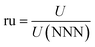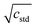The use of silver nanoparticles for the recovery of uranium from seawater by means of biofouling mitigation
Margaret Flicker
Byers
 *,
Sheldon
Landsberger
and
Erich
Schneider
*,
Sheldon
Landsberger
and
Erich
Schneider
University of Texas at Austin, Department of Mechanical Engineering, Nuclear and Radiation Engineering Program, I University Station C2200, Austin, Texas, USA 78712. E-mail: Margaret.Byers@austin.utexas.edu
First published on 17th August 2018
Abstract
Research currently being carried out by a consortium of national laboratory and university partners is leading to the development of advanced adsorbent materials suitable for the industrial-scale recovery of the essentially limitless supply of uranium naturally contained within the worlds oceans. Experiments have shown however that marine biofouling has decreased the uranium capacity of these adsorbent fibers by approximately 30%. Given the commensurate impact adsorbent capacity has on the projected cost of seawater uranium, this work seeks to mitigate the effects of marine biofouling in order to restore capacity to that of the unfouled fibers. A review of antifouling strategies showed that just one, incorporation of silver nanoparticles into the adsorbent matrix, was likely to meet cost and practicality goals. The ability of silver doped adsorbents to restore capacity to that of the unfouled adsorbents is quantified by means of a capacity restoration factor, seen to increase with silver content in the adsorbent. These results showed that the silver nanoparticles are extremely effective at eliminating the ca. 20% share of capacity loss that is directly attributed to the biological organisms. This work also identified the a-biotic conditioning film as a primary driver of loss in uptake due to biofouling, which provides finer resolved data and a deeper understanding of uranium uptake experiments, conducted externally, that showed a constant reduction in uptake at all measured data points, the smallest of which was 7 days. Data reduction showed that under the most extreme fouling conditions, approximately 80% of the uptake loss was ascribable to the fast-developing film, against which silver is observed to have little effect. Therefore this work was significant in proving that seawater uranium recovery efforts need to refocus on halting the formation of this abiotic film to improve uranium capacity.
1 Introduction
The worlds oceans naturally contain uranium in a concentration of 3 ng g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 representing a potential supply of tens of thousands of years of nuclear fuel. Although ample uranium currently exists from terrestrial sources, the ability to recover uranium in this capacity would provide fuel supply security for nuclear power generation, putting it on nearly equal footing with renewable energy technologies. Additionally the ability to recover this uranium at large scale would provide policy makers and energy investors with economic security by placing a ceiling on the cost of uranium. Lastly, uranium extraction from seawater circumvents the environmental impact associated with the removal of any land-based resource.
1 representing a potential supply of tens of thousands of years of nuclear fuel. Although ample uranium currently exists from terrestrial sources, the ability to recover uranium in this capacity would provide fuel supply security for nuclear power generation, putting it on nearly equal footing with renewable energy technologies. Additionally the ability to recover this uranium at large scale would provide policy makers and energy investors with economic security by placing a ceiling on the cost of uranium. Lastly, uranium extraction from seawater circumvents the environmental impact associated with the removal of any land-based resource.
Research currently being carried out by a consortium of national laboratory and university partners, led by Oak Ridge and Pacific Northwest National Laboratories (ORNL and PNNL) is leading to the development of advanced polymer-based materials suitable for large-scale deployment in the ocean.2,3 In the referenced strategy adsorbents fibers are then constructed into long braids so they may be moored to the bottom of the ocean floor where they are allowed to passively adsorb uranium.4–6 The adsorbent materials may be deployed for multiple use campaigns where the economically optimal number of times the adsorbent can be reused is determined by a numerical model7 and is primarily a function of the cost of each deployment event as well as the uranium uptake achieved on the given deployment cycle, which degrades overtime due to seawater exposure.
Experiments have proven that when uranium adsorbing fibers are placed in a bioactive marine environment, the mass of uranium recovered relative to those exposed to filtered, biologically inert seawater decreases due to the presence of micro-organisms.8 These experiments have shown that biomass, consisting of organic macromolecules as well as microorganisms, accumulates on the adsorbent surface, and this biofilm is suspected to act to physically block the uranium binding sites from making contact with the water, and the uranyl ions it contains. Since the uranium capacity of the adsorbent has been classified as a major cost driver of final uranium production cost6,9,10 there is a significant impetus to mitigate the effects of biofouling to maximize uranium uptake. Experiments by Park et al.8 have seen a loss in uranium uptake as high as 30% due to the effects of marine biofouling, but did not attempt to deconvolve the loss due to abiotic marine fouling from the loss in uptake due to microorganism growth. Subsequent economic analysis showed that this 30% loss in uptake results in an approximately 30% increase in the unit cost of seawater uranium.11
Therefore the aims of this research are to quantitatively determine the degree to which adsorbent capacity is hampered by the presence of microorganisms as compared to abiotic factors. Additionally a method for mitigating the loss due to one of these mechanisms, microorganism growth, was developed and demonstrated in an adsorbent similar to those synthesized by ORNL. The efficacy and uncertainty of the mitigation technique were quantified using a measure termed the capacity restoration factor as determined using neutron activation analysis (NAA) since previous publications,12,13 proved NAA to be the most suitable method for quantifying uranium uptake at these low levels and detail the specific methods utilized.
If this technique can successfully mitigate microorganism growth without hampering the ability of the polymer to be extruded, then it should be easily adaptable to all national laboratory and university adsorbent types that are melt-spun from a variety of thermoplastics. This is particularly important given the variety of adsorbent types and substrate materials under development.2,14,15 Furthermore, the ability to quantitatively attribute loss in uptake to the different marine loss mechanisms at play serves an important role in highlighting a capacity driver, thus guiding future work aiming to continually improve uranium recovery.
