-high surface energy of the MSNCs/SAs.5 As is known, the strong interaction or/and confinement effect between the MSNCs/SAs and supports can well stabilize these MSNCs/SAs and prevent them from aggregating during both materials synthesis and catalytic reactions. Thus, exploring effective strategies to immobilize decorated MSNCs/SAs on robust supports for advanced catalysis applications is highly desirable but remains extremely challenging.Metal–organic polyhedra (MOPs) are a new class of discrete coordination complexes built from self-assembly of inorganic metal ions and organic ligands.6 Owing to their well-defined structure and excellent symmetry, MOPs have demonstrated great potential for application in a variety of fields such as catalysis and molecular sensing.7 However, their practical applications are currently restricted due to instability of the MOP structures, which tend to collapse under harsh conditions such as high temperatures.8 Considering their ordered structures and isolated metal ions, we proposed that it could be feasible to employ MOPs as pyrolysis precursors for the fabrication of uniform functional composites such as C–N-decorated MSNCs/SAs by low-temperature thermolysis. During the pyrolysis procedure, the in situ formed C–N fragments derived from the organic ligands are suspected to play an important role in preventing serious aggregation of metal ions. Nevertheless, as far as we know, there is no report on employing MOPs as sacrificial precursors for the preparation of C–N-decorated MSNCs/SAs.
Herein, we report the fabrication of highly dispersed C–N-decorated MSNCs/SAs, which are encapsulated and stabilized in the pores of a metal–organic framework (MOF) by employing MOPs as a sacrificial template. MOFs are a newly developed class of porous materials featuring a uniform structure and well-defined porosity.9 These properties make MOFs one of the most fantastic hosts for the encapsulation of various guests.10 Moreover, it has also been demonstrated that the confinement could significantly enhance the stabilities and properties of the encapsulated guests.11 Our developed strategy employed MOPs as a sacrificial template, which was firstly encapsulated into the MOF pores and then pyrolyzed. By taking advantage of the thermal instability difference between the MOP and the MOF, the MOP guest collapsed to form C–N-decorated MSNCs/SAs while the MOF host preserved its crystalline framework. The residual C–N fragments derived from the organic ligands of the MOP were supposed to act as in situ formed stabilizers to prevent the metal from aggregating, achieving highly dispersed C–N-decorated MSNCs/SAs, which were further stabilized by the confinement effect offered by the MOF cages. This “benign by design” strategy may provide new insights into preparation of highly active and stable MSNC/SA systems.
Results and discussion
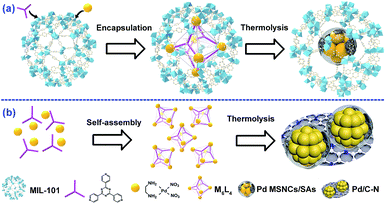
As a proof of principle, MIL-101(Cr) was employed as the host and M6L4 (M stands for (en)Pd(NO3)2; L stands for 1,3,5-tris(4-pyridyl)-2,4,6-triazine, also named TPT) was used as a model MOP. As a typical MOF, MIL-101(Cr) possesses two types of mesopores with internal free diameters of ca. 29 Å and 34 Å.12 M6L4 is a hollow octahedral self-assembly with six vertices occupied by six cationic M and eight triangular faces alternately occupied by four triangular ligands L.13 Typically, this strategy involves two steps (Scheme 1a): (1) encapsulating M6L4 into MIL-101 pores by a hydrophilicity-directed approach (HDA) to obtain the M6L4⊂MIL-101 hybrids,8a and (2) pyrolyzing the hybrids under a H2 flow. The obtained materials were denoted as Pd/C–N⊂MIL-101 (or PCN⊂M for simplicity).Thermogravimetric analysis (TGA) was first carried out to measure the thermal stability of M6L4 and MIL-101 in order to obtain the optimized pyrolysis temperature. As shown in Fig. S1,† the decomposition temperature for M6L4 and MIL-101 was 230 °C and 350 °C respectively. H2-TPR measurement was also performed to investigate the redox properties of M6L4 and M6L4⊂MIL-101. As shown in Fig. S1a,† M6L4 showed a main reduction peak at 215 °C, which can be ascribed to the reduction of Pd2+ to Pd0. Similarly, a strong reduction peak at ca. 250 °C was also detected for M6L4⊂MIL-101 due to the reduction of Pd2+ in its structure, indicating that Pd0 could be successfully prepared by reduction at 250 °C in a H2 atmosphere. Thus, we chose 250 °C as the optimal reduction temperature to prepare various PCN⊂M. In addition, M6L4 was also pyrolyzed at 250 °C under H2 flow (Scheme 1b) for comparison. The residual composite was named Pd/C–N due to the presence of Pd, C, and N elements, as indicated by the elemental analysis and AAS (Table S1†). The powder X-ray diffraction (PXRD) patterns of the Pd/C–N (Fig. S2†) showed eight diffraction peaks, demonstrating the characteristics of metallic Pd (JCPDS no. 46-1043). Representative transmission electron microscopy (TEM) images (Fig. S3a†) indicated that the Pd particles in Pd/C–N have an irregular morphology (10–30 nm) with some C–N fragments wrapped around them. High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and the corresponding EDX elemental mapping (Fig. S3b–e†) revealed a uniform distribution of C and N in Pd-enriched NPs, which was further confirmed by the elemental line-scanning spectra (Fig. S3f†). These results clearly indicated that after thermolysis in H2, the Pd centers in M6L4 aggregated and reduced to Pd particles while the organic components collapsed to C–N fragment layers.
