Bi-functional composite electrocatalysts consisting of nanoscale (La, Ca) oxides and carbon nanotubes for long-term zinc–air fuel cells and rechargeable batteries†
Nengneng
Xu
a,
Qi
Nie
a,
Yanan
Wei
a,
He
Xu
*a,
Yu-Dong
Wang
b,
Xiao-Dong
Zhou
*b and
Jinli
Qiao
 *a
*a
aCollege of Environmental Science and Engineering, Donghua University, 2999 Ren’min North Road, Shanghai 201620, China. E-mail: qiaojl@dhu.edu.cn; hexu@dhu.edu.cn; Fax: +86-21-67792159; Tel: +86-21-67792379
bDepartment of Chemical Engineering, University of South Carolina, Columbia, SC 29208, USA. E-mail: zhox@cec.sc.edu
First published on 6th November 2017
Abstract
Here, we report a new nanocomposite based on (La, Ca) oxides, which exhibits superior bi-functional activity and durability in a zinc–air battery with an everlasting discharge peak power density achieving long life (750 cycles). This work opens a new avenue for the design/fabrication of durable bi-functional electrocatalysts for scaleable applications in zinc–air batteries.
Current materials research on fuel cells and batteries is driven by the recognition of the necessity to develop clean, efficient and affordable power systems for portable electronics, and electrical vehicles.1–3 Amongst various batteries, rechargeable zinc–air batteries (ZABs) have attracted growing interest due to their high theoretical energy density (∼1086 W h kg−1), cost-effectiveness, and environmentally friendly operation.4–7 A generic rechargeable ZAB consists of four parts: a metallic zinc anode, an air electrode as the cathode, a 30% KOH solution as the electrolyte, and a membrane to separate the anode and cathode. Currently, there are two major barriers which hinder the practical use of ZABs. The first is the sluggish activity of the air electrode towards the oxygen reduction reaction (ORR) during the discharging process and the oxygen evolution reaction (OER) during the charging process, a so-called “bi-functional” electrode. The second barrier is the insufficient cycling life of the bi-functional air electrode.8,9 Its activity degrades rapidly during operation.
With respect to the ORR activity, Pt-based catalysts are satisfactory,10 however their OER activity is inadequate and the cost is a potential concern for large scale commercial applications.11 Perovskite-type oxides have been reported as promising catalysts for ZABs due to their bi-functionality and low cost. LaMnO3 is an exemplary air electrode for ZABs, due to its rich crystal structures, compositions, and oxidation states.11–14 Recent studies showed that LaMnO3 exhibited promising activity towards the ORR in 6 M KOH,15–17 however its electrochemical stability was poor in both ORR and OER processes due to the formation of lanthanum hydroxide. The negative effect of the presence of lanthanum hydroxide can be mitigated by modifying LaMnO3. More recent research focused on investigating the effects of substituting cations at the A-site, the synthetic conditions, heat treatment, and catalytic support on the catalytic stability and activity of La1−xAxMnO3−y.18–25 Hu et al.25 synthesized La1−xCaxMnO3−y (LCMO) graphene composites by a sol–gel method for use as the air electrode of a ZAB. A catalyst support was found to increase the performance and stability of LCMO. The design of the LCMO/carbon electrode is akin to the commercial carbon supported Pt in polymer electrolyte membrane fuel cells.21,26,27