2 Review of existing antifouling strategies
The desire to mitigate oceanic biofouling is by no means unique to the uranium from seawater project, and a literature review uncovers numerous measures taken to mitigate fouling by microorganisms.16 Various antifouling strategies were considered for the coupling with the recovery of seawater uranium via the passive scheme, but none of these strategies could be directly applied due to either their prevention mechanisms or implementation cost. Antifouling paints or coatings for example while widely available for commercial uses, which is potentially favorable from an economic standpoint, prevent the surface of the adsorbent from contacting the surrounding seawater, blocking the adsorption mechanism altogether.17,18 Active methods of biomass removal and prevention, including electrically or pneumatically induced surface perturbations and high velocity based removal, were also considered but would likely add exorbitant cost to seawater uranium recovery.19–21 Additional passive mechanical or chemical measures such as changing deployment configuration or fiber material or shape may exist but were considered out of scope as they would effectively negate the years of collaborative research working to optimize uranium uptake.The sole strategy that meets the viability criteria of minimizing cost and perturbations to the adsorbent and deployment scheme was the use of silver nanoparticles to introduce antimicrobial properties.22–26 This research therefore aims to take advantage of the proven capabilities of silver nanoparticles and extend them to a novel application, the recovery of uranium from seawater. While nanoparticles have been placed in polymer matrices for the introduction of antifouling properties to water treatment membranes,27,28 the application to these marine adsorbents and the method of synthesis are unique. The addition of silver nanoparticles to the polymer backbone would result in a new marine fouling resistant uranium adsorbent, thus achieving a higher uptake than its non-silver modified counterpart, ideally reducing the cost of seawater uranium recovery. Additionally, this study examines the ability of silver nanoparticles to mitigate the loss in capacity due to microorganism growth as well as the abiotic conditioning phase of marine biofouling. It can be seen in the literature that biofouling begins with the establishment of a chemical conditioning film before the colonization of micro-organisms.29–31 While there are many literature references confirming the antibacterial characteristics of silver nanoparticles,23–26 none could be found that specifically addressed the propensity of silver nanoparticles to prevent or eliminate the presence of macromolecules or dissolved organic matter.
There exists of course a trade-off between the increased uranium uptake and the cost incurred to mitigate biofouling, the economic analysis of silver nanoparticle use is beyond the scope of this paper which strictly aims to quantify this mitigation method and provide data that could feed into a cost analysis.
The novelty of this work is in quantifying the degree to which uranium capacity is reduced by the presence of marine microorganisms as opposed to marine macro-nutrients that exist to support that biological growth. It is already known that 2 mechanisms contribute to adsorbent surface colonization and thus presumably capacity loss: the marine organisms themselves and the organic macromolecules that serve as their nutrients.29–32 Both act by reducing the contact area between adsorbent and seawater should they adhere onto the surface, the degree to which each of these mechanisms is responsible for the loss in uptake is unknown. Additionally, the ability of silver-doped uranium adsorbents to restore the capacity loss due to microorganism growth will be quantified by means of a capacity restoration factor. More specifically, the ability of silver nanoparticles to restore the loss due to strictly microorganism growth is also quantified.
3 Methodology
3.1 Creation of surrogate adsorbents
Given the costly and time intensive nature of both the synthesis process and uptake kinetics of the ORNL adsorbent material it was decided that surrogate adsorbents would be used to allow for the timely completion of large numbers of fouling and uptake trials with minimal cost. The radiation induced graft polymerization process used to construct the ORNL adsorbents requires use of specialized equipment and spans several days, making it difficult to create the large number of highly reproducible adsorbents needed for these experiments. After testing a variety of synthesis methods it was concluded that reproducible surrogate adsorbents would best be represented by suspensions of coconut shell activated carbon in uranium spiked aqueous solution. While filament type adsorbents created by extruding fine activated carbon powder, <250 μm, in a high density polyethylene matrix were initially sought out for this work, as covered in great detail in ref. 12 and 13, those publications concluded that activated carbon granules, with no binding material, provided the most homogeneous surface area to volume ratio across batches. Therefore activated carbon granules of size 0.6 to 2 mm were used as received and deployed directly in uranium spiked solution.Time series data for adsorbents in suspensions was collected in order to quantify the relationship between uranium uptake and length of soaking campaign as well as to analyze consistency of performance within adsorbent batches. The uptake of all adsorbents was determined using the NAA methodology described in ref. 12 and 13. Following literature characterizations of the adsorption of uranyl ions onto activated carbon sites,33,34 the measured uptake data was transformed and fit to a pseudo-first order kinetics model. Although this uptake model is different than the one site ligand saturation model commonly used to characterized the uranium uptake of the ONRL adsorbents3,7 there is evidence to suggest that their uptake is driven by both diffusion rate and mass transfer effects across the adsorbent–water interface.35 Given that organisms and macromolecules influence uptake by reducing adsorbent surface area in contact with seawater, both diffusion and ligand-site limited uptake are affected, thus the use of the diffusion limited surrogate adsorbents is justified.
The pseudo-first order kinetics model, seen in eqn (1), is a function of the Lagergren rate constant, K1 and the adsorbent capacity at equilibrium, qe
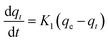 | (1) |
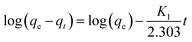 | (2) |
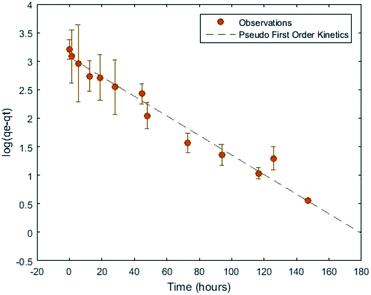 | ||
| Fig. 1 Adsorbent suspension uptake performance from ref. 13 fit to a pseudo first order kinetics model. | ||
Iterations of sample preparation and experimental procedures, described in ref. 12 and 13, were aimed at reducing aleatoric uncertainties and eliminating systematic sources of uncertainty. The largest remaining uncertainty derives from uptake variation within adsorbent batches. This uncertainty could be mitigated at the expense of time by running sufficient replicates to arrive at independent, low-variance statistical distributions of uptake for each batch. It will be shown that the aleatoric uncertainties reported in Section 4, which are typically on the order of 10%, are sufficiently small to support the main conclusions of this work.