The XPS and Fourier transform infrared spectroscopy (FT-IR) spectra of the M6L4⊂MIL-101 (Fig. S4†) were similar to those reported, indicating the successful encapsulation of M6L4 into the MIL-101 pores.8a By treating M6L4⊂MIL-101 at 250 °C under a H2 flow, M6L4 decomposed while MIL-101 remained stable due to its higher thermo-stability (Fig. S1†), forming the PCN⊂M with 0.64 wt% Pd loading (denoted as 0.64PCN⊂M). Three other PCN⊂M with different Pd loadings were also prepared, which were named 0.33PCN⊂M, 0.51PCN⊂M and 0.82PCN⊂M based on their actual Pd content (Table S2†). The PXRD patterns of all PCN⊂M (Fig. S5†) hybrids were similar to that of MIL-101 and no characteristic peak of the Pd phase was observed, suggesting the well-preserved structure of the parent MIL-101 and the high dispersion of Pd in PCN⊂M.12,14 The transformation of M6L4 in M6L4⊂MIL-101 after pyrolysis was further confirmed by FT-IR. As presented in Fig. S6,† the spectrum of M6L4⊂MIL-101 exhibited distinct characteristic bonds of M6L4 with the 715 cm−1 peak assigned to NO3− stretching in M, and the 673 cm−1 peak assigned to NH2 stretching in L.8a After heating in H2, these two peaks disappeared, indicating collapse of the M6L4 structure. However, the 1061 cm−1 peak assigned to C–N stretching remained even though its intensity decreased, which indicated that the C–N fraction was still preserved during the pyrolysis.
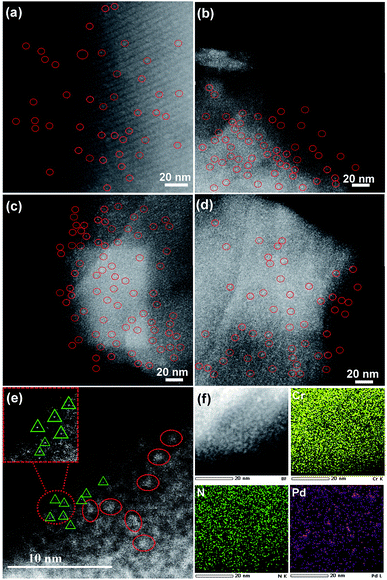
The structures of various samples were characterized in detail by HAADF-STEM. Clearly, M6L4⊂MIL-101 showed a smooth surface without clusters (Fig. S7†). After thermolysis, all PCN⊂M hybrids exhibited a uniform distribution of Pd sub-nanoclusters with ultrafine average particle sizes of ca. 0.8 nm (Fig. 1a–d and S8–S11†). Delightfully, further characterization of spherical aberration corrected electron microscopy revealed that 0.64PCN⊂M, as a representative, also showed plenty of bright spots besides bright clusters, which were assigned to the atomically dispersed Pd (Pd single atoms and Pd clusters are indicated by solid triangles and circles in Fig. 1e for clarity, respectively). The elemental mapping showed that Pd, N, and Cr were homogeneously dispersed (Fig. 1f). These results suggested that both Pd MSNCs and SAs were present and highly distributed in our system. In general, metal particles will aggregate seriously when the loading rises for support materials. However, we are delighted to mention that in our PCN⊂M system the main particle distribution was still located below the sub-nanoscale zone even when the Pd content was increased up to 0.82%.