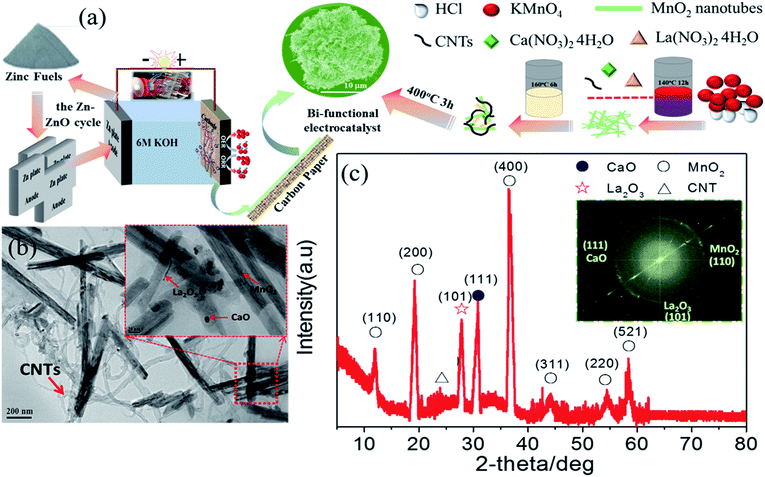
As previously mentioned, LCMO goes through a decomposition reaction in the presence of 6 M KOH during cycling. The question remains as to what the activity will be if the surface of LCMO is covered with constituent oxides. In this work, we present the electrochemical properties of a nanocomposite, consisting of CaO nanoparticles, La2O3 nanorods, and MnO2 nanotubes, supported on carbon nanotubes (CNTs) (Fig. 1(a) and Table S1†). This nanocomposite exhibits superior bi-functional activity towards ORR and OER in a ZAB with a peak power density of 203 mW cm−2 and an energy density of 826 W h kg−1, both of which are better than those of Pt/C and IrO2/C. The long-term performance of the composite electrode was demonstrated by replacing 30 pieces of zinc plate for continuous power generation.
Results and discussion
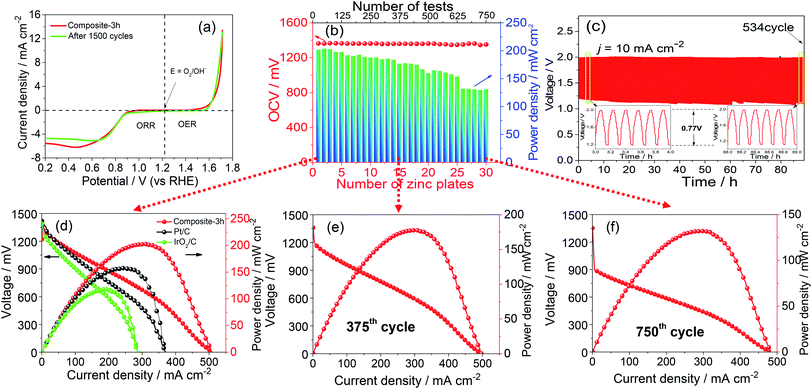
Fig. 1(a) shows the Zn–ZnO cycle for a sustainable energy system and a schematic illustration of the synthesis process of the composite-3h nanocomposite, which has a 3-D porous cinder type structure in the composite-3h electrode. The porous nature of the catalyst is a critical feature so it can tolerate volume changes during cycling.28,29 TEM was used to obtain more morphological information about the composite-3h electrode as shown in Fig. 1(b). It can be seen that both CaO nanoparticles and La2O3 nanorods were uniformly doped and coated on the surface of the MnO2 nanotubes and CNTs (Fig. 1(b)), and the MnO2 nanotubes have an average diameter of 40–60 nm and a length of 600–800 nm. The 5 nm CaO nanoparticles were embedded in the hybrid catalyst (inset of Fig. 1(b)), which is intertwined with CNTs. The inset of Fig. 1(c) shows the fast Fourier transform pattern, which includes three distinct diffraction spots assigned to the (110), (111) and (101) planes of the MnO2 nanotubes, CaO nanoparticles and La2O3 nanorods, respectively. These diffraction spots agree well with the results obtained from the XRD pattern shown in Fig. 1(c) and some of the peaks can be well indexed as La0.8Ca0.2MnO3 (JCPDS no: 46-0513).The ORR and OER activities and the durability of the nanocomposite catalysts were studied by using a half-cell in 0.1 M KOH. First of all, the electrochemical durability of the nanocomposite catalysts was evaluated by repeating the CV cycle 1500 times at a potential from 0.2 to 1.7 V vs. the reversible hydrogen electrode (RHE) (Fig. 2). As shown in Fig. 2(a), the ORR/OER onset potential and ORR half-wave potential (the E1/2 value, 0.83 V) of the composite-3h catalyst are accompanied by a diffusion-limited current plateau (5.8 mA cm−2) at the 1st cycle. After cycling, the ORR/OER onset potential and ORR half-wave potential of the composite-3h are not significantly changed, but the current density retention at 0.2 V has slightly decreased. It also exhibited a very stable ORR and OER durability. To further understand its stability, analysis of the content of the catalyst element and the combination state of the catalyst was carried out using XPS before and after full-range degradation testing. As shown in Fig. S1,† Ca always exists in the catalyst during full-range degradation testing. In particular, the O 1s spectra and the Ca 2p spectra are almost unchanged, proving that the catalyst (and especially the Ca) has a stable structure. As seen from Fig. S2(a) and (b),† the activity of the composite-3h catalyst can be compared to pure CNTs, pure MnO2, pure La2O3/MnO2, commercial 20 wt% IrO2/C (Johnson-Matthey), 20 wt% Pt/C (Johnson-Matthey), and composite-3h–CNT0 (without CNTs). Fig. S2(a)† shows the ORR onset potential (0.92 V) and half-wave potential (the E1/2 value, 0.83 V) of the composite-3h catalyst. The onset and half-wave potentials of Pt/C are 0.97 V and 0.85 V, respectively. IrO2/C exhibits onset and half-wave potentials of only 0.80 V and 0.60 V, much smaller than those of the composite-3h catalyst. The half-wave potential of the composite-3h catalyst is 200 mV more positive than that of IrO2/C and is close to that of Pt/C, indicating high oxygen reduction activity in the composite-3h catalyst. In addition, the composite-3h catalyst shows a better ORR performance than that of the catalyst without CNTs, pure MnO2, pure La2O3/MnO2 and pure CNTs (Fig. S2(b)†). As previously mentioned, the metal oxides studied here have poor electrical conductivity, while addition of CNTs can significantly improve the catalyst performance, particularly in the large current density region (Fig. S2(b)†), where a large number of electrons transfer in and out of the active sites during ORR and OER.17 Therefore, the CNTs/oxide catalyst promotes charge transfer at the oxide surfaces. Furthermore, CNTs suppress the agglomeration of oxides, thus augmenting the effective surface area and volume of the composite catalysts as shown in Fig. 1(a). Fig. S2(a) and (b)† show that the OER current density of the composite-3h catalyst is 14 mA cm−2 at 1.7 V, which is much better than for pure CNTs, pure MnO2, pure La2O3/MnO2, Pt/C and the composite-3h–CNT0. For a rechargeable ZAB, a satisfactory OER activity is mandatory to develop an efficient battery. IrO2/C is known as the best OER catalyst and is used as the baseline to study the composite-3h catalyst. The OER current density of the composite-3h catalyst is close to IrO2/C (Fig. S2(a) and (b)†). The composite-3h catalyst shows an onset potential for OER which is more positive than that of pure CNTs, pure MnO2, pure La2O3/MnO2, the composite-3h–CNT0, and Pt/C. Fig. S2(c)† shows the RDE measurement of the composite-3h catalyst loaded on a glassy carbon substrate in oxygen-saturated 0.1 M KOH at various rotation rates. The current density increases with increasing rotation rate (from 100 to 1600 rpm) due to the improved mass transport at high speeds. Koutecky–Levich analysis is shown in Fig. S2(d)† and a linear relationship can be seen, suggesting first-order reaction kinetics of the composite-3h catalyst towards ORR. The number of electrons transferred during the ORR was calculated to be between 3.76 and 3.93 at the potential from 0.6 to 0.8 V, indicating that the composite-3h catalyst proceeds via a nearly 4-electron oxygen reduction pathway.
As shown in Fig. S3(a) and (b),† the composite-3h catalyst shows a good ORR activity when compared to composite-3h-200 °C, composite-3h-300 °C, composite-3h-500 °C, composite-1h and composite-5h catalysts, where the E1/2 values are 0.83 V, 0.84 V, 0.41 V, 0.35 V and 0.61 V and the current densities only reach ∼3.6, 4.6, 1.7, 1.8 and 4.1 mA cm−2, respectively. The OER activity of the composite-3h catalyst was accompanied by an onset potential of 1.51 V, which is ∼25 mV, ∼17 mV, ∼115 mV, ∼30 mV and ∼97 mV more positive than that of composite-3h-200 °C, composite-3h-300 °C, composite-3h-500 °C, the composite-1h and composite-5h, suggesting that the optimized calcination time and temperature are essential. A shorter time or low temperature of the heat treatment results in incomplete formation of the phases in the composite-3h-200 °C, composite-3h-300 °C and composite-1h catalyst, while a longer treatment or high temperature leads to the collapse of the unique structure in the composite-5h catalyst and composite-3h-500 °C. The optimum annealing time seems to be 3 hours at 400 °C.
Fig. S3(c) and (d)† show the effect of CaO and CNTs on the ORR and OER activities. The ORR onset potential (0.92 V) and half-wave potential (the E1/2 value, 0.83 V) of the composite-3h catalyst suggest that its ORR activity is highest when the CaO/La2O3 ratio changes. The composite-3h also exhibits an onset potential for OER more positive than for the other three catalysts. At 1.7 V, the order of current densities is composite-3h > composite-3h–CaO0.5 > composite-3h–CaO2 > composite-3h–CaO0. It appears that the catalytic activity for ORR/OER can be improved by the addition of CaO into the composite electrode. Similarly, Fig. S3(d)† shows that composite-3h is the best catalyst when the CNT content in the electrode changes. The catalytic activity can be improved by the addition of CNTs into oxide catalysts; however, excessive CNT content reduces the catalytic activity. For instance, the composite-3h–CNT0.15 is wrapped in large quantities of CNTs, giving inferior activity to composite-3h which has a CNT/oxide ratio of 10%. The detailed comparison of key electrochemical properties is presented in Table S2.†
The performances of ZABs are shown in Fig. S3(b)–(f).† In order to further explain the catalyst activity and stability, a long-term test for continuous power generation was carried out, as shown in Fig. 2(b). Each zinc plate continuously generated electricity until after the 25th testing cycle, at which point the zinc plate and electrolyte were replaced. As shown in Fig. 2(b and d–f), the maximum power density reached 200 mW cm−2 at the beginning. The value gradually decreased, however 130 mW cm−2 was obtained after the 30th zinc plate was replaced (i.e. 750 cycles). On the other hand, the OCV of the single cell showed values of 1.37 V to 1.35 V during the whole operation period, and as can be seen in Fig. 2(d), the composite-3h shows the best open circuit voltage of ∼1.37 V. The current density is 150 mA cm−2 at 1.0 V, much higher than 108 mA cm−2 and 70 mA cm−2 which were obtained in commercial Pt/C and IrO2/C catalysts, respectively. In addition, the peak power density of the composite-3h (203 mW cm−2) is also higher than that of Pt/C (151 mW cm−2) and IrO2/C (117 mW cm−2) for the same conditions. The peak power density is then compared to those reported in the literature, including MnO2–LaNiO3/CNT composite (55.1 mW cm−2),29 CoO/N–CNT hybrid (265 mW cm−2)30 and Co3O4/MnO2 (36 mW cm−2).31 It should be noted that this work opens a new avenue to evaluate the stability of the catalyst and the durability of ZABs for future large-scale applications in sustainable energy and fuels. Fig. S4(a)† shows stable operation for 18 hours of the composite-3h electrode at a current density of 30 mA cm−2, superior to Pt/C (12 hours) for the same conditions. The specific capacities normalized by calculating the mass of consumed zinc material are 725 mA h g−1 and 550 mA h g−1, and the energy densities are 826 W h kg−1 and 574 W h kg−1 for the composite-3h