3.2 Adsorbent deployments
In an effort to reduce sources of uncertainty a number of adsorbent deployments were conducted using uranium spiked DI water as opposed to real or simulated seawater. Analogous to experiments conducted using the national laboratory adsorbents,2,15 the solution was spiked with uranium in the form of the uranyl ion, UO2+, in these experiments by means of uranyl acetate. In these experiments, a higher uranium concentration, 500 μg g−1, was utilized to achieve reasonably fast kinetics even in the limit of the low uranium affinity of the activated carbon.33,34The resulting time dependent uptake, transformed to account for the heteroskedasticity of the data observed in ref. 13 for these batch experiments can be seen in Fig. 1 where the kinetics were discussed. These experiments were conducted by exposing suspensions consisting of ratios of 0.5 grams of activated carbon per 50 mL of uranium spiked solution, at room temperature under constant rotation.
Before carrying out further experimentation to test the antifouling capabilities of silver nanoparticles it was necessary to first identify the conditions in which marine biofouling had a non-negligible effect on the uptake of uranium by adsorbent samples. To expedite the fouling process and mimic the pre-fouling of the adsorbent surface carried out by PNNL,3,8 a biphasic deployment process was used where in adsorbents were subjected to a pre-fouling stage, before being exposed to the uranium spiked solution.
The literature was reviewed to identify a fouling agent most appropriate for experiments moving forward and ultimately Vibrio fischeri, a bioluminescent marine bacteria known to contribute to biofilms, was selected.36,37 A pure stock culture of plated Vibrio fischeri (ATCC 49387) was obtained from PNNL and maintained in nutrient rich AB media.38
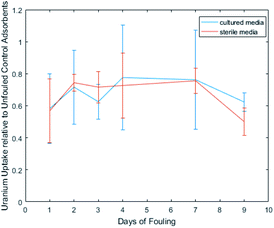
Adsorbent samples were exposed to either media cultured with Vibrio fischeri or sterile AB media, for varying time intervals, before exposure to uranium spiked solution in an effort to quantify the loss due to the two biofouling mechanisms. Fig. 2 depicts the uptake performance, relative to unfouled control adsorbents, achieved by adsorbents exposed to each condition, and it is apparent that the effects of biofouling are independent of time.
Ultimately a single phase deployment was also carried out by exposing adsorbents doped with varying degrees of silver, including a negative control, to real seawater provided by PNNL Marine Science Laboratory from Sequim Bay, WA. While seawater was again spiked to achieve 500 μg g−1 uranium at the initiation of these batch experiments, no pre-fouling was conducted and no media was added, thus closely approximating the experience of adsorbents placed in a true marine environment. A set of reference adsorbents representing the unfouled performance were likewise exposed to real uranium spiked seawater, but efforts were made to eliminate or reduce fouling as much as possible. Following methodologies used in PNNL fouling experiments8 the seawater was sterilized using a 0.22 μm vacuum filtration unit and not exposed to light, in order to limit photosynthetic growth.
3.3 Re-usability of adsorbents
Since the currently proposed deployment technique for recovering uranium from seawater involves the recycle of adsorbent, it is also important to examine the re-usability of the silver doped adsorbents. While there is no evidence to suggest the addition of silver nanoparticles would degrade the adsorbents durability, there does exist the potential that the silver nanoparticles dissolve or are physically removed from the adsorbent, diminishing the biofouling mitigation capabilities upon reuse. Since the elution process is assumed to remove all or a majority of the microorganisms such that the loss due to biofouling starts at or near 0% with each reuse, it could then be reasonably concluded that the biofouling mitigation capabilities would be present on subsequent reuses of the adsorbent so long as the silver nanoparticles remain present.Some number of silver ions will presumably be lost via the biofouling mechanisms, either by attaching to or entering bacterial cells.24,39–41 It is hypothesized however that this loss mechanisms would be on the order of ions and thus negligible, resulting in the majority of silver nanoparticles remaining embedded in the polymer matrix.
Unlike the true uranium from seawater adsorbents, no elution method is used to remove adsorbed metals from the surrogate adsorbent. Since the only concern of this particular project is uptake ability, and not actual collection of adsorbed material, the concentration of uranium is tested while still on the adsorbent in order to avoid the introduction of another source of variability. Therefore, reuse studies cannot be conducted by simply removing uranium and redeploying adsorbents. Instead the rate at which silver nanoparticles have vacated the adsorbent was examined by comparing the silver content of adsorbents before and after deployment.
Given the high likelihood of naturally occurring silver isotopes to interact with neutrons of various energies, silver, even at the small concentrations used in these adsorbents, is a good candidate for quantification via NAA. The irradiation parameters, 10 second epithermal irradiations, already in use for determination of uranium uptake12,13 resulted in substantial activation of the silver nanoparticles. Gamma spectroscopy analysis of the 108Ag content resulting from activation and use of certified reference materials allowed for quantification of the silver content with detection limits as low as 10 ppm. This method of testing re-usability was conducted using filament type adsorbents, rather than suspensions. Although the filament adsorbents, consisting of activated carbon particles in a high density polyethylene matrix,12 were not ultimately analyzed for capacity restoration, as discussed in a previous publication13 they were believed to be better suited for these reusability experiments given that their form was more reflective of the fibrous adsorbents described in the literature2,6,7 to be used for industrial scaled seawater uranium recovery.
4 Results and discussion
4.1 Qualitative
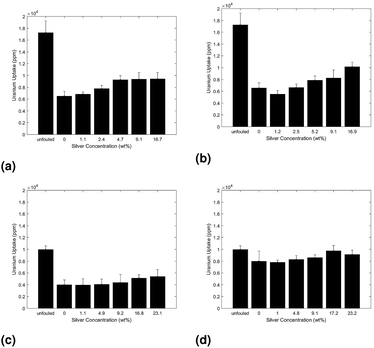
The uptake performance was analyzed for a variety of different fouling conditions to first qualitatively examine the loss, and subsequent restoration by silver nanoparticles, of adsorbent uptake as a function of hydrocarbon concentration. In all trials ANalysis Of VAriance (ANOVA) was used to test the statistical significance of the increase in uptake of the silver doped adsorbents as compared to their unmodified control adsorbents.The uptake for adsorbents prefouled in solutions with depreciating concentrations of AB media is displayed in Fig. 3. Parts 3a and 3b of Fig. 3 were subsequently exposed to uranium spiked DI water while parts 3c and 3d were exposed to uranium spiked real seawater.
In all three trials the uptake increases with appreciating silver concentration, indicating the silver nanoparticles offer some degree of capacity restoration. Additionally, the uptake increases with decreasing media concentration, as seen in Fig. 3, suggesting the hydrocarbon conditioning phase plays a non-trivial role in the loss in uptake due to biofouling.
The ability of the silver nanoparticles to mitigate biofouling by the microorganisms was also qualitatively analysed by monitoring adsorbents for signs of impeded growth. Fig. 4 depicts the qualitative difference in opacity of the pre-fouling solution, indicative of bacterial growth, for the negative control (left) as compared to silver doped adsorbents (right). Visual inspection clearly indicates that the silver nanoparticles are in fact retarding bacterial growth.
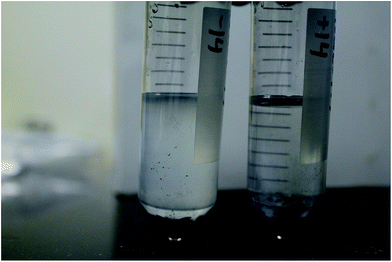 | ||
| Fig. 4 Comparison of bacterial growth occurring in pre-fouling solution during deployment of negative control (left) and silver doped adsorbents (right). | ||
Finally, the use of abiotic media was eliminated in an additional trial to reduce the hydrocarbon concentration as well as most closely replicate true marine conditions. This single phase exposure trial, described in the methodology, consisted of adsorbents exposed to uranium spiked seawater without a pre-fouling phase. The resulting uptake as a function of adsorbent formulation can be seen in part 3d of Fig. 3.
The trend in uptake as a function of silver content in adsorbent again aligns with previous trials but more closely approaches that of the unfouled adsorbents. These qualitative results all suggest that the silver nanoparticles are in fact capable of restoring the loss in uptake due to microorganism growth, but do not yet provide a clear picture of the extent to which each of the two loss mechanisms plays a role. Therefore the next section quantitatively analyses performance data to separate the loss due to microorganisms from the loss due to abiotic media, so the restoration capabilities of silver may be quantified.
4.2 Quantitative
The collected data enabled calculation of the key measure of the effectiveness of silver nanoparticles. The results displayed in Fig. 3 and 4 indicated that two mechanisms, the presence of the nutrient-rich abiotic media and the growth of microorganisms themselves, act to reduce uranium capacity. While plenty of data exists in the literature supporting the ability of silver nanoparticles to inhibit the growth of the microorganisms themselves, none could be found to suggest the silver nanoparticles would inhibit the establishment of an a-biotic chemical conditioning film that precedes the colonization of a surface by microorganisms.22–26 Therefore it was first necessary to deconvolve the share of loss due to the two different mechanisms in order to test the hypothesis that silver nanoparticles restore the capacity loss ascribed to the organisms but have little or no effect on loss due to the media.This was done by collecting experimental data while varying the relevant independent variables (pre-fouling with the abiotic media, introduction of the microorganisms, and the addition of silver nanoparticles to the adsorbent) while holding the conditions affecting uranium uptake, such as uranium concentration and immersion time, constant. Each data set collected is described in Table 1, indicating: exposure to each condition (Y = yes, N = no) the number of replicates in the set, N, the average uptake, U [ppm], the standard deviation, σ, obtained by fitting the data to a normal distribution, and the standard error, SE, obtained by multiplying the standard deviation by (1 + N1/2).
The second to last column in Table 1, ru, normalize all of the raw uptake data against the uptake for case 1, which effectively serves as the base case having no media, organisms, or silver present. The two data sets denoted with an * in Table 1 consisted of adsorbent exposed to uranium spiked solution for shorter amounts of time, in the face of facility and personnel constraints, so they must be effectively scaled to be used in conjunction with the other cases. Earlier experimentation, as seen in Fig. 2, indicates that the relative reduction in uptake for any case becomes time invariant for fouling periods greater than 1 day. Therefore datasets 3 (U3 = 8795 ppm) and 4 (U4 = 2423 ppm) are postulated to have incurred equal relative reductions in capacity. Hence, the uptakes of datasets 4 and 5 were first scaled by the ratio of observed uptake in case 3 to case 4 (U3/U4), such that once normalized against the base case, they both result in the same degree of uptake reduction.
The loss in uptake due to strictly the macromolecules in the media was isolated by comparing the loss in uptake observed in case 2, with only hydrocarbons present, to the loss observed in the presence of both media and microorganisms, case 3 (eqn (3)).
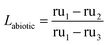 | (3) |
Considering both of those uptakes in light of the negative control adsorbents, case 1, shows that 80 ± 9.5% of the loss in uptake due to biofouling is a result of strictly the initial abiotic conditioning phase.
Secondly, to test the efficacy of silver nanoparticles, the restoration of capacity attributable to the introduction of silver (case 5 as compared to case 4) is compared against the loss of capacity accountable to the introduction of organisms (case 3 as compared to case 2). The portion of capacity lost as a result of strictly biological organism, LV. fischeri can thus be isolated from the capacity loss due to the combination of organisms and media by eqn (4).
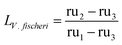 | (4) |
Employing standard uncertainty propagation techniques, this is found to be LV. fischeri = 0.20 ± 0.09. The same normalization can then be used to calculate the capacity restoration accountable to the use of silver nanoparticles seen in eqn (5) for these cases, where it is determined that R = 0.3 ± 0.2.
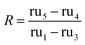 | (5) |
The ratio of these two factors, specifically the statistical likelihood that the ratio is different from zero, supports the working hypothesis concerning the efficacy of the silver:silver nanoparticles are very useful against organism growth, possibly even entirely removing their effect on uptake, but are not effective for disrupting the formation of the hydrocarbon coating. Assuming independence of errors between all sets of trials, the ratio of restoration by silver nanoparticles to loss due to strictly V. fischeri is 1.52 ± 1.03 Application of the t-test yields a 93.1% confidence that this ratio is different from zero, the point at which the silver nanoparticles have no effect on capacity.
Therefore, these experiments have proven, with over 90% confidence, that the silver nanoparticles restore the loss in adsorbent capacity resulting from the growth of V. fischeri. Since the relative restoration of capacity when the silver-doped adsorbent is used in the presence of organisms is greater than the relative loss when the organisms are added (i.e. 1.52 > 1) it is tempting to conclude that the silver nanoparticles also restore part of the capacity loss due to introduction to the media. Statistical uncertainties however prevent such a conclusion from being reached with any degree of confidence. While the uncertainty values could have been reduced with repeated experimentation, time limitations as well as equipment availability, namely nuclear reactor availability, imposed a maximum number of feasible experimental trials.
It is worth noting however that instances were seen in the literature of uranyl ions adsorbing onto silver nanoparticle surfaces.42,43 Therefore it is entirely possible that some degree of the enhanced uptake achieved by the silver doped adsorbents resulted from uranyl adsorption to the silver nanoparticles directly. This mechanism of capacity increase could be considered a desirable by-product of silver nanoparticles as it still works toward the overarching goal of decreasing the production cost of seawater uranium by improving adsorbent uptake, and is not believed to be the major driver of increased uptake.
The resulting weight gain, as a fraction of initial adsorbent weight, for the series of adsorbent formulations fouled in cultured AB media diluted with 75% sterile NaCl can be seen in Fig. 5.
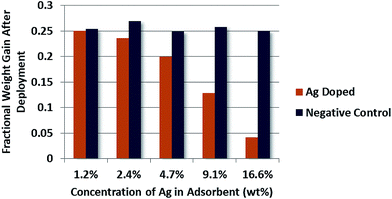 | ||
| Fig. 5 Quantitative analysis of bioaccumulation mass on adsorbent surfaces pre-fouled in diluted AB media cultured with Vibrio fischeri as a function of silver content tin adsorbent. | ||
It is clear from these results that the silver doped adsorbents experience a lower degree of bioaccumulation than their non-doped counter parts. One exception may be present however in the case of the adsorbent population doped with 1.2% silver by weight.
Although the fractional increase in weight gain is in fact lower for the silver doped population, it does not differ by more than the standard deviation of all negative control adsorbents, which in theory should have identical performance. Therefore, while it appears that increasing the silver content of adsorbents does enhance the bio-inhibition abilities, this effect does not necessarily extend down to very low silver contents, on the order of 1% by mass.
Upon first observation difficulty may arise in reconciling the large decrease in bioaccumulation experienced by the doped adsorbents, especially with high silver contents, as compared to the smaller increase in restoration of capacity seen in Fig. 3. It is thus worth pointing out that experiments by PNNL also observed what may appear as a discrepancy between mass of accumulated biofouling material and loss in uptake, where fouled adsorbents increased in mass by 80% relative to their initial weight while only reducing uranium uptake by 30%, while the unfouled adsorbents gained only 20% of their initial dry mass.8 The substantial weight gain by the fouled PNNL adsorbents may be expected to observe a more severe loss due to biofouling, indicating that while bioaccumulation is correlated to loss in uptake, sufficient evidence does not exist to suggest the two are linearly proportional. This further supports the conclusion drawn in Section 4.2, that the establishment of a very low mass conditioning film, composed of the macro-nutrients present in the media, contributes a non-trivial component of the capacity loss due to biofouling while the silver nanoparticles prevent further bacterial growth outward of the filmed surface.
To further conclude that the effects observed in uptake restoration are in fact a result of biofouling mitigation, the viability of microorganisms existing on the adsorbent surface post-deployment was explored. After adsorbents had been deployed and their uptake quantified they were placed in sterile AB media and incubated for 2 days in an effort to analyze the viability of growth by residual Vibrio fischeri remaining on the adsorbent surface. The observed opacity of the AB media in Fig. 6 shows significantly more growth resulting from the negative control adsorbents (left) as compared to the silver doped adsorbents (right).
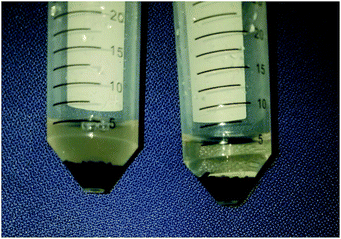 | ||
| Fig. 6 Qualitative analysis of bioaccumulation on the negative control adsorbent (left) as opposed to silver doped (right) adsorbent surfaces post-deployment. | ||
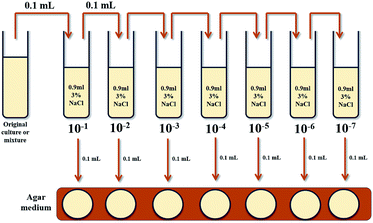
Given the difficulty of manually counting bacteria in solution, even under a microscope, a technique common in microbiology to evaluate the number of Colony Forming Units (CFU), referred to as a serial dilution, was implemented to examine the extent of the growth differences. A serial dilution was conducted on the solutions pictured in Fig. 6 in order to isolate a collection of bacterial colonies small enough to be accurately counted. The methodology for a serial dilution can best be described pictorially by Fig. 7.
The initial cultured solution undergoes a series of 10-fold dilutions, in sequences, in sterile 3% NaCl solution. Each resulting solution is spread onto an AB agar plate for incubation at room temperature so the number of viable colonies may be determined. The three plates that have observable colony formation most suitable for manual counting are selected for analysis to back-calculate the number of CFU in the original solutions.
In the case of the negative control adsorbents the last three solutions were used for average colony quantification. The AB agar plates spread with these solutions after the incubation were determined to contain approximately 3.5 × 108 CFU mL−1 original solution.
Analysis of the plates spread with varying dilutions of the media exposed to silver doped adsorbents was likewise carried out. Unlike the negative control adsorbents however, growth could only be observed on the plates spread with the initial media exposed directly to the adsorbents and the first solution in the series. The concentration of Vibrio fischeri contained in the original solution was calculated to be 375 CFU mL−1, a much lower value than that of the negative control adsorbents. It should be noted that the bacterial colonies identified on the plate are not guaranteed to all be Vibrio fischeri given that the exposure of the non-sterile adsorbents to the media potentially introduced any ambient bacteria species already present on the non-sterile adsorbent.
It is clear from both these qualitative and quantitative analyses that the presence of silver nanoparticles has hampered the ability of Vibrio fischeri to grow on the surface of the activated carbon adsorbents. These observations, coupled with the increased uptake characteristic of the silver doped adsorbents certainly indicate that the silver nanoparticles were acting to mitigate some of the effects of marine biofouling. Furthermore, the confirmed presence of biofouling hampered uptake and subsequent impeded growth of marine bacteria in the presence of silver nanoparticles clearly indicates that the abiotic conditioning film, against which silver nanoparticles appeared to provide little relief, play a key role in biofouling of adsorbents.
The quantitative divergence between restoration of capacity and bioaccumulation as a function of silver doping could likewise be explained by the notion that the loss in uptake observed is dictated by the presence of the chemical conditioning film. If microorganism growth was in fact the primary cause of uptake degradation, then increasing silver concentrations in adsorbent would correspond with decreased bioaccumulation and increased capacity recover factors. Additionally, the rate at which these two phenomena were observed would be expected to be quite similar if a decrease in bacterial growth correlated directly with enhanced uranium recovery. While the first criteria of qualitative agreement of trends is met, the latter is not, thus indicating some other, abiotic, factor contributes to the loss due to biofouling experienced by adsorbents deployed in nutrient rich media.
4.3 Re-usability
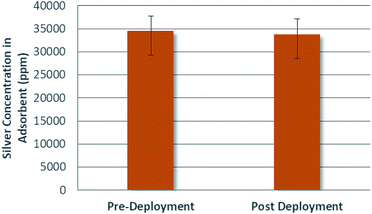
The concentration of silver in a given population of adsorbents was analyzed before and after deployment in uranium spiked real seawater. The average silver concentrations for the adsorbents can be seen in Fig. 8.Inspection of Fig. 8 yields no evidence of concentration loss. Supporting ANOVA analyses were carried out and could be extended, for instance by way of the two one-sided t-test (TOST) approach to prove this hypothesis that concentration loss was negligible.
4.4 Uncertainty quantification
At the onset of experimental design significant effort was made to identify all possible sources of uncertainty. The general strategy was to first attempt to reduce the sources of uncertainty, and then those which could not be fully eliminated were quantified by the use of replicates. This sub-section details each of the identified sources contributing to the uncertainty in the capacity and means taken to alleviate or quantify them.In doing so, the distinction is first made between epistemic and aleatoric uncertainty, where the latter is the focus of this section. Epistemic uncertainty can be considered analogous to systematic uncertainty, while aleatoric uncertainties are those that differ each time an identical experiment is run and include statistical effects. Aleatoric uncertainties must be quantified so that they may be propagated through final calculations of capacity restoration of uptake, and eventually seawater uranium production cost. In the case of both aleatoric and epistemic uncertainties there is a desire to mitigate them as much as possible.
The tables below identify all of the uncertainties present in the ultimate determination of the performance enhancement offered by the use of silver nanoparticles, as determined using uranium uptake by activated carbon, along with plans for alleviating them. In the case of the aleatoric uncertainties listed in Table 2 the quantification, which may be an analytical calculation applied to each sample or a value observed across populations, is also included. Table 3 enumerates epistemic uncertainties, which will not be quantified, and the efforts to reduce them.
| Source of uncertainty | Mitigation method |
|---|---|
| Flow/water contact difference amongst different adsorbents due to location in container | Will be theoretically similar issue across both conditions since speed of mixing was consistent across tanks so keeping track of adsorbent placement in tank will allow comparison between samples of the same placement in different conditions |
| Drying technique of adsorbents post deployment (some U may be in the water on the surface but not truly adsorbed) | Dry adsorbent using vacuum filtration rather than pat-drying or rinsing with DI water, which may shift the equilibrium to removing U off the adsorbents |
Additional sources of aleatoric uncertainty that were small relative to the drivers listed in Table 2, such as those resulting from measurements of weight and time were considered negligible. These would be relevant regarding the uncertainty in the mass of any adsorbent sample along with the mass fraction of its constituents. The high precision of laboratory scales results in uncertainties on the order of ±0.00009 g; even with adsorbent samples weighing as little as 0.1 g this amounts to a less than 0.1% uncertainty. Similarly time measurements will contribute very litter uncertainty when incorporated into NAA calculations and deployment kinetics. The irradiation and counting facilities at the Nuclear Engineering Teaching Laboratory make use of automated timers that are quite precise such that even on short irradiations, uncertainty on the order of fractions of second would not make significant contributions. Exposure and deployment periods of adsorbent will however be timed manually. The introduction of uncertainty form this procedure is estimated to be on the order of a minute, which can be considered in the noise of deployments on the order of days. Therefore these uncertainties will be neglected on the basis that mass and time measurements will introduce little uncertainty compared to the others listed in Table 2.
Conservatively assuming independence of the enumerated sources of aleatoric uncertainty, they were propagated to the final capacity restoration factor values using standard propagation of uncertainty formulas. The calculation of uncertainty in the capacity restoration factor, σR can be seen in eqn (6) to be a function of the uptake for the unfouled, Uunfouled, silver-doped, UAg, and unmodified fouled control adsorbents, Ucontrol.
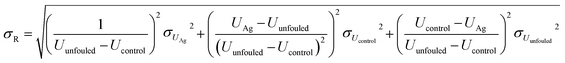 | (6) |
It is clear that a major contributor to uncertainty is the compounding of the non-trivial, although significantly reduced, aleatoric variation in adsorbent performance.
5 Conclusions and recommendations
This work clearly demonstrated that silver nanoparticles could be used to improve the uranium capacity of the surrogate activated carbon adsorbents. Given their proven ability to introduce antimicrobial properties to a variety of materials and their ability to partially restore the uptake loss seen here to be a result of marine biofouling, it can also be concluded that the ORNL adsorbents would likewise experience a partial restoration of uptake if doped with silver nanoparticles.In addition to confirming the hypothesis, this work also acts like many of the other adsorbent technology developments to provide experimental data to be analyzed for the economic impacts on the recovery of seawater uranium.8,35,44–48 While the equipment costs associated with the synthesis of silver doped adsorbents is not expected to deviate from that of the unmodified adsorbents, the material cost of silver nanoparticles will be non-negligible. Therefore future work will include the modeling of the production cost of seawater uranium recovered using silver doped ORNL adsorbents and the investigation of the economic trade-offs between increased silver nanoparticle content in adsorbent and increasingly restored capacity.
This work has also been important in concluding that the abiotic conditioning film makes a non-trivial contribution to the reduction of uranium uptake. While it is unfortunate that the silver nanoparticles did not appear to offer a means of preventing or reversing the settling of the macromolecules, the ability to identify that particular stage of biofouling as impactful in reducing uranium uptake by adsorbents is important in guiding future work. Given this new insight, future efforts can be made to mitigate this phenomenon specifically, by identifying or developing techniques for the prevention and/or removal of dissolved organic material. Future work in this area may include changing the chemistry of the adsorbent fibers themselves as to not support the adsorption of organic material, or by targeting marine regions with low dissolved organic carbon content for deployment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge Dr Gary Gill and Dr Jiyeon Park for all of their support. This work was supported by Battelle Pacific Northwest National Laboratory under Contract No. 6B0012.References
- T.-l. Ku, K. G. Knauss and G. G. Mathieu, Deep-Sea Res., 1977, 24, 1005–1017 CrossRef.
- S. Das, Y. Oyola, R. T. Mayes, C. J. Janke, L.-J. Kuo, G. Gill, J. R. Wood and S. Dai, Ind. Eng. Chem. Res., 2015, 55, 4110–4117 CrossRef.
- G. A. Gill, L.-j. Kuo, C. J. Janke, J. Park, R. T. Jeters, G. T. Bonheyo, H.-b. Pan, C. Wai, T. Khangaonkar, L. Bianucci, J. R. Wood, M. G. Warner, S. Peterson, D. G. Abrecht, R. T. Mayes, C. Tsouris, Y. Oyola, J. E. Strivens, N. J. Schlafer, R. S. Addleman, W. Chouyyok, S. Das, J. Kim, K. Buesseler, C. Breier and E. D. Alessandro, Ind. Eng. Chem. Res., 2016, 55, 4264–4277 CrossRef.
- T. Sugo, M. Tamada, T. Seguchi, T. Shimizu, M. Uotani and R. Kashima, J. At. Energy Soc. Jpn., 2010, 43, 1010–1016 CrossRef.
- M. Tamada, N. Seko, N. Kasai and t. Shimizu, Trans. At. Energy Soc. Jpn., 2006, 5, 358–363 CrossRef.
- E. Schneider and D. Sachde, Sci. Global Secur., 2013, 21, 134–163 CrossRef.
- M. F. Byers and E. a. Schneider, Ind. Eng. Chem. Res., 2016, 55, 4351–4361 CrossRef.
- J. Park, G. A. Gill, J. E. Strivens, L.-J. Kuo, R. Jeters, A. Avila, J. Wood, N. J. Schlafer, C. J. Janke, E. A. Miller, M. Thomas, R. S. Addleman, G. Bonheyo, R. T. Jeters, J.-D. R. Wood, C. J. Janke and G. T. Bonheyo, Ind. Eng. Chem. Res., 2016, 55, 4328–4338 CrossRef.
- J. Kim, C. Tsouris, R. T. Mayes, Y. Oyola, T. Saito, C. J. Janke, S. Dai, E. Schneider and D. Sachde, Sep. Sci. Technol., 2013, 48, 367–387 CrossRef.
- H. Lindner and E. Schneider, Energy Econ., 2015, 49, 9–22 CrossRef.
- M. Flicker Byers and E. Schneider, Proceedings of the American Nuclear Society 2017 Annual Meeting, 2017, vol. 116, pp. 85–88 Search PubMed.
- S. Eder, M. Byers and E. Schneider, Proceedings of the American Nuclear Society 2017 Annual Meeting, 2017, vol. 116 Search PubMed.
- M. F. Byers, S. Landsberger, E. Schneider and S. Eder, Appl. Radiat. Isot., 2018, 133, 4–8 CrossRef PubMed.
- S. Brown, Y. Yue, L.-j. Kuo, N. Mehio, M. Li, G. Gill, C. Tsouris, R. T. Mayes, T. Saito and S. Dai, Ind. Eng. Chem. Res., 2016, 55(15), 4139–4148 CrossRef.
- S. Das, Y. Oyola, R. T. Mayes, C. J. Janke, L.-J. Kuo, G. Gill, J. R. Wood and S. Dai, Ind. Eng. Chem. Res., 2016, 55(15), 4110–4117 CrossRef.
- M. Byers, Ph.D. thesis, University of Texas, 2017.
- L. D. Chambers, K. R. Stokes, F. C. Walsh and R. J. K. Wood, Surf. Coat. Technol., 2006, 201, 3642–3652 CrossRef.
- D. M. Yebra, S. Kiil and K. Dam-Johansen, Prog. Org. Coat., 2004, 50, 75–104 CrossRef.
- M. Candries, M. Atlar and C. D. Anderson, Conference on Marine Research for Environmental Sustainability, Tyne, UK, 2000, pp. 88–95 Search PubMed.
- B. M. Forrest and K. A. Blakemore, Aquaculture, 2006, 257, 333–345 CrossRef.
- P. Shivapooja, Q. Wang, B. Orihuela, D. Rittschof, G. P. Lopez and X. Zhao, Adv. Mater., 2013, 25, 1430–1434 CrossRef PubMed.
- J. P. Ruparelia, A. K. Chatterjee, S. P. Duttagupta and S. Mukherji, Acta Biomater., 2008, 4, 707–716 CrossRef PubMed.
- J. S. Kim, E. Kuk, K. N. Yu, J. H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C. Y. Hwang, Y. K. Kim, Y. S. Lee, D. H. Jeong and M. H. Cho, Nanomedicine, 2007, 3, 95–101 CrossRef PubMed.
- Y. Lv, H. Liu, Z. Wang, S. Liu, L. Hao, Y. Sang, D. Liu, J. Wang and R. I. Boughton, J. Membr. Sci., 2009, 331, 50–56 CrossRef.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef PubMed.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef PubMed.
- S. Mahendra, D. Li, A. Zhang, K. Zodrow, Q. Li and P. J. J. Alvarez, Water Res., 2009, 43, 715–723 CrossRef PubMed.
- J. Mansouri and V. Chen, J. Mater. Chem., 2010, 4567–4586 RSC.
- M. Lejars, A. Margaillan and C. Bressy, Chem. Rev., 2012, 112, 4347–4390 CrossRef PubMed.
- M. E. Callow and R. L. Fletcher, Int. Biodeterior. Biodegrad., 1994, 34, 333–348 CrossRef.
- P. Y. Qian, S. C. K. Lau, H. U. Dahms, S. Dobretsov and T. Harder, Mar. Biotechnol., 2007, 9, 399–410 CrossRef PubMed.
- A. Rosenhahn, S. Soren, H. Jurgen Kreuzer and M. Grunze, Phys. Chem. Chem. Phys., 2010, 12, 4275–4286 RSC.
- A. Mellah, S. Chegrouche and M. Barkat, J. Colloid Interface Sci., 2006, 296, 434–441 CrossRef PubMed.
- M. Caccin, F. Giacobbo, M. Da, R. Luigi and B. Mario, J. Radioanal. Nucl. Chem., 2013, 297, 9–18 CrossRef.
- A. Ladshaw, L.-j. Kuo, J. Strivens, J. Wood, N. Schlafer, S. Yiacoumi, C. Tsouris and G. Gill, Ind. Eng. Chem. Res., 2017, 12, 2205–2211 CrossRef.
- A. Carbon and L. Jakob, Environ. Sci. Technol., 2013, 47, 8674–8683 CrossRef PubMed.
- A. Chavez-dozal, C. Gorman, M. Erken, P. D. Steinberg, D. Mcdougald and M. K. Nishiguchi, Appl. Environ. Microbiol., 2016, 79, 553–558 CrossRef PubMed.
- S. Girotti, L. Bolelli, A. Roda, G. Gentilomi and M. Musiani, Anal. Chim. Acta, 2002, 471, 113–120 CrossRef.
- V. K. Sharma, R. A. Yngard and Y. Lin, Adv. Colloid Interface Sci., 2009, 145, 83–96 CrossRef PubMed.
- U. Samuel and J. P. Guggenbichler, Int. J. Antimicrob. Agents, 2004, 23, 75–78 CrossRef PubMed.
- S. Shrivastava, T. Bera, A. Roy, G. Singh, P. Ramachandrarao and D. Dash, Nanotechnology, 2010, 18, 1–9 Search PubMed.
- D. Bhandari, S. M. Wells, S. T. Retterer and M. J. Sepaniak, Anal. Chem., 2009, 81, 8061–8067 CrossRef PubMed.
- J. Jiang, L. Ma, J. Chen, P. Zhang, H. Wu, Z. Zhang, S. Wang, W. Yun, L. Yingru, J. Jia and J. Liao, Microchim. Acta, 2017, 1–23 Search PubMed.
- S. Das, W. P. Liao, M. Flicker Byers, C. Tsouris, C. J. Janke, R. T. Mayes, E. Schneider, L. J. Kuo, J. R. Wood, G. A. Gill and S. Dai, Ind. Eng. Chem. Res., 2016, 55(15), 4303–4312 CrossRef.
- S. D. Alexandratos, X. Zhu, M. Florent and R. Sellin, Ind. Eng. Chem. Res., 2016, 55(15), 4208–4216 CrossRef.
- L.-J. Kuo, H.-B. Pan, C. M. Wai, M. F. Byers, E. Schneider, J. E. Strivens, C. J. Janke, S. Das, R. T. Mayes, J. R. Wood, N. Schlafer and G. A. Gill, Ind. Eng. Chem. Res., 2017, 56(40), 11603–11611 CrossRef.
- H.-B. Pan, L.-J. Kuo, C. M. Wai, N. Miyamoto, R. Joshi, J. R. Wood, J. E. Strivens, C. J. Janke, Y. Oyola, S. Das, R. T. Mayes and G. A. Gill, Ind. Eng. Chem. Res., 2016, 55, 4313–4320 CrossRef.
- L.-J. Kuo, G. Gill, C. Tsouris, L. Rao, H.-B. Pan, C. Wai, C. Janke, J. Strivens, J. Wood, N. Schlafer and E. D'Alessandro, Chemistry select., 2018, 3(2), 843–848 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |