A plasmonic interfacial evaporator for high-efficiency solar vapor generation
Fujun
Tao
a,
Yuliang
Zhang
 *a,
Kuan
Yin
a,
Shengjia
Cao
a,
Xueting
Chang
a,
Yanhua
Lei
a,
Dongsheng
Wang
a,
Runhua
Fan
a,
Lihua
Dong
a,
Yansheng
Yin
a and
Xiaobo
Chen
*a,
Kuan
Yin
a,
Shengjia
Cao
a,
Xueting
Chang
a,
Yanhua
Lei
a,
Dongsheng
Wang
a,
Runhua
Fan
a,
Lihua
Dong
a,
Yansheng
Yin
a and
Xiaobo
Chen
 *b
*b
aCollege of Ocean Science and Engineering, Shanghai Maritime University, Shanghai 201306, P. R. China. E-mail: ylzhang@shmtu.edu.cn
bDepartment of Chemistry, University of Missouri-Kansas City, Kansas City, MO 64110, USA. E-mail: chenxiaobo@umkc.edu
First published on 18th October 2018
Abstract
The increasing energy and environmental concerns have spurred enormous research interest towards developing various renewable energy and sustainable environmental solutions. Photothermal conversion for interfacial solar vapor generation is a promising, green energy technology and efficient route for desalination and purification of seawater, i.e. for those parts where freshwater shortage is a severe concern and clean energy is not available. Eco-friendly, highly efficient and low-cost interfacial evaporators are highly desirable for the practical and widespread application of this technology. In this work, we have demonstrated a novel interfacial evaporator employing Cu9S5 nanonets with heterogeneous hexagonal holes as the photothermal conversion material and a microporous poly(vinylidene fluoride) membrane (PVDFM) as the supporting material. The Cu9S5/PVDFM evaporator displays a broadband (from 250 to 2000 nm) and large (∼91.7%) solar absorptance. The porous structures of Cu9S5 nanonets and PVDFM facilitate the water transportation, and the large optical absorption of Cu9S5/PVDFM converts most of the solar energy to thermal energy, producing water vapor with high efficiency. The Cu9S5/PVDFM evaporator exhibits solar vapor generation efficiencies of 80.2 ± 0.6% and 91.5 ± 1.1% under one-sun and four-sun irradiation, respectively, making it among the best copper sulphide-based solar evaporators reported so far. This Cu9S5/PVDFM evaporator is reusable, flexible, highly efficient, easy to prepare, easy to scale up, and controllable for tailoring, showing a promising future for interfacial solar vapor generation.
Introduction
The ever-increasing clean energy and environmental needs have spurred enormous research interest around the world towards developing various renewable energy and environmental solutions. Efficient utilization of solar energy in various forms has provided good promise and also expanded our vision on the means of using solar energy, which is clean and free of the concerns of depletion. Among the various possible uses of solar energy,1–6 photothermal conversion is one of the most promising and efficient approaches that can efficiently produce hot water and distilled clean water with high efficiency. Today, because of the increasing population growth, and extensive industrial and environmental contamination, many parts of the world are suffering from a serious freshwater shortage problem.7,8 Interfacial solar vapor generation is becoming very attractive and efficient for seawater desalination, compared to the conventional route of heating up bulk water to generate steam/vapor. Interfacial solar vapor generation can localize the solar energy at the interface where vapor is generated and decrease the heat loss in the water evaporation process, enhancing the solar vapor generation efficiency and rate under low intensity of solar irradiation,9 because it does not need to heat the bulk water beneath the air–water interface that does not take part in generating vapor but also consumes the absorbed solar energy.The structures of the interfacial evaporators are critical to achieve high efficiency.10–14 Many types, such as integrated structure,10,11 bi-layer structure12,13 and multi-layer structure,14 have been reported. Photothermal conversion materials and supporting materials play key roles in these structures. Ideal photothermal conversion materials should have a large and wide absorption in the solar spectrum and be able to convert the absorbed solar energy into heat efficiently, and ideal supporting materials should have low density, low thermal conductivity, high porosity and excellent thermal stability. Common photothermal conversion materials include carbon-based materials (e.g., exfoliated graphite,15 carbon nanotube,16 graphite powder,17 graphene,18 graphene oxide,19 and reduced graphene oxide12,20), plasmonic-metals (e.g., Au,21–26 Ag,27,28 Al,29 and Pd30), plasmonic-ceramics (e.g., TiN31 and Ti3C2 (ref. 32 and 33)), plasmonic-semiconductors (e.g., Cu2−xS,34–38 Cu2−xSe,39 Cu2−xTe,40 W18O49,41,42 and WO3−x (ref. 43)), and other light-harvesting materials (e.g., Ti2O3,44 MoS2,45,46 and carbonized mushroom47). And the supporting materials include paper-,24,25,48 wood-,30,49,50 foam-,15 membrane-,10,12,17 gel-,51–53 and gauze-based materials.54 Although higher efficiencies are becoming feasible due to continuous development in this field, an ideal system needs not only high efficiency, but also an eco-friendly, efficient, cost-effective interfacial solar evaporator. The components inside should be inexpensive and non-toxic, and have long lifetime.
Gold nanoparticles have been investigated extensively in the solar-energy-harvesting field due to their good capability in absorbing sunlight effectively in a wide wavelength range from localized surface plasmon resonance (LSPR). For instance, Bae et al.21 reported a black gold membrane for solar vapor generation with a water evaporation efficiency of ∼57% under 20 sun. Naik et al.22 reported a plasmonic Au aerogel for water evaporation with a steam generation efficiency of ∼76.3% under 808 nm laser irradiation with a power density of 51 kW m−2. Zhu et al.23 reported an Au/nanoporous alumina template composite for solar vapor generation with a water evaporation efficiency of ∼65% under 4 sun. Deng et al.24 reported an airlaid-paper-based Au nanoparticle film for solar vapor generation with a water evaporation efficiency of ∼77.8% under 4.5 sun. He et al.25 reported an Au/filter paper composite for solar steam generation with an efficiency value of ∼85% under 10 sun. He et al.26 reported an Au/poly(p-phenylene benzobisoxazole) nanofiber composite for solar steam generation with an efficiency value of ∼83% under 1 sun. Despite the gradual increase in the solar vapor generation efficiency of plasmonic-Au based evaporators, one major demerit is that the gold nanoparticles are easily aggregated and fused together at high temperature resulting from a long duration of solar irradiation, causing degradation of the water evaporation performance over time. The other major obstacle for gold-based evaporators is the high cost, which limits their widespread, large-scale production, even if the efficiency can be very high.
Eying to solve the high cost problem that gold-based evaporators have, other solar radiation absorbing materials are sought with LSPR as well as with some transition metal semiconducting materials.31,32,55,56 For example, various solid phases of Cu2−xS (0 ≤ X ≤ 1) have been reported, ranging from copper-rich Cu2S to copper-poor CuS (e.g. chalcocite Cu2S,57 djurleite Cu1.96S,58 digenite Cu1.8S,36 anilite Cu1.75S,38 and covellite CuS34) and with various nanostructures and morphologies (e.g., nanorods,35 nanoplates,59 nanonets,36 nanowires,60 nanoflowers,34 nanotubes,61 and nanovesicles62). Compared with other plasmonic materials (e.g., Au21–26 and Ag27,28), copper sulphide semiconductor materials display low cost, low cytotoxicity, and outstanding light-stability features, and have been regarded as some of the most promising green and sustainable energy materials for solar vapor generation,13,34,35,63etc. For example, Wang et al.63 reported an evaporator composed of CuS nanoparticles and a polyethylene hybrid membrane for solar vapor generation with a water evaporation efficiency of ∼63.5% under 1 sun. In our previous work,34 an evaporator comprising CuS nanoflowers and a semipermeable collodion membrane (SCM) had a water evaporation efficiency of ∼68.6% under 1 sun. Wang et al.13 reported bi-layer structural evaporators composed of CuxS and mixed cellulose ester (MCE) film for solar vapor generation, e.g., an octahedral Cu9S5 nanocrystal/MCE composite with an evaporation efficiency of ∼60.1%, and granular CuS nanocrystal/MCE composite with an appreciable efficiency of ∼80% under 1 sun, respectively. The photothermal conversion material of CuxS nanoparticles was filtered on the surface of MCE film by a vacuum filtration method to form a bi-layer structural evaporation system.13 Although high water evaporation efficiency has been demonstrated, the CuxS nanoparticle located at the top layer could detach from the bottom layer (the MCE film) after a long duration of solar irradiation or/and high-usage levels due to the low adhesion between the top and bottom layers. Besides, the effect of natural water evaporation is very important and should be subtracted when calculating the water evaporation efficiency under solar irradiation, which was, however, neglected in some previous studies.13,34,63 In view of this, the actual evaporation efficiencies of the interfacial evaporators in the above-mentioned studies are lower than what they reported. Therefore, it is essential to prepare an evaporator with high solar water evaporation efficiency and excellent reusability for solar vapor generation.
In this work, we report an integrative membraneous interfacial evaporator comprising Cu9S5 nanonets with heterogeneous hexagonal holes as a light-harvesting material and PVDFM as a supporting material for solar vapor generation. PVDFM has already been previously reported as an outstanding supporting material to prepare several desalination devices due to its major benefits of low density, microporous structure, and good hydrophilicity.10,27,32,64,65 Here, our Cu9S5/PVDFM device has several advantages: (1) broadband (250–2000 nm) and large solar absorptance (∼91.7%). (2) Unique porous structures. Both Cu9S5 and PVDFM are porous, i.e., the Cu9S5 nanonets have a unique heterogeneous hexagonal hole structure, and the PVDFM has a microporous structure. These unique porous structures can together facilitate the water transportation. (3) Excellent physical compatibility. Cu9S5 is distributed uniformly in the integrative membraneous Cu9S5/PVDFM composite, including the interior or/and the surface of PVDFM. (4) Easy fabrication process. The preparation procedure of the Cu9S5/PVDFM composite is easy and simple. (5) Low cost. The production cost of Cu9S5/PVDFM is low. (6) Excellent durability and reusability. This Cu9S5/PVDFM has been reused over 20 cycles without any decrease of water evaporation performance, showing outstanding reusability. Impressively, high water evaporation efficiencies of 80.2 ± 0.6% and 91.5 ± 1.1% were obtained under 1 sun and 4 sun irradiation, respectively. Therefore, it has shown great potential for solar vapor generation, and may be beneficial for other solar-heating applications such as sterilization, power generation, and solar distillation.
Results and discussion
The Cu9S5 nanonets showed a wide optical-absorption in the visible wavelength region of 300–700 nm due to the bandgap (∼1.5 eV) absorption and an increased light absorption in the NIR region due to the LSPR of the free carrier in Cu9S5 nanonets as shown in Fig. 1A. It is well known that an ideal solar-harvesting material should have an electromagnetic radiation absorption as high as possible over the full solar spectrum wavelength range (300–2500 nm), in which the visible wavelength region is from 400 to 700 nm, occupying ∼45% of the solar energy, and the IR wavelength region is from 700 to 2500 nm, accounting for ∼52% of solar energy.44,53 The inset of Fig. 1B shows a photo of the Cu9S5/PVDFM disk with a diameter of 33.5 mm. To confirm its light-absorption capability, the UV-vis-NIR absorption spectrum was measured, calculated from its transmittance and diffuse reflectance spectra. The pure PVDFM was white and opaque with near-zero transmittance, high (∼95%) diffuse reflectance and very low (5%) absorption in the entire solar spectrum region, consistent with previous reports,10,32,64 while the Cu9S5/PVDFM was black and had near-zero transmission, low reflection (<9%), and high (>91%) absorption, as shown in the transmission/reflection/absorption spectra in Fig. 1B. Specifically, it can absorb ∼91.4% of UV, ∼92.4% of visible, and ∼90.9% of infrared solar irradiation energy with a total solar absorptance of ∼91.7% in the 250–2000 nm wavelength region, as shown in Table 1.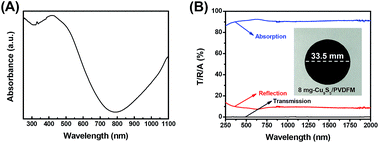 | ||
| Fig. 1 (A) UV-vis-NIR absorption spectrum of the as-obtained Cu9S5 nanonets. (B) UV-vis-NIR transmission/reflection/absorption spectra and digital photograph (inset of (B)) of 8 mg-Cu9S5/PVDFM. | ||
| Sample | UV (250–400 nm) | Visible (400–760 nm) | Infrared (760–2000 nm) | Solar absorptance |
|---|---|---|---|---|
| AM 1.5 | ∼7% | ∼50% | ∼39.7% | ∼91.7% |
| Cu9S5/PVDFM | ∼6.4% | ∼46.2% | ∼36.1% |
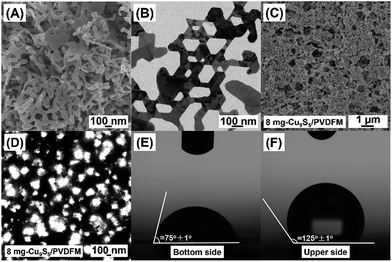
Furthermore, the structure and morphology of Cu9S5/PVDFM were analyzed using a field-emission scanning electron microscope and a Leica microscope, respectively. A unique porous nanonet structure of Cu9S5 with heterogeneous hexagonal holes was seen in Fig. 2A and B, and it could effectively facilitate the transport of water from the bulk water to the water–air interface where water vapor generates. Meanwhile, the surface morphology of Cu9S5/PVDFM was a poly-porous structure with an average open size of 200 nm as shown in Fig. 2C and D. As shown in Fig. 2E, the initial surface contact angle was 75 ± 1° for the bottom side, showing a hydrophilicity which could facilitate the transport of the water from the bulk beneath the film to the water–air interface. The contact angle for the upper side was 125 ± 1° (see Fig. 2F), which demonstrated a good hydrophobicity that could benefit for localizing heat around the water drops on the film. The unique hydrophilic–hydrophobic characteristics of the film were easily recognized in that the bottom side (i.e., hydrophilic side) was smooth and the upper side (i.e., hydrophobic side) was rough, in accordance with a previous report.10
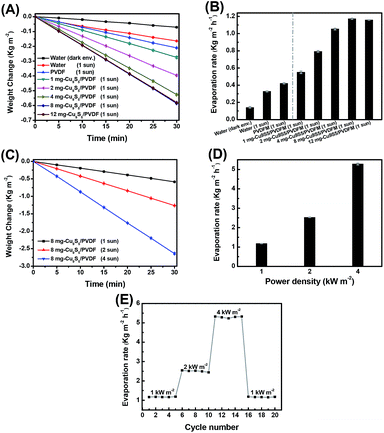
To study the solar vapor generation performance, water evaporation tests were performed on a series of Cu9S5/PVDFMs with different amounts of Cu9S5 nanonets added (i.e., 1, 2, 4, 8 and 12 mg per disk). Fig. 3A shows the weight changes of pure water covered with Cu9S5/PVDFMs under 1 sun irradiation. For comparison, the weight changes of pure water and pure water covered with pure PVDFM under 1 sun were also measured. Here, it is important to note that the weight change of pure water under a dark environment was also determined and subtracted from all the measured weight changes under solar irradiation to eliminate the effect of natural water evaporation. The average weight changes (i.e., the water evaporated weights) increased from 0.275 to 0.396, 0.526, 0.586 and 0.580 kg m−2 when the amount of Cu9S5 nanonets increased from 1 mg to 12 mg. The increased content of Cu9S5 in Cu9S5/PVDFMs from 1 mg to 8 mg improved the amount of water evaporated by the film; however, adding excess amounts of Cu9S5 nanonets (e.g. 12 mg) may block the microporous channels of Cu9S5/PVDFM and inhibit the water transport and the release of water vapor, leading to a decrease of the amount of water evaporated. Therefore, the 8 mg-Cu9S5/PVDFM showed the maximum water evaporation weight, which was 2.8 and 3.6 times higher than that of pure PVDFM under 1 sun and pure water under 1 sun, respectively.
To evaluate the water evaporation performance, the water evaporation rates (v) were calculated using the following equation (eqn (1))17,34,35
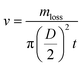 | (1) |
To further investigate the solar vapor generation performance of Cu9S5/PVDFM, water evaporation tests were carried out under various solar intensities (i.e., from 1 sun to 4 sun). As shown in Fig. 3C, the average weights of the water evaporated by the 8 mg-Cu9S5/PVDFM were 0.586, 1.264 and 2.640 kg m−2 under 1, 2 and 4 sun, respectively. Obviously, the water evaporation weights increased with the increased irradiation light intensity, so did the water evaporation rates (see Fig. 3D). As shown, the average water evaporation rates were 1.173, 2.527 and 5.280 kg m−2 h−1 under 1, 2, and 4 sun, respectively. Also, the solar evaporation rate of 5.280 kg m−2 h−1 under 4 sun was 12.5 and 16.1 times higher than that of pure PVDFM under 1 sun and pure water under 1 sun, respectively. This indicated an outstanding solar evaporation performance of the Cu9S5/PVDFM. To evaluate the reusability and durability of Cu9S5/PVDFM, the water evaporation tests of 8 mg-Cu9S5/PVDFM were repeated 20 times under different light irradiation densities. As showed in Fig. 2E, the corresponding water evaporation rates were close to 1.173, 2.527 and 5.280 kg m−2 h−1 under 1, 2 and 4 sun, respectively, indicating a stable performance of solar vapor generation of Cu9S5/PVDFM.
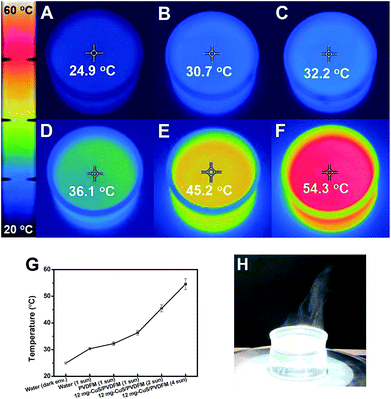
The IR photos of interfacial evaporators were further used to determine the surface temperatures of the evaporator under different intensities of solar irradiation. The initial temperature of pure water was about 25 °C for each solar vapor generation test. Fig. 4A–F show the IR photos of the temperatures after 30 min for pure water under a dark environment, pure water under 1 sun, pure water covered with pure PVDFM under 1 sun, and pure water covered with the as-prepared 8 mg-Cu9S5/PVDFM under 1, 2 and 4 sun irradiation, respectively. Accordingly, the surface temperatures were about 24.9, 30.7, 32.2, 36.1, 45.2 and 54.3 °C, after the corresponding solar vapor generation tests, as shown in the plot of temperatures change of Fig. 4G. As shown in Fig. 4H, the vapor generated was clearly observed under 4 sun.
As is well known, another important parameter to evaluate the solar vapor generation performance is solar vapor generation efficiency (η), calculated based on the following equation (eqn (2))46,48
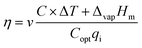 | (2) |
| C opt | Sample | v (kg m−2 h−1) | ΔT (°C) | CΔT (kJ kg−1) | ΔvapHm (kJ kg−1) | η (%) |
|---|---|---|---|---|---|---|
| 1 | Pure water | 0.328 ± 0.006 | 5.6 ± 0.5 | 23.41 ± 2.09 | 2427 | 22.2 ± 0.4 |
| 1 | PVDFM | 0.421 ± 0.007 | 7.3 ± 0.7 | 30.51 ± 2.93 | 2424 | 28.7 ± 0.5 |
| 1 | 8 mg-Cu9S5/PVDFM | 1.173 ± 0.007 | 11.4 ± 0.9 | 47.65 ± 3.76 | 2415 | 80.2 ± 0.6 |
| 2 | 8 mg-Cu9S5/PVDFM | 2.527 ± 0.010 | 20.6 ± 1.3 | 86.11 ± 5.43 | 2393 | 87.0 ± 0.7 |
| 4 | 8 mg-Cu9S5/PVDFM | 5.280 ± 0.050 | 29.6 ± 2.1 | 123.73 ± 8.78 | 2371 | 91.5 ± 1.1 |
In Table 3, we compared some of the water evaporation efficiencies of recent reports using different interfacial plasmonic evaporators (e.g., plasmonic-metal-based,21–26,29,30 plasmonic-ceramic-based,31–33 and plasmonic-semiconductor-based13,34,35,38,41,63). Here, it is noteworthy that the values of efficiency labelled by an asterisk are inaccurate due to neglecting the effect of natural water evaporation. To our knowledge, the effect of natural water evaporation is considerable and should be subtracted while calculating the water evaporation efficiency under solar irradiation. We estimate that the data could decrease ca. 10%. For instance, in this work, the efficiency of water evaporation of 8 mg-Cu9S5/PVDFM is ∼80.2% under 1 sun; however, the corresponding evaporation efficiency is ∼90% if the evaporation rate of pure water under a dark environment is not subtracted. In view of this, the water evaporation efficiency of our Cu9S5/PVDFM of ∼80.2% under 1 sun irradiation is top-ranking among the three plasmonic evaporators reported so far. Furthermore, with the advantages of the straightforward fabrication process, the inexpensive materials, and the excellent reusability, the Cu9S5/PVDFM demonstrated its strong competitiveness over other materials for solar vapor applications.
| Interfacial plasmonic evaporators | Classification of light-harvesting materials | Light sources | Power density (kW m−2) | Efficiency | Ref. |
|---|---|---|---|---|---|
| a The symbol “*” refers to the data without subtracting natural water evaporation in their works. | |||||
| Au/Al2O3 composite | Plasmonic-metal | Solar simulator | 1 | ∼49%* | 23 |
| Al/Al2O3 composite | Plasmonic-metal | Solar simulator | 1 | ∼57.5% | 29 |
| Au/filter paper composite | Plasmonic-metal | Solar simulator | 1 | ∼62.5%* | 25 |
| Pd/wood composite | Plasmonic-metal | Solar simulator | 1 | ∼69% | 30 |
| Au/PBO nanofiber composite | Plasmonic-metal | Solar simulator | 1 | 83% | 26 |
| Paper-based AuNP film | Plasmonic-metal | Solar simulator | 4.5 | ∼77.8%* | 24 |
| Black Au membrane | Plasmonic-metal | Solar simulator | 20 | ∼57% | 21 |
| Au aerogel | Plasmonic-metal | 808 nm laser | 51 | 76.3%* | 22 |
| TiN/microfiber composite | Plasmonic-ceramic | Solar simulator | 1 | >80%* | 31 |
| Ti3C2/PVDFM | Plasmonic-ceramic | Solar simulator | 1 | 84%* | 32 |
| Ti3C2/MCE membrane | Plasmonic-ceramic | Solar simulator | 1 | 71% | 33 |
| W18O49/PTFE membrane | Plasmonic-semiconductor | Solar simulator | 1 | 80.7%* | 41 |
| Cu7S4 nanocrystals film | Plasmonic-semiconductor | Infrared lamp | 1 | ∼77.1% | 38 |
| CuS/PE membrane | Plasmonic-semiconductor | Solar simulator | 1 | ∼63.9%* | 63 |
| CuS/SCM | Plasmonic-semiconductor | Solar simulator | 1 | ∼68.6%* | 34 |
| Cu9S5/MCE membrane | Plasmonic-semiconductor | Solar simulator | 1 | ∼60.1%* | 13 |
| CuS/MCE membrane | Plasmonic-semiconductor | Solar simulator | 1 | 80 ± 2.5%* | 13 |
| CdS–Cu7S4 film | Plasmonic-semiconductor | Solar simulator | 1.5 | ∼48.4%* | 35 |
| Cu9S5/PVDFM | Plasmonic-semiconductor | Solar simulator | 1 | 80.2 ± 0.6% | This work |
Conclusions
In summary, we have demonstrated a Cu9S5/PVDFM with an integrative porous membrane structure, for solar vapor generation. The Cu9S5/PVDFM exhibits a large solar absorptance of ∼91.7% in the 250–2000 nm wavelength region, and enables the water evaporation efficiencies to reach 80.2 ± 0.6% and 91.5 ± 1.1% under the solar irradiation intensities of 1 sun and 4 sun, respectively. It has been reused over 20 times without performance degradation, exhibiting excellent reusability and durability. With its simple preparation technology, low-cost feature and high water evaporation efficiency, the Cu9S5/PVDFM composite shows great potential for solar vapor generation, and likely for other applications as well, such as water purification, sterilization, distillation, and desalination.Experimental
Materials
N,N-Dimethylformamide (DMF) was purchased from Shanghai Sinopharm Chemical Reagent Co. Ltd. Poly (vinylidene fluoride) (PVDF) was purchased from Shandong Xiya Reagent Co. Ltd. Deionized water was purified using a Milli-Q system (Millipore).Preparation of the Cu9S5/PVDF membrane
The preparation and characterization of Cu9S5 nanonets were based on the report in our previous work.36 Specifically, the Cu9S5 obtained at a reaction time of 18 h was chosen as the photothermal conversion material. The Cu9S5/PVDFMs were prepared as follows.10,27,65 Firstly, 2 g PVDF powder was added into 30 mL DMF solution and stirred to get a PVDF/DMF solution. Then, 116 mg Cu9S5 was added into a 10 mL beaker containing 1 mL PVDF/DMF solution to form a Cu9S5/PVDF/DMF solution mixture under sonication. The Cu9S5/PVDF/DMF solution was transferred with a 3 mL pipette to a flat glass surface, and cast with a 150 μm gap scraper to form the Cu9S5/PVDFM composite. The flat glass coated with Cu9S5/PVDFM was immersed in a deionized water bath for 0.5 h and the Cu9S5/PVDFM film was peeled off from the flat glass, washed with ethanol several times and dried in a vacuum oven at 45 °C for 1.5 h. Finally, the Cu9S5/PVDFM was trimmed into a round disk shape with a diameter of 33.5 mm. Hence, the Cu9S5/PVDFM with 8 mg Cu9S5 nanonets added (i.e., 8 mg-Cu9S5/PVDFM) was fabricated. The same method was used to prepare other samples with various amounts of Cu9S5 light absorber, e.g., 1 mg-Cu9S5/PVDFM, 2 mg-Cu9S5/PVDFM, 4 mg-Cu9S5/PVDFM, 12 mg-Cu9S5/PVDFM, and pure PVDFM (i.e., without any Cu9S5 nanonets added).Characterization
The morphology and structure were analyzed with a field-emission scanning electron microscope (JSM-6700F, Japan) and Leica microscope (DM500, Germany). The contact angle was measured with a contact-angle analyzer (JC2000D1, China). The UV-vis-NIR transmittance and diffuse reflectance spectra were measured on a Lambda 950 UV-vis-NIR scanning spectrophotometer (PerkinElmer, America). The IR digital pictures were captured with an FLIR camera (E40, America).Solar vapor generation
The solar vapor generation tests were conducted using a homemade chamber optical measurement system, with a PLS-SXE300/300UV solar simulator and other optical components (Perfect Light Technology Co. Ltd, Beijing). The optical irradiation intensities from 1 to 4 sun (kW m−2) were measured with an optical power meter (VLP-2000, China). The Cu9S5/PVDFM was placed in a 40 mm × 25 mm weighing bottle containing 20 mL deionized water as the solar vapor generation setup. Here, it is important to note that due to the difference of the hydrophilic–hydrophobic properties of the upper and bottom sides of the Cu9S5/PVDFM, the hydrophilic surface (called the bottom side) of the Cu9S5/PVDFM was placed face down so that it can establish contact with bulk water directly, so the hydrophobic surface (called the upper side) was placed upward. The weight change during solar vapor generation was recorded using a 4 decimal electronic precision balance (OHRUS FR224CN, accuracy: 0.1 mg). For cycling water evaporation tests, after each cycle, the wetted Cu9S5/PVDFM was dried under ambient conditions to simulate the actual usage.Author contributions
F. Tao, K. Yin, and S. Cao prepared the samples. X. Chang, Y. Lei and D. Wang characterized the samples. F. Tao, K. Yin, S. Cao, and D. Wang performed the solar vapor generation tests. F. Tao wrote this manuscript. R. Fan, L. Dong, and Y. Yin discussed and critically read the manuscript. Y. Zhang and X. Chen designed this project and polished this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
X. C. is thankful for the support from the U.S. National Science Foundation (DMR-1609061). Y. Z. and X. C. are thankful for the support from the National Key R & D Program of China (no. 2016YFB0300704). Y. Y. acknowledges the State Key Development Program for Basic Research of China (no. 2014CB643306). X. C. appreciates the NSF of Shanghai (no. 17ZR1440900). Y. L. acknowledges the National Nature Science Foundation of China (no. 51602195). F. T. is thankful for the support from the Doctoral Innovation Foundation of SMU (no. 2016ycx037), and the Doctoral Excellent Thesis Project Foundation of SMU (no. 2017BXLP005).Notes and references
- Z. Wang, C. Yang, T. Lin, H. Yin, P. Chen, D. Wan, F. Xu, F. Huang, J. Lin, X. Xie and M. Jiang, Energy Environ. Sci., 2013, 6, 3007–3014 RSC.
- T. Lin, C. Yang, Z. Wang, H. Yin, X. Lü, F. Huang, J. Lin, X. Xie and M. Jiang, Energy Environ. Sci., 2014, 7, 967–972 RSC.
- Z. Wang, C. Yang, T. Lin, H. Yin, P. Chen, D. Wan, F. Xu, F. Huang, J. Lin, X. Xie and M. Jiang, Adv. Funct. Mater., 2013, 23, 5444–5450 CrossRef CAS.
- X. Chen, L. Liu, P. Yu and S. Mao, Science, 2011, 331, 746–750 CrossRef CAS PubMed.
- V. Zardetto, B. Williams, A. Perrotta, F. Giacomo, M. Verheijen, R. Andriessen, W. Kessels and M. Creatore, Sustainable Energy Fuels, 2017, 1, 30–55 RSC.
- G. Ni, G. Li, S. Boriskina, H. Li, W. Yang, T. Zhang and G. Chen, Nat. Energy, 2016, 1, 16126 CrossRef CAS.
- M. Elimelech, J. Water Supply: Res. Technol.--AQUA, 2006, 55, 3–10 CrossRef.
- M. Mekonnen and A. Hoekstra, Sci. Adv., 2016, 2, e1500323 Search PubMed.
- W. Shang and T. Deng, Nat. Energy, 2016, 1, 16133 CrossRef CAS.
- L. Yi, S. Ci, S. Luo, P. Shao, Y. Hou and Z. Wen, Nano Energy, 2017, 41, 600–608 CrossRef CAS.
- V. Kashyap, A. Al-Bayati, S. Sajadi, P. Irajizad, S. Wang and H. Ghasemi, J. Mater. Chem. A, 2017, 5, 15227–15234 RSC.
- G. Wang, Y. Fu, X. Ma, W. Pi, D. Liu and X. Wang, Carbon, 2017, 114, 117–124 CrossRef CAS.
- Z. Guo, X. Ming, G. Wang, B. Hou, X. Liu, T. Mei, J. Li, J. Wang and X. Wang, Semicond. Sci. Technol., 2018, 33, 025008 CrossRef.
- Y. Li, T. Gao, Z. Yang, C. Chen, W. Luo, J. Song, E. Hitz, C. Jia, Y. Zhou, B. Liu, B. Yang and L. Hu, Adv. Mater., 2017, 29, 1700981 CrossRef PubMed.
- H. Ghasemi, G. Ni, A. Marconnet, J. Loomis, S. Yerci, N. Miljkovic and G. Chen, Nat. Commun., 2014, 5, 4449 CrossRef CAS PubMed.
- Y. Wang, L. Zhang and P. Wang, ACS Sustainable Chem. Eng., 2016, 4, 1223–1230 CrossRef CAS.
- F. Tao, Y. Zhang, B. Wang, F. Zhang, X. Chang, Y. An, R. Fan, L. Dong and Y. Yin, Sol. Energy Mater. Sol. Cells, 2018, 180, 34–45 CrossRef CAS.
- P. Zhang, J. Li, L. Lv, Y. Zhao and L. Qu, ACS Nano, 2017, 11, 5087–5093 CrossRef CAS PubMed.
- X. Li, W. Xu, M. Tang, L. Zhou, B. Zhu, S. Zhu and J. Zhu, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13953–13958 CrossRef CAS PubMed.
- L. Shi, Y. Wang, L. Zhang and P. Wang, J. Mater. Chem. A, 2017, 5, 16212–16219 RSC.
- K. Bae, G. Kang, S. Cho, W. Park, K. Kim and W. Padilla, Nat. Commun., 2015, 6, 10103 CrossRef CAS PubMed.
- L. Tian, J. Luan, K. Liu, Q. Jiang, S. Tadepalli, M. Gupta, R. Naik and S. Singamaneni, Nano Lett., 2016, 16, 609–616 CrossRef CAS PubMed.
- L. Zhou, S. Zhuang, C. He, Y. Tan, Z. Wang and J. Zhu, Nano Energy, 2017, 32, 195–200 CrossRef CAS.
- Y. Liu, S. Yu, R. Feng, A. Bernard, Y. Liu, Y. Zhang, H. Duan, W. Shang, P. Tao, C. Song and T. Deng, Adv. Mater., 2015, 27, 2768–2774 CrossRef CAS PubMed.
- X. Wang, Y. He, X. Liu, G. Cheng and J. Zhu, Appl. Energy, 2017, 195, 414–425 CrossRef CAS.
- M. Chen, Y. Wu, W. Song, Y. Mo, X. Lin, Q. He and B. Guo, Nanoscale, 2018, 10, 6186–6193 RSC.
- A. Politano, P. Argurio, G. Profio, V. Sanna, A. Cupolillo, S. Chakraborty, H. Arafat and E. Curcio, Adv. Mater., 2017, 29, 1603504 CrossRef PubMed.
- H. Wang, L. Miao and S. Tanemura, Sol. RRL, 2017, 1, 1600023 CrossRef.
- L. Zhou, Y. Tan, J. Wang, W. Xu, Y. Yuan, W. Cai, S. Zhu and J. Zhu, Nat. Photonics, 2016, 10, 393–398 CrossRef CAS.
- M. Zhu, Y. Li, F. Chen, X. Zhu, J. Dai, Y. Li, Z. Yang, X. Yan, J. Song, Y. Wang, E. Hitz, W. Luo, M. Lu, B. Yang and L. Hu, Adv. Energy Mater., 2017, 8, 1701028 CrossRef.
- M. Kaur, S. Ishii, S. Shinde and T. Nagao, ACS Sustainable Chem. Eng., 2017, 5, 8523–8528 CrossRef CAS.
- R. Li, L. Zhang, L. Shi and P. Wang, ACS Nano, 2017, 11, 3752–3759 CrossRef CAS PubMed.
- J. Zhao, Y. Yang, C. Yang, Y. Tian, Y. Han, J. Liu, X. Yin and W. Que, J. Mater. Chem. A, 2018, 6, 16196–16204 RSC.
- F. Tao, Y. Zhang, S. Cao, K. Yin, X. Chang, Y. Lei, R. Fan, L. Dong, Y. Yin and X. Chen, Mater. Today Energy, 2018, 9, 285–294 CrossRef.
- F. Tao, Y. Zhang, F. Zhang, K. Wang, X. Chang, Y. An, L. Dong and Y. Yin, ChemistrySelect, 2017, 2, 3039–3048 CrossRef CAS.
- F. Tao, Y. Zhang, F. Zhang, Y. An, L. Dong and Y. Yin, RSC Adv., 2016, 6, 63820–63826 RSC.
- F. Tao, Y. Zhang, F. Zhang, L. Yang, Y. An and Y. Yin, Mater. Lett., 2015, 140, 166–169 CrossRef CAS.
- C. Zhang, C. Yan, Z. Xue, W. Yu, Y. Xie and T. Wang, Small, 2016, 12, 5320–5328 CrossRef CAS PubMed.
- S. Zhang, C. Sun, J. Zeng, Q. Sun, G. Wang, Y. Wang, Y. Wu, D. Dou, M. Gao and Z. Li, Adv. Mater., 2016, 28, 8927–8936 CrossRef CAS PubMed.
- A. Poulose, S. Veeranarayanan, M. Mohamed, R. Aburto, T. Mitcham, R. Bouchard, P. Ajayan, Y. Sakamoto, T. Maekawa and D. Kumar, Sci. Rep., 2016, 6, 35961 CrossRef CAS PubMed.
- Y. Chang, Z. Wang, Y. Shi, X. Ma, L. Ma, Y. Zhang and J. Zhan, J. Mater. Chem. A, 2018, 6, 10939–10946 RSC.
- Z. Chen, Q. Wang, H. Wang, L. Zhang, G. Song, L. Song, J. Hu, H. Wang, J. Liu, M. Zhu and D. Zhao, Adv. Mater., 2013, 25, 2095–2100 CrossRef CAS PubMed.
- T. Chala, C. Wu, M. Chou, M. Gebeyehu and K. Cheng, Nanomaterials, 2017, 7, 191 CrossRef PubMed.
- J. Wang, Y. Li, L. Deng, N. Wei, Y. Weng, S. Dong, D. Qi, J. Qiu, X. Chen and T. Wu, Adv. Mater., 2017, 29, 1603730 CrossRef PubMed.
- X. Yang, Y. Yang, L. Fu, M. Zou, Z. Li, A. Cao and Q. Yuan, Adv. Funct. Mater., 2018, 28, 1704505 CrossRef.
- Z. Guo, G. Wang, X. Ming, T. Mei, J. Wang, J. Li, J. Qian and X. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 24583–24589 CrossRef CAS PubMed.
- N. Xu, X. Hu, W. Xu, X. Li, L. Zhou, S. Zhu and J. Zhu, Adv. Mater., 2017, 29, 1606762 CrossRef PubMed.
- Z. Deng, P. Liu, J. Zhou, L. Miao, Y. Peng, H. Su, P. Wang, X. Wang, W. Cao, F. Jiang, L. Sun and S. Tanemura, Sol. RRL, 2018, 1800073 CrossRef.
- P. Liu, L. Miao, Z. Deng, J. Zhou, H. Su, L. Sun, S. Tanemura, W. Cao, F. Jiang and L. Zhao, Mater. Today Energy, 2018, 8, 166–173 CrossRef.
- K. Kim, S. Yu, C. An, S. Kim and J. Jang, ACS Appl. Mater. Interfaces, 2018, 10, 15602–15608 CrossRef CAS PubMed.
- F. Zhao, X. Zhou, Y. Shi, X. Qian, M. Alexander, X. Zhao, S. Mendez, R. Yang, L. Qu and G. Yu, Nat. Nanotechnol., 2018, 13, 489–495 CrossRef CAS PubMed.
- J. Song, C. Chen, Z. Yang, Y. Kuang, T. Li, Y. Li, H. Huang, I. Kierzewski, B. Liu, S. He, T. Gao, S. Yuruker, A. Gong, B. Yang and L. Hu, ACS Nano, 2018, 12, 140–147 CrossRef CAS PubMed.
- X. Yin, Y. Zhang, Q. Guo, X. Cai, J. Xiao, Z. Ding and J. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 10998–11007 CrossRef CAS PubMed.
- Y. Liu, J. Chen, D. Guo, M. Cao and L. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 13645–13652 CrossRef CAS PubMed.
- Q. Liu, Y. Liu and Y. Yin, Natl. Sci. Rev., 2018, 5, 128–130 CrossRef.
- L. Zhu, M. Gao, C. Peh and G. Ho, Mater. Horiz., 2018, 5, 323–343 RSC.
- M. Sigman, A. Ghezelbash, T. Hanrath, A. Saunders, F. Lee and B. Korgel, J. Am. Chem. Soc., 2003, 125, 16050–16057 CrossRef CAS PubMed.
- N. Morimoto, Mineral. J., 1962, 3, 338–344 CrossRef.
- J. Park, S. Kim, J. Chang, H. Seo, J. Lee and J. Yuk, Nat. Commun., 2018, 9, 922 CrossRef PubMed.
- Y. Hsu, Y. Chen and Y. Lin, Electrochim. Acta, 2014, 139, 401–407 CrossRef CAS.
- C. Tan, Y. Zhu, R. Lu, P. Xue, C. Bao, X. Liu, Z. Fei and Y. Zhao, Mater. Chem. Phys., 2005, 91, 44–47 CrossRef CAS.
- Q. Lu, F. Gao and D. Zhao, Nano Lett., 2002, 2, 725–728 CrossRef CAS.
- M. Shang, N. Li, S. Zhang, T. Zhao, C. Zhang, C. Liu, H. Li and Z. Wang, ACS Appl. Energy Mater., 2018, 1, 56–61 CrossRef CAS.
- Y. Wang, C. Wang, X. Song, M. Huang, S. Megarajan, S. Shaukat and H. Jiang, J. Mater. Chem. A, 2018, 6, 9874–9881 RSC.
- E. Fontananova, J. Jansen, A. Cristiano, E. Curcio and E. Drioli, Desalination, 2006, 192, 190–197 CrossRef CAS.
- X. Wu, G. Chen, W. Zhang, X. Liu and H. Xu, Adv. Sustainable Syst., 2017, 1, 1700046 CrossRef.
- L. Zhang, J. Xing, X. Wen, J. Chai, S. Wang and Q. Xiong, Nanoscale, 2017, 9, 12843–12849 RSC.
| This journal is © The Royal Society of Chemistry 2018 |