and commercial Pt/C electrodes, respectively. It is known that the toxicity tolerance of noble metal catalysts is poor, leading to the performance degradation in the Pt/C electrode. The rechargeable ZAB performance is shown in Fig. 2(c) during charging and discharging cycles (5 minutes in each state) using a recurrent pulse current method (10 mA cm−2). The charge–discharge voltage gap of the composite-3h is only ∼0.77 V. The first six cycles show almost the same charge and discharge voltage. Even after 530 cycles, the last six cycles still retain almost the same charge and discharge voltage, as shown in the inserted figure in Fig. 2(c). During the full cycling process, the charge potential of the 534th cycle is higher by 2% compared to the 1st cycle, and the discharge potential change of the 534th cycle is lower by 12% (only 0.1 V) compared to the 1st cycle. Comparing with results in the literature,31–33 what we observed suggests that the composite-3h exhibits an excellent charge and discharge performance, thus making it an ideal candidate for zinc–air batteries. Fig. S4(b)† shows that compared with commercial Pt/C and IrO2/C, the composite-3h electrode exhibits an adequate discharging potential, thus a satisfactory ORR activity, which agrees well with the LSV and RDE results. More importantly, during the charging process at a large current density of 60 mA cm−2, the composite-3h possesses a smaller cell voltage (2.2 V) than that of Pt/C (2.5 V) and IrO2/C (2.3 V), as shown in Fig. S4(b),† suggesting the presence of a lower overpotential in the composite-3h electrode. For commercial Pt/C and IrO2/C, the voltage quickly degraded, which may be due to side reactions such as the agglomeration of the particles and the detachment of carbon at a higher potential. Our composite-3h catalyst is ideally suited for practical ZABs owing to the exceptional electrochemical activity and durability (Fig. S5†).
Conclusions
In summary, CaO/La2O3/MnO2-CNT nanocomposite catalysts were successfully synthesized through a facile hydrothermal process. The effects of calcination time, CaO/La2O3 ratio, and CNT fraction on the activity of the catalysts were studied. The composite electrode annealed at 400 °C for 3 hours shows a highly active bi-functionality and an everlasting discharge peak power density for both ORR and OER in ZABs. The ZABs fabricated with this catalyst provide prominent performance over hundreds of charge–discharge cycles. All tests suggest that the composite-3h nanocomposite could be used as a bi-functional cathode catalyst for practical ZABs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (U1510120); Natural Science Foundation of Shanghai Science and Technology Committee (14ZR1400700) and the International Academic Cooperation and Exchange Program of Shanghai Science and Technology Committee (14520721900).References
- M. T. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- B. J. Waghmode, S. N. Bhange, S. M. Unni, K. R. Patil and D. D. Malkhede, Sustainable Energy & Fuels, 2017, 1, 1329–1338 CAS.
- H. Y. Duan, T. T. Yan, Z. Y. Li, G. R. Chen, J. P. Zhang, L. Y. Shi and D. S. Zhang, Sustainable Energy & Fuels, 2017, 1, 1557–1567 CAS.
- M. A. Rahman, X. Wang and C. Wen, J. Electrochem. Soc., 2013, 160, 1759–1771 CrossRef.
- R. G. Cao, J. S. Lee, M. L. Liu and J. Cho, Adv. Energy Mater., 2012, 2, 816–829 CrossRef CAS.
- N. N. Xu, Y. Y. Liu, X. Zhang, X. M. Li, A. J. Li, J. L. Qiao and J. J. Zhang, Sci. Rep., 2016, 6, 33590–33600 CrossRef CAS PubMed.
- J. S. Lee, S. T. Kim, R. G. Cao, N. S. Choi, M. L. Liu, K. T. Lee and J. Cho, Adv. Energy Mater., 2011, 1, 34–50 CrossRef CAS.
- J. S. Lee, G. S. Park, H. I. Lee, S. T. Kim, R. G. Cao, M. Liu and J. Cho, Nano Lett., 2011, 11, 5362–5366 CrossRef CAS PubMed.
- N. N. Xu, X. M. Li, H. R. Li, Y. N. Wei and J. L. Qiao, Sci. Bull., 2017, 62, 1216–1226 CrossRef.
- Z. W. Chen, D. Higgins, A. P. Yu, L. Zhang and J. J. Zhang, Energy Environ. Sci., 2011, 4, 3167–3192 CAS.
- Y. Gorlin and T. F. Jaramillo, J. Am. Chem. Soc., 2010, 132, 13612–13614 CrossRef CAS PubMed.
- C. A. Hancock, A. L. Ong, P. R. Slater and J. R. Varcoe, J. Mater. Chem. A, 2014, 9, 3047–3056 Search PubMed.
- L. Q. Mao, D. Zhang, T. Sotomura, K. Nakatsu, N. Koshiba and T. Ohsaka, Electrochim. Acta, 2003, 48, 1015–1021 CrossRef CAS.
- Z. Chen, A. P. Yu, R. H. Ahmed, H. J. Wang, H. Li and Z. W. Chen, Electrochim. Acta, 2012, 69, 295–300 CrossRef CAS.
- J. Sunarso, A. A. Torriero, W. Zhou, P. C. Howlett and M. Forsyth, J. Phys. Chem. C, 2012, 116, 5827–5834 CAS.
- M. Yuasa, N. Yamazoe and K. Shimanoe, J. Electrochem. Soc., 2011, 158, A605–A610 CrossRef CAS.
- K. Miyazaki, K. I. Kawakita, T. Abe, T. Fukutsuka, K. Kojima and Z. Ogumia, J. Mater. Chem., 2011, 21, 1913–1917 RSC.
- X. X. Zhang, Q. Q. Xiao, Y. X. Zhang, X. Jiang, Z. Y. Yang, Y. F. Xue, Y. M. Yan and K. N. Sun, J. Phys. Chem. C, 2014, 118, 20229–20237 CAS.
- Y. F. Dong, W. L. Wang and K. J. Liao, Sens. Actuators, B, 2000, 67, 254–257 CrossRef CAS.
- C. M. Chiu and Y. H. Chang, Thin Solid Films, 1999, 342, 15–19 CrossRef CAS.
- Y. G. Li and H. J. Dai, Chem. Soc. Rev., 2014, 43, 5257–5275 RSC.
- S. Muller, K. Striebel and O. Haas, Electrochim. Acta, 1994, 39, 1661–1668 CrossRef.
- N. L. Wu, W. R. Liu and S. J. Su, Electrochim. Acta, 2003, 48, 1567–1571 CrossRef CAS.
- N. N. Xu, J. L. Qiao, X. Zhang, C. Y. Ma, S. S. Jian, Y. Y. Liu and P. C. Pei, Appl. Energy, 2016, 175, 495–504 CrossRef CAS.
- J. Hu, L. N. Wang, L. N. Shi and H. Huang, J. Power Sources, 2014, 269, 144–151 CrossRef CAS.
- E. J. Lim, S. M. Choi, M. H. Seo, Y. Kim, S. Lee and W. B. Kim, Electrochem. Commun., 2013, 28, 100–103 CrossRef CAS.
- F. B. Wang, J. Wang, L. Shao, Y. Zhao and X. H. Xia, Electrochem. Commun., 2014, 38, 82–85 CrossRef CAS.
- A. C. C. Tseung and S. Jasem, Electrochim. Acta, 1977, 22, 31–34 CrossRef CAS.
- H. Y. Ma and B. G. Wang, RSC Adv., 2014, 4, 46084–46092 RSC.
- Y. G. Li, M. Gong, Y. Y. Liang, J. Feng, J. E. Kim, H. L. Wang, G. S. Hong, B. Zhang and H. J. Dai, Nat. Commun., 2013, 4, 1805–1812 CrossRef PubMed.
- G. J. Du, X. G. Liu, Y. Zong, T. S. A. Hor, A. S. Yu and Z. L. Liu, Nanoscale, 2013, 5, 4657–4661 RSC.
- M. Prabu, P. Ramakrishnan and S. Shanmugam, Electrochem. Commun., 2014, 41, 59–63 CrossRef CAS.
- Y. Zhan, G. J. Du, S. L. Yang, C. H. Xu, M. H. Lu, Z. L. Liu and J. Y. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 12930–12936 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, ORR and OER polarization curves, charge–discharge polarization and the real zinc–air battery. See DOI: 10.1039/c7se00444c |
| This journal is © The Royal Society of Chemistry 2018 |