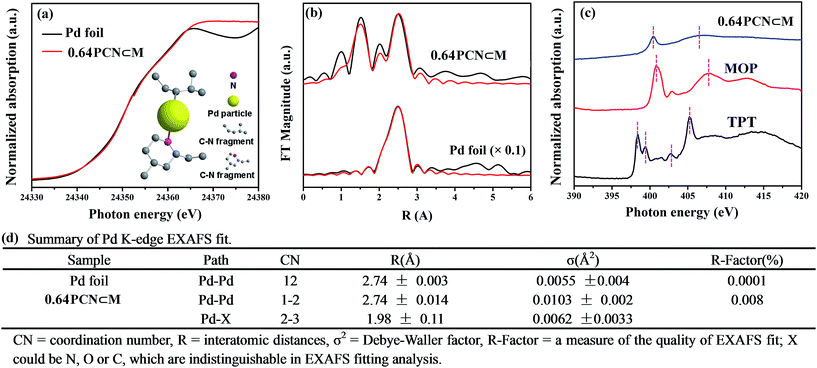
The fine-structure information and chemical bonding environment were further studied by the extended X-ray absorption fine structure (EXAFS) and near-edge X-ray absorption fine structure (NEXAFS) (Fig. 2). In the Pd K-edge, the absorption threshold appearing between 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 and 24
330 and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 eV, called the white line, corresponds to the 1s–4p electron transitions and is sensitive to variations of the electron occupancy in the valence band and ligand field environments of the absorber.3a,15 As shown in Fig. 2a, the white-line intensity of PCN⊂M was mostly similar to that of Pd foil except that the intensity was a little bit higher. This indicated that the Pd valence in PCN⊂M was similar to that in Pd foil but in a more oxidized form.16Fig. 2b shows the EXAFS Fourier transform (without phase correction) of Pd foil as a reference and PCN⊂M together with the results of the curve-fitting analysis. The coordination numbers (CNs) of Pd–Pd bonding (CNPd–Pd ≈ 1 to 2) in the PCN⊂M were as low as 1 to 2, and were significantly lower than that of bulk Pd foil (CNPd–Pd ≈ 12) (Fig. 2d), confirming the high dispersion of the Pd MSNCs/SAs encapsulated in the MIL-101.17 This result was also consistent with the HAADF-STEM results. PCN⊂M presented (Fig. 2d) two evident peaks at around 1.98 Å and 2.74 Å (after phase correction, the bond lengths R were larger than those without phase correction in Fig. 2b), belonging to Pd–X (X = N or C) and Pd–Pd, respectively. These results suggested the coexistence of single-atom Pd and Pd clusters, which were partly coordinated with N and/or C atoms of the residual C–N fragment derived from the organic components.18 This interaction was suggested to stabilize the Pd MSNCs/SAs, preventing them from aggregating.
365 eV, called the white line, corresponds to the 1s–4p electron transitions and is sensitive to variations of the electron occupancy in the valence band and ligand field environments of the absorber.3a,15 As shown in Fig. 2a, the white-line intensity of PCN⊂M was mostly similar to that of Pd foil except that the intensity was a little bit higher. This indicated that the Pd valence in PCN⊂M was similar to that in Pd foil but in a more oxidized form.16Fig. 2b shows the EXAFS Fourier transform (without phase correction) of Pd foil as a reference and PCN⊂M together with the results of the curve-fitting analysis. The coordination numbers (CNs) of Pd–Pd bonding (CNPd–Pd ≈ 1 to 2) in the PCN⊂M were as low as 1 to 2, and were significantly lower than that of bulk Pd foil (CNPd–Pd ≈ 12) (Fig. 2d), confirming the high dispersion of the Pd MSNCs/SAs encapsulated in the MIL-101.17 This result was also consistent with the HAADF-STEM results. PCN⊂M presented (Fig. 2d) two evident peaks at around 1.98 Å and 2.74 Å (after phase correction, the bond lengths R were larger than those without phase correction in Fig. 2b), belonging to Pd–X (X = N or C) and Pd–Pd, respectively. These results suggested the coexistence of single-atom Pd and Pd clusters, which were partly coordinated with N and/or C atoms of the residual C–N fragment derived from the organic components.18 This interaction was suggested to stabilize the Pd MSNCs/SAs, preventing them from aggregating.
NEXAFS spectroscopy was also employed to examine the N environment in the PCN⊂M samples using the N K-edge by taking TPT and MOP as references. As shown in Fig. 2c, the resonances of π* at 398.4 and 399.4 eV in pure TPT were assigned to nitrogen species in the form of pyridine (C–N (p)) and aromatic C–N–C coordination of tri-triazine, respectively. The π* resonance at 402.8 eV was attributed to charging effects or π excitations. The resonance of σ* at 405.2 eV was assigned to σ* transitions.19a,b Compared with TPT, the shift of the N 1s of PCN⊂M toward a higher binding energy indicated a decrease in the electron density of N.20 As compared to MOP, N 1s of PCN⊂M shifted to a lower binding energy. These two comparisons implied that the N electron density in PCN⊂M was between that in TPT and MOP. This indicated that there existed a Pd–N (pyridine type) in the PCN⊂M, but the bond strength was lower than that in MOP. This result was also in agreement with the Pd K-edge study, demonstrating the existence of positive coordination interaction between the Pd and N in the residual C–N fragments.
XPS measurements were also carried out to determine the palladium environment in 0.64PCN⊂M. As shown in Fig. 3a, 0.64PCN⊂M exhibited a Pd 3d5/2 band at around 336.9 eV and a Pd 3d3/2 band at around 342.4 eV, both of which were nearly 2 eV lower than those of the M6L4⊂MIL-101 (the M6L4⊂MIL-101 exhibited a Pd 3d5/2 band at 338.6 eV and a Pd 3d3/2 band at 343.9 eV). This result implied again that the PdII in M6L4 was reduced under the investigated conditions. The N 1s spectra of pure TPT showed two binding energies at around 398.3 eV and 398.6 eV, which were related to pyridine-type and triazine-type nitrogens, respectively.19c After assembly and reduction, the N environment of PCN⊂M underwent some changes. The N 1s bands of 0.64PCN⊂M were located at 398.9 and 400.2 eV, matching well with the character of pyridine-type (C–N) and triazine-type (C–N–C) nitrogens,19c and were 0.6 and 1.6 eV higher than those of pure L, respectively (Fig. 3b). The changes in binding energies suggested an electron interaction between N and Pd, which played an important role in preventing Pd from aggregating, as also demonstrated by the TEM, EXAFS and NEXAFS results.21 Furthermore, based on XPS data, the Pd content of 0.64PCN⊂M was calculated to be ca. 0.01%, which was much lower than the AAS result (0.64%). This result further confirmed that most of the Pd NPs were indeed encapsulated in the pores of MIL-101 since XPS is only able to detect the surface properties of few-atom layers.
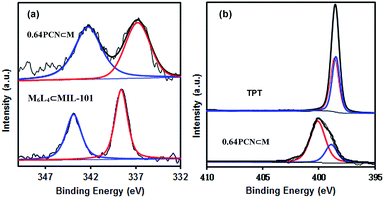 | ||
| Fig. 3 (a) Pd 3d and (b) N 1s XPS spectra of 0.64PCN⊂M, M6L4⊂MIL-101 and 2,4,6-tri(pyridin-4-yl)-1,3,5-triazine (TPT). | ||
The surface area and porosity of the hybrids were measured by N2 adsorption and desorption at 77 K. All PCN⊂M samples (Fig. S12†) showed similar isotherms to that of the parent MIL-101, indicating that the PCN⊂M materials still retained the porous structure of MIL-101. However, obvious decreases in the BET surface area and pore volume (Table S3†) were observed as compared with MIL-101, which were caused by the pore occupation by encapsulated Pd MSNCs/SAs. Reasonably, the BET surface area and pore volume of the PCN⊂M hybrids decreased gradually with an increase in Pd content.
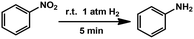
Combining all these results, it can be confirmed that we have successfully fabricated and functionalized C–N decorated Pd MSNCs/SAs in the pores of MIL-101. The catalytic performance was then examined by taking hydrogenation as a model reaction due to its importance in the industrial production of aniline.22 Reactions were carried out under atmospheric pressure of H2 at room temperature. The parent MIL-101 showed almost no conversion (Table 1, entry 1), confirming the necessity of Pd to perform the hydrogenation. The influence of solvents on the catalytic performance was firstly examined using 0.64PCN⊂M as the catalyst. The results showed that CH3OH was the best solvent for this transformation under the investigated conditions (Table S4,† entries 2–6). Then the Pd loading effect was investigated. 0.64PCN⊂M showed the best catalytic performance with a TOF of 800 (Table 1, entry 4), which provided an almost complete conversion to aniline within only 5 min. 0.82PCN⊂M also showed excellent performance, giving a TOF of 776 (Table 1, entry 5). Obviously, this catalytic system represents an exceptional example for the quantitative hydrogenation of nitrobenzene under atmospheric pressure of H2 and room temperature, and it outperformed the most active Pd-based catalysts reported in the literature (Table S5†). More importantly, the catalyst could be recycled without any efficiency loss and Pd leaching for at least five runs (Fig. S13a†). To our delight, there was also no obvious Pd aggregation of the reused catalyst observed even after five runs (Fig. S13b†). These investigations showed that the PCN⊂M hybrids are stable catalysts for hydrogenation transformations, offering great potential in industrial applications under mild conditions.
| Entry | Catalyst | Solvent | Yield (%) | TOF (h−1) |
|---|---|---|---|---|
| a Reaction conditions: nitrobenzene (0.1 mmol), 1 atm H2, room temperature, 5 min, 2 mL solvent, Pd/substrate = 1.5 mol%. | ||||
| 1 | MIL-101 | CH3OH | <1 | — |
| 2 | 0.33PCN⊂M | CH3OH | 56 | 448 |
| 3 | 0.51PCN⊂M | CH3OH | 76 | 655 |
| 4 | 0.64PCN⊂M | CH3OH | >99 | 800 |
| 5 | 0.82PCN⊂M | CH3OH | 97 | 776 |
| 6 | Pd/MIL-101 | CH3OH | 16 | 128 |
| 7 | Pd/C | CH3OH | 6 | 51 |
For comparison, a traditional impregnation method was also employed for the implantation of Pd into MIL-101, i.e., Pd/MIL-101. Pd/MIL-101 was synthesized by soaking MIL-101 in an M6L4 aqueous solution, followed by H2 treatment. The loading efficiency was as low as 26% (Table S2†). The M6L4 was believed to be adsorbed on the MIL-101 surface as the M6L4 size was larger than the MIL-101 window, which was also verified by the TEM images, demonstrating a wide range of particle sizes (4–20 nm) and severe aggregation (Fig. S14†). As a result, Pd/MIL-101 showed much lower catalytic activity than PCN⊂M under the same conditions, affording only 16% yield (TOF: 128) of aniline (Table 1, entry 6). The TOF of PCN⊂M was also much higher than that of commercial 5% Pd/C (TOF: 51, Table 1, entry 7). Considering the similar active sites but different structures of PCN⊂M, Pd/MIL-101 and Pd/C, it is reasonable to suggest that the outstanding catalytic activity over PCN⊂M can be directly ascribed to its highly exposed Pd atoms and the positive synergistic effect between Pd MSNCs/SAs and C–N fragments, which was also verified by the EXAFS and XPS results.
The PCN⊂M also showed excellent catalytic performance towards the selective hydrogenation of biomass-derived furfural (FFA) to cyclopentanone (CPO). Due to the abundance of FFA sources and high added-value of CPO, the selective transformation of FFA to CPO has received increasing interest in recent years.23 Even though various catalyst systems for FFA hydrogenation have been developed, the harsh reaction conditions (such as ultrahigh H2 pressure) and low selectivity are still a significant bottleneck which hinders their practical application (Table S6†). Consequently, the need to design a highly selective but mild catalytic system towards sustainable development is urgent. As expected, our PCN⊂M system also showed unprecedented catalytic performance in aqueous hydrogenation of FFA to CPO under a low H2 pressure. The parent MIL-101 gave essentially no reactivity (Table 2, entry 1). In sharp contrast, the 0.64PCN⊂M catalyst showed amazing performance with both ultra-high conversion (>99%) and selectivity (>99%) (Table 2, entry 4). Other PCN⊂M catalysts with different loadings also showed good performance with high selectivity (Table 2, entries 2, 3 and 5). Different reaction temperatures were subsequently screened (Fig. S15†). As the temperature increased, the CPO yield increased gradually. It is worth mentioning that even when the reaction temperature was 200 °C, the CPO selectivity was still >99% with few over-hydrogenated products (i.e., CPL).
| Entry | Catalyst | Conv. (%) | CPO sel. (%) | CPL sel. (%) |
|---|---|---|---|---|
| a Reaction conditions: FFA (0.52 mmol), Pd/FFA = 1.15 × 10−3, water (4 mL), 180 °C, 0.8 MPa H2, 24 h. | ||||
| 1 | MIL-101 | — | — | — |
| 2 | 0.33PCN⊂M | 85 | >99 | <1 |
| 3 | 0.51PCN⊂M | 91 | >99 | <1 |
| 4 | 0.64PCN⊂M | >99 | >99 | <1 |
| 5 | 0.82PCN⊂M | 94 | >99 | <1 |
| 6 | Pd/MIL-101 | 46 | 83 | 17 |
| 7 | Pd/C | 28 | 71 | 29 |
We also investigated the effect of H2 pressure on CPO yield (Fig. S16†). Under the investigated conditions, the reaction could also proceed even when the pressure was as low as 1 atm (Fig. S17†), which was much lower as compared to other reported systems (Table S6†). Generally speaking, selectivity would decrease with increasing H2 pressure. To our delight, the CPO yield over 0.64PCN⊂M was still excellent (>99%) even when the pressure reached 1.2 MPa.
In general, the stability and reusability of MOF catalysts can be particularly challenging under aqueous reaction conditions at moderate to high temperatures. However, the PCN⊂M could be used up to 5 times without any significant decrease in catalytic performance, which was checked by terminating the reaction at 12 h (Fig. S18†). Furthermore, no metal leaching was observed by AAS analysis of the liquid phase after the reaction. All these investigations indicated the robustness and effectiveness of this newly developed catalytic system.
Conclusions
In summary, we have developed a facile strategy to fabricate highly dispersed C–N-decorated MSNCs/SAs that were encapsulated in the pores of a MOF. The obtained PCN⊂M hybrids contained both Pd sub-nanoclusters and Pd single atoms, which were stabilized by the in situ formed C–N fragments and the confinement effect offered by the MOF pores. The confined C–N-decorated MSNCs/SAs exhibited superior catalytic activity and stability in important catalytic reactions. This approach offers a versatile approach for the encapsulation of a broad range of similar MSNCs/SAs into MOF pores and broadens the library of decorated MSNCs/SAs. Studies aimed at extending this strategy for the encapsulation of other types of MSNCs/SAs into MOFs for advanced catalysis applications are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21436005, 21576095, and 21606087), the Guangdong Natural Science Funds for Distinguished Young Scholar (2018B030306050), the Fundamental Research Funds for the Central Universities (2017PY004 and 2017MS069), the Science and Technology Program of Guangzhou (201804020009), the Pearl River S&T Nova Program of Guangzhou (201806010140), the State Key Laboratory of Pulp and Paper Engineering (2017ZD04 and 2018TS03), and the Natural Science Foundation of Guangdong Province (2016A050502004 and 2017A030312005). We acknowledge beamlines BL14W1 and BL08U (Shanghai Synchrotron Radiation Facility) for providing the beam time.Notes and references
- (a) L. Chen, R. Luque and Y. Li, Chem. Soc. Rev., 2017, 46, 4614–4630 RSC; (b) Z. Li and Q. Xu, Acc. Chem. Res., 2017, 50, 1449–1458 CrossRef CAS PubMed; (c) P. Wang, Z. Lin, X. Su and Z. Tang, Nano Today, 2017, 12, 64–97 CrossRef CAS.
- (a) P. Falcaro, R. Ricco, A. Yazdi, I. Imaz, S. Furukawa, D. Maspoch, R. Ameloot, J. D. Evans and C. J. Doonan, Coord. Chem. Rev., 2016, 307, 237–254 CrossRef CAS; (b) S. Zhang, C. Chang, Z. Huang, J. Li, Z. Wu, Y. Ma, Z. Zhang, Y. Wang and Y. Qu, J. Am. Chem. Soc., 2016, 138, 2629–2637 CrossRef CAS PubMed.
- (a) X. Wang, W. Chen, L. Zhang, T. Yao, W. Liu, Y. Lin, H. Ju, J. Dong, L. Zheng, W. Yan, X. Zheng, Z. Li, X. Wang, J. Yang, D. He, Y. Wang, Z. Deng, Y. Wu and Y. Li, J. Am. Chem. Soc., 2017, 139, 9419–9422 CrossRef CAS PubMed; (b) W. Liu, L. Zhang, X. Liu, X. Liu, X. Yang, S. Miao, W. Wang, A. Wang and T. Zhang, J. Am. Chem. Soc., 2017, 139, 10790–10798 CrossRef CAS PubMed; (c) V. Rajenahally, R. V. Jagadeesh, K. Murugesan, A. S. Alshammari, H. Neumann, M.-M. Pohl, J. Radnik and M. Beller, Science, 2017, 358, 326–332 CrossRef PubMed.
- (a) X. Li and M. Antonietti, Chem. Soc. Rev., 2013, 42, 6593–6604 RSC; (b) V. Perazzolo, R. Brandiele, C. Durante, M. Zerbetto, V. Causin, G. A. Rizzi, I. Cerri, G. Granozzi and A. Gennaro, ACS Catal., 2018, 8, 1122–1137 CrossRef CAS.
- Y. V. Kaneti, J. Tang, R. R. Salunkhe, X. Jiang, A. Yu, K. C.-W. Wu and Y. Yamauchi, Adv. Mater., 2017, 29, 1604898 CrossRef PubMed.
- (a) T. R. Cook, Y. Zheng and P. J. Stang, Chem. Rev., 2012, 113, 734–777 CrossRef PubMed; (b) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed; (c) N. Ahmad, A. H. Chughtai, H. A. Younus and F. Verpoort, Coord. Chem. Rev., 2014, 280, 1–27 CrossRef CAS; (d) Y. Han, J. Li, Y. Xie and G. Guo, Chem. Soc. Rev., 2014, 43, 5952–5981 RSC; (e) W. J. Ramsay, F. T. Szczypiński, H. Weissman, T. K. Ronson, M. M. Smulders, B. Rybtchinski and J. R. Nitschke, Angew. Chem., Int. Ed., 2015, 54, 5636–5640 CrossRef CAS PubMed.
- (a) N. Ahmad, H. A. Younus, A. H. Chughtai and F. Verpoort, Chem. Soc. Rev., 2015, 44, 9–25 RSC; (b) Y. Kohyama, T. Murase and M. Fujita, J. Am. Chem. Soc., 2014, 136, 2966–2969 CrossRef CAS PubMed; (c) J. Roukala, J. Zhu, C. Giri, K. Rissanen, P. Lantto and V. V. Telkki, J. Am. Chem. Soc., 2015, 137, 2464–2467 CrossRef CAS PubMed.
- (a) X. Qiu, W. Zhong and Y. Li, J. Am. Chem. Soc., 2016, 138, 1138–1141 CrossRef CAS PubMed; (b) L. Sun, J. Li, W. Lu, Z. Gu, Z. Luo and H. Zhou, J. Am. Chem. Soc., 2012, 134, 15923–15928 CrossRef CAS PubMed.
- (a) K. Shen, L. Zhang, X. Chen, L. Liu, D. Zhang, Y. Han, J. Chen, J. Long, R. Luque, Y. Li and B. Chen, Science, 2018, 359, 206–210 CrossRef CAS PubMed; (b) M. O'Keeffe, Chem. Soc. Rev., 2009, 38, 1215–1217 RSC; (c) J. A. Howarth, Y. Liu, P. Li, Z. Li, T. C. Wang, J. T. Hupp and O. K. Farha, Nat. Rev. Mater., 2016, 1, 15018 CrossRef; (d) A. Dhakshinamoorthy, A. M. Asiri and H. García, Angew. Chem., Int. Ed., 2016, 55, 5414–5445 CrossRef CAS PubMed; (e) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982–5993 RSC; (f) L. Wang, Y. Han, X. Feng, J. Zhou, P. Qi and B. Wang, Coord. Chem. Rev., 2016, 307, 361–381 CrossRef CAS; (g) B. Li, M. Chrzanowski, Y. Zhang and S. Ma, Coord. Chem. Rev., 2016, 307, 106–129 CrossRef CAS; (h) B. Y. Xia, Y. Yan, N. Li, H. B. Wu, X. W. Lou and X. Wang, Nat. Energy, 2016, 1, 15006 CrossRef CAS.
- (a) Q. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC; (b) C. R. Kim, T. Uemura and S. Tang, Chem. Soc. Rev., 2016, 45, 3828–3845 RSC.
- (a) A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, ACS Catal., 2017, 7, 2896–2919 CrossRef CAS; (b) P. Hu, J. V. Morabito and C.-K. Tsung, ACS Catal., 2014, 4, 4409–4419 CrossRef CAS; (c) H. Liu, L. Chang, C. Bai, L. Chen, R. Luque and Y. Li, Angew. Chem., Int. Ed., 2016, 55, 5019–5023 CrossRef CAS PubMed.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef PubMed.
- (a) M. Fujita, D. Ogura, M. Miyazawa, H. Oka, K. Yamaguchi and K. Ogura, Nature, 1995, 378, 469–471 CrossRef CAS; (b) M. Fujita, M. Tominaga, A. Hori and B. Therrien, Acc. Chem. Res., 2005, 38, 369–378 CrossRef CAS PubMed.
- M. Zhao, K. Yuan, Y. Wang, G. Li, J. Guo, L. Gu, W. Hu, H. Zhao and Z. Tang, Nature, 2016, 539, 76–80 CrossRef CAS PubMed.
- S. Wang, L. Shang, L. Li, Y. Yu, C. Chi, K. Wang, J. Zhang, R. Shi, H. Shen, G. I. N. Waterhouse, S. Liu, J. Tian, T. Zhang and H. Liu, Adv. Mater., 2016, 28, 8379–8387 CrossRef CAS PubMed.
- (a) S. Schuster, E. Klemm and M. Bauer, Chem.–Eur. J., 2012, 18, 15831–15837 CrossRef CAS PubMed; (b) X. Fang, Q. Shang, Y. Wang, L. Jiao, T. Yao, Y. Li, Q. Zhang, Y. Luo and H. Jiang, Adv. Mater., 2018, 30, 1705112 CrossRef PubMed.
- P. Yin, T. Yao, Y. Wu, L. Zheng, Y. Lin, W. Liu, H. Ju, J. Zhu, X. Hong, Z. Deng, G. Zhou, S. Wei and Y. Li, Angew. Chem., Int. Ed., 2016, 55, 10800–10805 CrossRef CAS PubMed.
- (a) T. Zhou, Y. Du, A. Borgna, J. Hong, Y. Wang, J. Han, W. Zhang and R. Xu, Energy Environ. Sci., 2013, 6, 3229–3234 RSC; (b) C. J. Doonan, W. Morris, H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 9492–9493 CrossRef CAS PubMed.
- (a) Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2016, 138, 16174–16181 CrossRef CAS PubMed; (b) M. Jeon, D. J. Han, K. S. Lee, S. H. Choi, J. Han, S. W. Nam, S. C. Jang, H. S. Park and C. W. Yoon, Int. J. Hydrogen Energy, 2016, 41, 15453–15461 CrossRef CAS; (c) I. Bertóti, M. Mohai and K. László, Carbon, 2015, 84, 185–196 CrossRef.
- K. Koh, M. Jeon, D. M. Chevrier, P. Zhang, C. W. Yoon and T. Asefa, Appl. Catal., B, 2017, 203, 820–828 CrossRef CAS.
- P. Chen, A. Khetan, F. Yang, V. Migunov, P. Weide, S. Stürmer, P. Guo, K. Kähler, W. Xia, J. Mayer, H. Pitsch, U. Simon and M. Muhler, ACS Catal., 2017, 7, 1197–1206 CrossRef CAS.
- (a) A. Corma and P. Serna, Science, 2006, 313, 332–334 CrossRef CAS PubMed; (b) F. A. Westerhaus, R. V. Jagadeesh, G. Wienhöfer, M.-M. Pohl, A.-E. Surkus, J. Rabeah, K. Junge, H. Junge, M. Nielsen, A. Brückner and M. Beller, Nat. Chem., 2013, 5, 537–543 CrossRef CAS PubMed; (c) L. Chen, H. Chen, R. Luque and Y. Li, Chem. Sci., 2014, 5, 3708–3714 RSC.
- R. Fang, H. Liu, R. Luque and Y. Li, Green Chem., 2015, 17, 4183–4188 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc03549k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |