A versatile platform to achieve mechanically robust mussel-inspired antifouling coatings via grafting-to approach†
Youbing
Mu
 ab,
Zelin
Wu
a,
Danfeng
Pei
a,
Jiming
Wang
a and
Xiaobo
Wan
ab,
Zelin
Wu
a,
Danfeng
Pei
a,
Jiming
Wang
a and
Xiaobo
Wan
 *ab
*ab
aThe Key Laboratory of Bio-based Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, 189 Songling Road, Qingdao, 266101, P. R. China. E-mail: wanxb@qibebt.ac.cn
bUniversity of Chinese Academy of Sciences, 19A Yuquan Road, Beijing 100049, P. R. China
First published on 28th November 2017
Abstract
Although significant progress has been made in mussel-inspired antifouling coatings, most of them suffer from low mechanical stability. Herein, we present a facile and efficient method to fabricate mechanically robust mussel-inspired antifouling coatings. A polyvinyl alcohol (PVA)-based mussel-inspired coating material, which exhibits the highest adhesion capability (always at 5B level in a tape adhesion test based on the ASTM D3359 method) and excellent anti-abrasive properties (little mass loss after 1000 abrasion cycles), is used as a universal platform for further modification to introduce antifouling properties. Intriguingly, the hydroxyl groups in this PVA-based coating material are used as the anchor for the installation of either hydrophilic or hydrophobic segments (or both) via a grafting-to approach. Single modifiers, methoxypolyethylene glycol (MPEG), sodium 2-hydroxyethanesulfonate (SHS) and 1H,1H,2H,2H-perfluorooctan-1-ol (PFO), and complex modifiers, MPEG/PFO, are tethered onto the coating through an effective urethane bond formation reaction to endow the surfaces with antifouling efficacy. The functionalized surfaces are shown to be effective in preventing bovine serum albumin (BSA) adsorption and in reducing bacterial (Gram-positive bacteria S. aureus and Gram-negative bacteria E. coli) adhesion. More importantly, such modification does not influence the strong adhesion and excellent anti-abrasion properties of the coating. To the best of our knowledge, this is the first example of merging excellent mechanical properties and antifouling capability for mussel-inspired coatings, which might find niches in a broad range of applications in the industrial and biomedical fields.
Introduction
Nonspecific protein adsorption, bacterial adhesion, and subsequent biofilm formation at solid surfaces, namely biofouling, may have catastrophic consequences and are of great concern for biomedical implant devices, biosensors and food packing and storage.1,2 Biofouling may result in inflammation, irritation of the surrounding tissue, and improper function of medical devices, which not only may lead to costly retreatment or reoperation, but also in some cases may be life-threatening to the patient. Therefore, much attention has been focused on the alteration of the surface chemistry of material surfaces to prevent protein adsorption or bacterial adhesion in order to decrease the potential for biofilm development.3–9 A common strategy to limit nonspecific protein adsorption or bacterial adhesion is to introduce antifouling polymers. Accumulating evidence has demonstrated that both hydrophilic polymers such as PEG and hydrophobic polymers such as perfluoroalkyl-containing polymers exhibit good antifouling ability to resist nonspecific protein adsorption, bacterial adhesion, and biofilm formation.2,10 However, they are subject to certain restrictions in a variety of substrates. For example, to immobilize such hydrophilic or hydrophobic polymers onto surfaces, various anchors, which can produce specific interactions between the interfacial modifier and the surfaces, have been designed depending on the substrates.2,10–14 For instance, thiolate interacts specifically with noble metals, phosphonic acid interacts specifically with titanium oxides and other oxides and organosilane interacts specifically with various oxides.10–14Recently, mussels have attracted widespread interest due to their strong adhesion to almost all types of surfaces. Although the real mechanism for mussel adhesion is not clear, a particular amino acid, 3,4-dihydroxyphenylalanine (DOPA), present in their foot proteins, is generally considered to be the critical element accounting for the strong adhesion.15,16 The ortho-dihydroxyphenyl (catechol) moiety of DOPA plays multiple roles in mussel adhesion. Dopamine (DA), which can be directly deposited on a wide range of inorganic and organic substrates via self-polymerization under basic conditions (pH = 8.5) to give the polydopamine (PDA) coating, is the most frequently used catecholic compound.15,17,18 The formed PDA coating contains an abundance of catechol, amino, and quinone groups, which can be used as a precursor for conjugation of other molecules to a PDA-modified surface. Many antifouling ingredients such as zwitterions,19,20 zinc ions,21 phosphorylcholines,22 peptides,23 perfluorinated alkyl chains,24 silver nanoparticles,25,26 and oligo(ethylene glycol) (OEG),27,28 have been conjugated to the PDA-modified surface. Although the present strategies display good antifouling ability, the PDA-based coatings suffer from a low polymerization degree (which corresponds to low molecular weight) and rigidity of the polymer backbone, which makes the coating thin (the maximum thickness is only up to 50 nm) and brittle,18 and its mechanical strength (such as being anti-abrasive and hard) and long-term stability is highly questionable. A recent publication indicated that thicker coatings might have better long-term antifouling stability.29 Another problem is that the efficiency of forming a PDA coating on a surface is rather low. During the polymerization process, most of the formed PDA stays in the solution, and only a small percentage deposits onto the substrate surface. Given the high cost of dopamine, such a coating-formation strategy is not practical in real applications.
Another strategy is to use more flexible catechol-containing polymers with controllable molecular weight, which could be deposited onto various surfaces. For this purpose, many polymers such as PEG,30–32 poly(acrylate-co-acrylamide),33,34 polysaccharides,35–38 polyethylenimine,39 and peptides,40 were modified with pendent catechols and antifouling groups (e.g. OEG,41,42 zwitterions,43 perfluorinated alkyl chains,44 phosphorylcholine,45,46 cardanol,33etc.). These biomimetic polymers are versatile since the properties (e.g. antifouling ability, mechanical properties, etc.) of the formed coating can be adjusted by varying the backbone structure, molecular weight, catechol content and the type and content of antifouling composition. However, sophisticated synthetic and fabrication procedures are generally involved in the fabricating process, which limited their large scale synthesis and practical application. Also, in most cases, the long-term stability of these coatings against abrasion and other mechanical damage has not been investigated, which makes the real application of these coatings questionable.
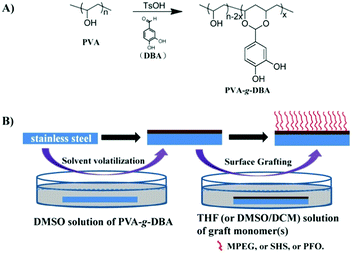
In a previous work, we have developed a simple and efficient method (one step) to synthesize a polyvinyl alcohol (PVA)-based biomimetic polymer (PVA-g-DBA) with pendent catechol groups via the acetal formation reaction from two cheap commercially available materials: PVA and 3,4-dihydroxybenzaldehyde (DBA).47 This biomimetic polymer shows excellent adhesion properties, mechanical hardness and high abrasion resistance on different substrates, including metal, glass and plastic, when being used as an adhesive and in controlled-release coatings.47,48 Herein, we report the expansion of this polymer into the antifouling coating field to solve the problems mentioned above. We anticipate that after the formation of a robust coating on various substrates, the residual abundant hydroxyl groups in PVA-g-DBA can be used as the anchors to conjugate with antifouling molecules to endow the coating with antifouling properties. Thus, a grafting-to approach, which is simple and effective for installing antifouling components onto the coating, was employed to modify the PVA-g-DBA coating, without deteriorating its excellent mechanical properties. Specifically, both hydrophilic components such as methoxypolyethylene glycol (MPEG) and sodium 2-hydroxyethanesulfonate (SHS) and the hydrophobic component 1H,1H,2H,2H-perfluorooctan-1-ol (PFO) were terminated with isocyanate groups, which reacted easily with the hydroxyl groups in the PVA-g-DBA coatings to adjust their antifouling properties. The antifouling coatings with a single antifouling monomer and complex antifouling monomers on the surface were prepared and the mechanical properties, surface morphology, wettability, and antifouling efficiency of the formed coatings were systematically investigated. All the coatings retain the highest adhesion (5B level) capability to surfaces and excellent anti-abrasive properties (less than 20 mg loss after 1000 abrasion cycles), while exhibiting good to excellent antifouling properties. It is worthwhile to point out that the grafting-to approach is also a more convenient methodology for practical applications.32
Experimental section
Materials
Polyvinyl alcohol (PVA) (PVA1799, average molecular weight (Mw) ∼75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, hydrolysis degree 99 mol%), Luria-Bertani (LB), anhydrous dimethyl sulfoxide (DMSO), p-toluenesulfonic acid monohydrate (TsOH), methoxypolyethylene glycol (MPEG, Mw = 750), sodium 2-hydroxyethanesulfonate (SHS), 1H,1H,2H,2H-perfluorooctan-1-ol (PFO), hexamethylene diisocyanate (HDI) and dibutyltindilaurate were purchased from Aladdin Industrial Co. 3,4-Dihydroxybenzaldehyde (DBA) was purchased from Linhai Xinghua Chemical Co., Ltd. Escherichia coli (E. coli, ATCC25922) and Staphylococcus Aureus (S. aureus, ATCC25923) were purchased from Shanghai Luwei Technology Co., Ltd. Albumin–fluorescein isothiocyanate conjugate (BSA-FITC, product no. A9771) was purchased from Sigma-Aldrich Chemical Co. Dioxane, tetrahydrofuran (THF), and dichloromethane (DCM) were dried and distilled before use. Stainless steel (SS) (0.3 mm thick) with a smooth and flat surface was selected as the substrate and it was obtained from the local market. The average roughness of the SS is about 1.6 nm. Deionized water (18 MΩ) was made in our laboratory. All the chemicals were used as received unless otherwise stated.
000, hydrolysis degree 99 mol%), Luria-Bertani (LB), anhydrous dimethyl sulfoxide (DMSO), p-toluenesulfonic acid monohydrate (TsOH), methoxypolyethylene glycol (MPEG, Mw = 750), sodium 2-hydroxyethanesulfonate (SHS), 1H,1H,2H,2H-perfluorooctan-1-ol (PFO), hexamethylene diisocyanate (HDI) and dibutyltindilaurate were purchased from Aladdin Industrial Co. 3,4-Dihydroxybenzaldehyde (DBA) was purchased from Linhai Xinghua Chemical Co., Ltd. Escherichia coli (E. coli, ATCC25922) and Staphylococcus Aureus (S. aureus, ATCC25923) were purchased from Shanghai Luwei Technology Co., Ltd. Albumin–fluorescein isothiocyanate conjugate (BSA-FITC, product no. A9771) was purchased from Sigma-Aldrich Chemical Co. Dioxane, tetrahydrofuran (THF), and dichloromethane (DCM) were dried and distilled before use. Stainless steel (SS) (0.3 mm thick) with a smooth and flat surface was selected as the substrate and it was obtained from the local market. The average roughness of the SS is about 1.6 nm. Deionized water (18 MΩ) was made in our laboratory. All the chemicals were used as received unless otherwise stated.
Synthesis of PVA-based biomimetic polymers
PVA-g-DBA was synthesized via a simple acid-catalyzed acetal formation reaction between PVA and DBA as reported before.47 The degree of grafting (DG) of DBA was fixed at around 10 mol%. The DG was calculated from the integral ratio of the peak of the acetal proton to peaks of methylene protons on the polymer backbone in the 1H-NMR spectrum of PVA-g-DBA. The calculation methods have been discussed in detail in our previous publication47 and will not be covered in this report.Synthesis of isocyanate-terminated grafting monomers
Deposition of PVA-g-DBA coatings on SS substrates
The SS sheet was cut into 90 mm long, 15 mm wide rectangular foils. Then, the SS foils were soaked in a detergent solution for 30 minutes, washed with tap water, ethanol, acetone, deionized water and dried under ambient conditions before use. The PVA-g-DBA polymer (2.0 g) was dissolved in 40 mL of DMSO solvent and stirred until it was completely dissolved. The solution was poured into a PTFE petri dish with the cleaned SS foils inside and the petri dish was placed into a drying oven at 110 °C for 18 h. After cooling to room temperature, the SS foils were taken out and tailored to remove the residues on the edge and back. The thickness of the obtained coating was controlled at around 50 μm. The resulting PVA-g-DBA-coated substrates were denoted as SS-PVA-g-DBA.Grafting of MPEG, SHS and PFO on PVA-g-DBA coatings
MPEG, SHS and PFO were grafted onto SS-PVA-g-DBA via a grafting-to approach. Solutions for grafting were prepared by dissolving the isocyanate-terminated grafting monomers in the corresponding solvent (THF for isocyanate-terminated MPEG and PFO, DMSO/DCM (VDMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VDCM = 1
VDCM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) for isocyanate-terminated SHS). The concentration of the monomer was about 0.10 g mL−1. Specifically, the SS-PVA-g-DBA substrates were immersed into the corresponding solvent in a pre-dried glass bottle with a bracket and a magnetic stirrer bar at the bottom and then the bottle was sealed. The grafting reaction was allowed to run for 48 h to reach equilibrium. After that, the substrates were thoroughly rinsed with ethanol, DCM and acetone, and dried under a stream of compressed air. The resulting MPEG-, SHS- and PFO-grafted substrates were denoted as SS-PVA-g-DBA-MPEG, SS-PVA-g-DBA-SHS and SS-PVA-g-DBA-PFO. The SS-PVA-g-DBA substrates were also immersed in a mixture system of isocyanate-terminated MPEG and isocyanate-terminated PFO (0.1 g mL−1 each) at room temperature for 48 h to achieve a surface containing both MPEG and PFO. The resulting substrates were denoted as SS-PVA-g-DBA-MPEG/PFO. At least five samples were prepared with each recipe and preserved in a desiccator before the performance test.
4) for isocyanate-terminated SHS). The concentration of the monomer was about 0.10 g mL−1. Specifically, the SS-PVA-g-DBA substrates were immersed into the corresponding solvent in a pre-dried glass bottle with a bracket and a magnetic stirrer bar at the bottom and then the bottle was sealed. The grafting reaction was allowed to run for 48 h to reach equilibrium. After that, the substrates were thoroughly rinsed with ethanol, DCM and acetone, and dried under a stream of compressed air. The resulting MPEG-, SHS- and PFO-grafted substrates were denoted as SS-PVA-g-DBA-MPEG, SS-PVA-g-DBA-SHS and SS-PVA-g-DBA-PFO. The SS-PVA-g-DBA substrates were also immersed in a mixture system of isocyanate-terminated MPEG and isocyanate-terminated PFO (0.1 g mL−1 each) at room temperature for 48 h to achieve a surface containing both MPEG and PFO. The resulting substrates were denoted as SS-PVA-g-DBA-MPEG/PFO. At least five samples were prepared with each recipe and preserved in a desiccator before the performance test.
Protein adsorption tests on the antifouling surfaces
Static protein adsorption and a quartz crystal microbalance with dissipation (QCM-D, Q-sense E4, Sweden) experiment were conducted to evaluate the protein resistance capability of the coatings. The former was carried out according to the reported method.49 Briefly, the pristine and grafted substrates were placed in a 24-well plate and immersed into BSA-FITC solution (2.0 mg mL−1 in phosphate buffered saline (PBS, 10 mM, pH 7.4)) for 12 h at 37 °C in a dark environment. After washing with PBS and deionized water, the substrates were then viewed under a Nikon Eclipse Ti microscope for green fluorescence. The fluorescence intensity, which is indicative of the amount of protein adsorbed, was calculated by NIH ImageJ software. The QCM-D experiment was carried out according to a previous report, using a QCM crystal chip as the coating substrate.32 The system was first run in PBS solution at 25 °C for 1 h to attain a stable baseline, then the pristine and grafted substrates were exposed to BSA solution (5 wt% in PBS) introduced at a flow rate of 50 μL min−1, finally followed by a rinse with PBS for 20 min. All the experiments were repeated at least three times.Bacterial adhesion tests on the antifouling surfaces
E. coli and S. Aureus were used to evaluate the antibacterial adhesion characteristics of the grafted coatings. The test was carried out according to the reported method.50 Specifically, cultures of S. aureus and E. coli were grown aerobically for 24 h at 37 °C in sterile LB broth. These active growing cultures were diluted in saline (NaCl 8.5 g L−1) to a concentration of 105 bacteria per mL. Each substrate was then immersed into 1.0 mL of bacterial suspension in a 24-well plate under static conditions at 37 °C for 4 h. Quantification of bacterial adhesion on the pristine and grafted substrates was determined using the spread plate method. The bacteria suspension in each well was gently removed by aspiration using a pipette, and 1 mL of deionized water was slowly added to each well along the wall. The deionized water was then gently pipetted away. After washing with deionized water three times, the substrates were put into 3.0 mL of deionized water and subjected to ultrasonication for 7 min, followed by vortexing for 20 s to release the cells. The bacterial solution was then serially diluted, spread on an agar plate, and cultured overnight to quantify the number of bacterial cells. All experiments were performed in triplicate with three samples and the mean values were calculated. The number of adherent bacterial cells was expressed as cells per mm2 of substrate surface.Characterization
Statistical analysis
Statistical analysis was performed by using ANOVA with SPSS 21.0 software. The Fisher Least Significant Difference (LSD) post hoc test was chosen as the means for comparison with the statistically significant level (p < 0.05) defined. Experiments were carried out at least three times unless otherwise noted.Results and discussion
Synthesis of isocyanate-terminated grafting monomers
It is generally accepted that the initial step in biofilm formation is a reversible and nonspecific attachment. The interactions between the attaching substances and the surface are noncovalent bonds, and generally weak. Subsequent attachment of macroorganisms is permanent and irreversible. Therefore, inhibiting the initial adhesion of proteins and bacteria to a surface is one of the most critical and effective strategies to prevent the formation of biofilms.10,43A hydrophilic surface, which can be prepared via the formation of a hydration layer on the surfaces, increases the steric repulsion effect of the surface and exhibits good antifouling ability to resist nonspecific protein adsorption, bacterial adhesion, and biofilm formation.51 On the other hand, a hydrophobic surface, especially a surface tethered with fluorine-containing segments, also shows good antifouling ability due to its low surface energy.10 Thus, in this study, MPEG, SHS and PFO were selected as the surface modifier to modulate the hydrophilicity/hydrophobicity of the coatings. In order to tether these segments onto PVA-g-DBA coating surfaces, a highly reactive isocyanate group was incorporated into the surface modifiers. The synthesis routes toward these isocyanate-terminated monomers were straightforward and are shown in Fig. S1 (ESI†). Specifically, aliphatic diisocyanate, HDI, was reacted with the hydroxyl group in the MPEG, SHS and PFO monomers, using dibutyltindilaurate as the catalyst, and the resultant isocyanate-terminated monomers were obtained in quantitative yield. The chemical structures of these monomers were confirmed by 1H NMR spectroscopy, as shown in Fig. 1. The peak b at 4.2 ppm in Fig. 1A (4.18 ppm in Fig. 1B and 4.38 ppm in Fig. 1C) is assigned to the protons on the methylene group adjacent to the formed urethane bond, and the peaks between 1.2 ppm and 1.8 ppm (d, e, f, g) are assigned to the four middle methylene groups in HDI. The integrals of peak b and peaks d–g match well in all three graftable monomers, indicating that the corresponding isocyanate-terminated monomers were successful synthesized.
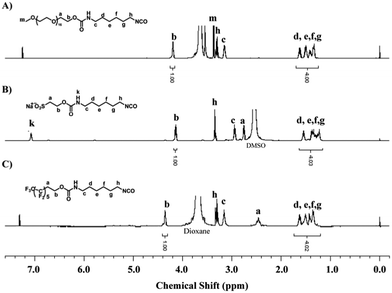 | ||
| Fig. 1 1H NMR spectra of isocyanate-terminated MPEG (in CDCl3), SHS (in DMSO-d6) and PFO (in CDCl3). | ||
Preparation of polymer and coating
PVA-g-DBA polymer was synthesized via a simple acid-catalyzed acetal formation reaction from two low-cost, commercially available materials, PVA and DBA and the synthesis scheme is shown in Scheme 1A. The synthesis and the structural characterization of this polymer have been discussed in our previous publication,47 and will not be covered in this report. Fully hydrolyzed PVA was used to guarantee the maximum hydroxyl group content available on the surface once the corresponding coating was formed. Meanwhile, PVA with Mw around 75 kDa was selected and the DG of catechol was fixed at approximately 10 mol% since it shows the best binding ability based on our previous optimized results.47 The polymer was dissolved in DMSO, and the corresponding solution was cast onto the substrate surfaces and then evaporated and cured at 110 °C for 18 h to form the coating (Scheme 1B). Previous results show that the formed coating exhibits excellent adhesion properties, mechanical hardness and high abrasion resistance on different substrates, including metal, glass and plastic.48 Compared to the reported mussel-inspired coatings using noneconomic biomimetic polymers described before, such an engineered coating that combines low-cost materials and excellent mechanical properties might be closer to actual application. In this study, SS, the most commonly used material in the industrial and medical fields, was selected as the representative substrate to fabricate the SS-PVA-g-DBA coating substrates for further modification.Functionalization of SS-PVA-g-DBA substrate surfaces with MPEG, SHS and PFO
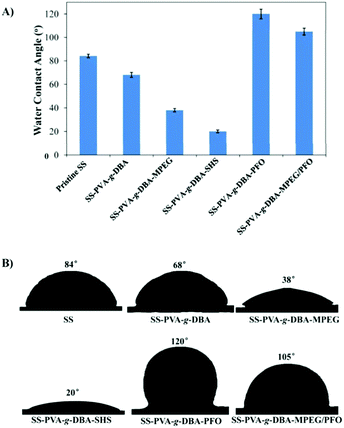
The formed PVA-g-DBA coating contains a large number of hydroxyl groups (about 80 mol%, except for a small percentage of hydroxyl groups that participated in the crosslinking via Michael addition with quinone groups during the formation of the coating) hanging on the PVA backbone that are suitable for further modification, while the oxidized and subsequently crosslinked catechol groups (which have been verified in our previous report) during the hot-curing process provide the coating with strong mechanic properties. Thus, the large amount of unreacted hydroxyl groups on the PVA backbone could be used as a precursor to conjugate with antifouling molecules to endow the coating with antifouling properties (Scheme 1B). For this purpose, highly reactive hydrophilic (MPEG and SHS) and hydrophobic (PFO) segments with terminal isocyanate groups were chosen to react with the PVA-g-DBA coating.Due to the high efficiency of the reaction of the isocyanate and hydroxyl groups, MPEG, SHS and PFO can be easily grafted onto the surface under mild conditions. FT-IR spectroscopy evolution (Fig. 2) provides direct evidence for the occurrence of the grafting reaction: a clear absorption at 1705 cm-1 appears for SS-PVA-g-DBA-MPEG, SS-PVA-g-DBA-SHS and SS-PVA-g-DBA-PFO (sample taken from the corresponding coating on the SS substrate) and is assigned to the carbonyl symmetric stretching in the formed urethane bond, which is not observed for pristine SS-PVA-g-DBA or PVA. The element analysis of the coating samples shows that all grafted coatings contain around 2% nitrogen (the detailed values are shown in Table S1 in ESI†). This further confirms that MPEG, SHS and PFO have been grafted onto the SS-PVA-g-DBA substrate surfaces. Moreover, contact angle measurements provide further supporting evidence that the surfaces have been successfully modified. Fig. 3 shows the static water contact angles and the corresponding images of the grafted SS, PVA-g-DBA-coated SS and pristine SS surfaces. The pristine SS surfaces are relatively hydrophobic, with a contact angle of around 84°. After coating with PVA-g-DBA, the contact angle of SS-PVA-g-DBA surface slightly decreases to around 68°, due to the unreacted hydroxyl groups on the PVA-g-DBA backbone. After grafting with MPEG and SHS, the contact angles of the SS-PVA-g-DBA-MPEG and SS-PVA-g-DBA-SHS surfaces decrease to around 38° and 20°, respectively. This obvious decrease is due to the following reasons: one is the hydrophilic nature of MPEG and SHS, the other is the formed polar urethane bond. The contact angles of the SS-PVA-g-DBA-MPEG surfaces are higher than that of the corresponding SS-PVA-g-DBA-SHS surfaces, which is understandable since the sulfonic acid ion is a more hydrophilic group compared to the OEG segment. After PFO grafting, the SS-PVA-g-DBA-PFO surface becomes more hydrophobic, with contact angles of 120°, due to the good hydrophobic nature of the perfluorinated alkyl segment. However, the surface did not reach the same level of hydrophobicity as other reported perfluoroalkyl group modified surfaces. This might be due to the formed polar urethane bond, which can weaken the contribution of the perfluorinated alkyl segment to hydrophobicity.
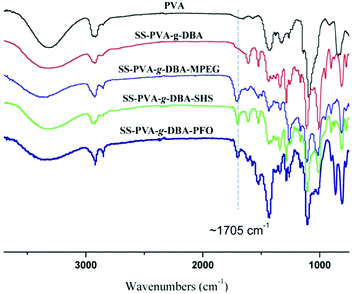 | ||
| Fig. 2 FT-IR spectra of pristine PVA, SS-PVA-g-DBA, SS-PVA-g-DBA-MPEG, SS-PVA-g-DBA-SHS and SS-PVA-g-DBA-PFO coating samples (sample taken from the corresponding coating on the SS substrate). | ||
In addition, besides the surface tethering with single MPEG (or SHS or PFO), a surface with both MPEG and PFO was also prepared. The FT-IR spectrum and the element content of the obtained coating are shown in Fig. S2 and Table S1 in ESI.† The contact angle of the SS-PVA-g-DBA-MPEG/PFO surface is 105° (Fig. 3), which is lower than that of the SS-PVA-g-DBA-PFO surface and higher than that of the SS-PVA-g-DBA-MPEG surface. Since the SS-PVA-g-DBA surface has a relatively “smooth” surface (0.34 nm of roughness based on the AFM measurement, Fig. S3, ESI†), the thickness of the grafted coatings on the SS-PVA-g-DBA surface was measured by using a step profiler. By comparing the thickness of coatings before and after grafting, the thickness increase of the MPEG, SHS, PFO and MPEG/PFO grafted coatings on the SS-PVA-g-DBA surface was found to be 27.5 ± 3.2 nm, 23.1 ± 2.4 nm, 25.7 ± 3.1 nm and 26.6 ± 2.8 nm, respectively.
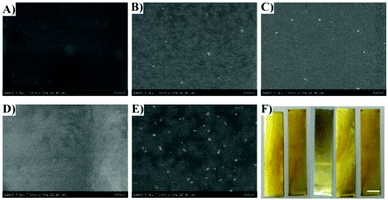
AFM and SEM techniques were employed to evaluate the surface morphology of the coating. The surface of the pristine coating, SS-PVA-g-DBA, is relatively smooth with an average roughness of 0.34 nm (Fig. 4A and Fig. S3, ESI†). After MPEG, SHS and PFO grafting, the SS-PVA-g-DBA-MPEG, SS-PVA-g-DBA-SHS and SS-PVA-g-DBA-PFO surfaces become relatively rougher than the surface before grafting (the average roughness of these surfaces is about 22 nm, 17 nm and 10 nm, respectively, Fig. S3, ESI†) and slight wrinkles can be found on the grafted-coating surface (Fig. 4B–D). Furthermore, no obvious aggregates and convex blocks can be found on the surfaces modified with a single modifier, indicating that the grafting modification is homogeneous. However, for the surface with two modifiers, the SS-PVA-g-DBA-MPEG/PFO surface, a significantly different surface morphology was observed. We can clearly see that the coating surface is discontinuous with separate domains, containing unevenly distributed small bumps (Fig. 4E). This might be due to the amphiphilicity of the formed MPEG (hydrophilic segment)/PFO (hydrophobic segment)-grafted coating, which is a common phenomenon for amphiphilic copolymer coatings.44 The average roughness of the SS-PVA-g-DBA-MPEG/PFO surface is about 102 nm (Fig. S3, ESI†), which is much higher than the surface tethering with a single modifier. The OM images with 4 times magnification also show similar results (Fig. S4, ESI†). Although a larger roughness was obtained, all the surfaces after grafting are still smooth and flat at the visual scale (Fig. 4F), which is very important for antifouling coatings in practical application, since a smoother surface can reduce the contact area of the adhesion protein, decrease its adhesion strength and enhance the antifouling ability.
Protein adsorption
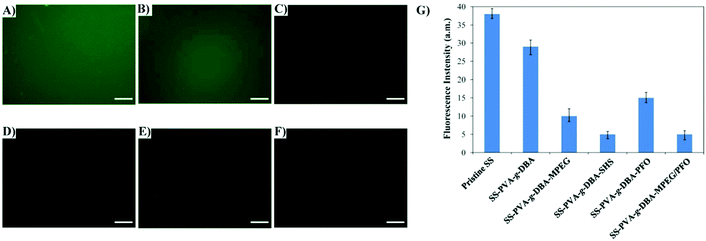
Antifouling surfaces, which can resist protein adsorption, also tend to be less susceptible to bacterial adhesion.49 Thus, the ability of our engineered coatings to resist protein was first assessed. BSA, a common protein used in protein adsorption assay, was chosen for this purpose. The antifouling ability was examined by incubating the surfaces in a solution containing fluorescent BSA-FITC via the static protein adsorption test, and subsequently imaging with a fluorescence microscope. Fig. 5 shows the respective fluorescence images and fluorescence intensities (bar graph) of the pristine SS, SS-PVA-g-DBA, SS-PVA-g-DBA-MPEG, SS-PVA-g-DBA-SHS, SS-PVA-g-DBA-PFO and SS-PVA-g-DBA-MPEG/PFO surfaces after exposure to the PBS solution of BSA-FITC. The weaker the fluorescence intensity, the higher the antifouling ability. A uniform and intense fluorescence is observed across the pristine SS surface (Fig. 5A), suggesting the significant extent of BSA-FITC adsorption on the substrate surface. For the hydrophilic surface, one can see very clearly that the fluorescence intensity is strongly dependent on the hydrophilicity of the surface. Compared to the pristine SS surface, a slight decrease was observed for the SS-PVA-g-DBA surface (Fig. 5B). With the further decrease of surface hydrophilicity, much weaker fluorescence is present on the SS-PVA-g-DBA-MPEG surface (Fig. 5C), while almost no fluorescence is observed on the SS-PVA-g-DBA-SHS surface (Fig. 5D). These results indicate that the adsorption of BSA-FITC protein on the engineered SS surfaces is significantly inhibited due to the repulsive forces arising from the formation of a hydration layer on the hydrophilic surface. For the hydrophobic surface, the SS-PVA-g-DBA-PFO surface also shows a decreased intensity (Fig. 5E and G) compared to the pristine SS and SS-PVA-g-DBA surfaces, indicating that a self-cleaning surface derived from the hydrophobic coating also has antifouling ability. However, the fluorescence intensity for SS-PVA-g-DBA-PFO was higher than that of the SS-PVA-g-DBA-MPEG and SS-PVA-g-DBA-SHS surfaces (Fig. 5G). This is due to the degree of contribution to their respective hydrophilicity/hydrophobicity after grafting, which is in line with the observations of water contact angle. In our case, the contribution to the surface hydrophobicity for the PFO modifier is weaker than the contribution to the surface hydrophilicity for the MPEG and SHS modifiers, due to the weakening effect of the formed urethane bond on the surface hydrophobicity. Very interestingly, when MPEG and PFO were used together to modify the coating surface, a much better antifouling property (than when the two modifiers were used separately) was observed, as indicated by the fact that no fluorescence was observed on the SS-PVA-g-DBA-MPEG/PFO surface (Fig. 5F). This excellent antifouling effect might be due to the surface microphase separation derived from the amphiphilic surface, which is in agreement with other reported research.44 QCM-D measurements (Fig. S5, ESI†) also show a similar tendency to the change of fluorescence intensity in the static protein adsorption test, indicating the good antifouling ability of the modified surfaces.Bacterial adhesion
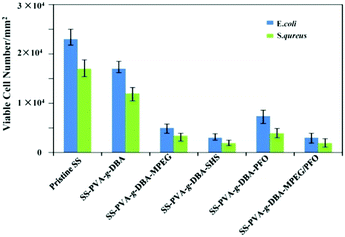
To further examine the antifouling properties of the engineered SS surfaces, the resistance to bacterial adhesion on the pristine SS, SS-PVA-g-DBA, SS-PVA-g-DBA-MPEG, SS-PVA-g-DBA-SHS, SS-PVA-g-DBA-PFO and SS-PVA-g-DBA-MPEG/PFO surfaces was assayed. E. coli and S. aureus were chosen for this purpose. The number of adhered bacteria on the pristine and engineering SS surfaces was assayed quantitatively by the spread plate method, as shown in Fig. 6. The lower the number, the higher the antifouling ability. Compared to the pristine SS and SS-PVA-g-DBA surfaces, the presence of MPEG, SHS, PFO and MPEG/PFO can significantly improve the efficiency of the resultant coatings to resist bacterial adhesion, and the antifouling efficiency is strongly dependent on the coating components. With the increase of surface hydrophilicity, the number of viable E. coli cells on the SS-PVA-g-DBA, SS-PVA-g-DBA-MPEG and SS-PVA-g-DBA-SHS surfaces decreases to 73%, 22% and 13%, respectively, as compared to that of the pristine SS surface. Similar results were also observed in the S. aureus tests. The amphiphilic surface again shows excellent antifouling efficiency. The number of viable E. coli and S. aureus cells on the SS-PVA-g-DBA-MPEG/PFO surface decreased to 13% and 11%, respectively, relative to that on the pristine SS surfaces. However, the antifouling efficiency of SS-PVA-g-DBA-PFO is weaker than that of MPEG- and SHS-grafted hydrophilic and MPEG/PFO-grafted amphiphilic surfaces, due to similar reasons to those described above. The number of viable E. coli and S. aureus cells on the SS-PVA-g-DBA-PFO surface decreased to 32% and 27%, respectively, relative to that on the pristine SS surfaces. Moreover, one can also find that the grafted coatings exhibit more effective antifouling activity for S. aureus than for E. coli. This may be due to the complex cell membrane composition of E. coli.Mechanical properties
In our previous studies, the SS-PVA-g-DBA coating has shown excellent adhesion properties, mechanical hardness and high abrasion resistance.48 Although it is widely believed that the grafting-to approach does not alter the structures and mechanical properties of the original coating, additional evidence is still needed to testify this. Thus, a systemic study on the mechanical properties of the grafted coatings in our case is still necessary. The cross-cut test (according to the ASTM D3359 standards), abrasion test (according to ASTM D4060 standards) and pencil test (according to ASTM D3363 standards) were employed to evaluate the adhesion, abrasion resistance and hardness of the formed coatings, respectively. The test methods have been discussed in detail in our previous publication,48 and will not be covered in this report. The comparison of the results before and after grafting is shown in Table 1. As can be seen, all the grafted coatings show excellent adhesion (5B, which is the highest level for adhesion), outstanding anti-abrasive properties (weight loss less than 20 mg after 1000 cycles of abrasion) and adequate hardness comparable to that of the pristine SS-PVA-g-DBA surface, indicating that the grafting-to approach does not alter the mechanical properties of the original coating in our case. The photo images of the coating samples before and after the cross-cut test are shown in Fig. S6 (ESI†). It is worthwhile mentioning that such robust coatings with excellent adhesion properties, mechanical hardness and high abrasion resistance surpassed many reported cases.27,32,52 As discussed before, the mechanical properties of the coating are important parameters for long-term antifouling. In our case, both coatings are mechanically hard, highly adhesive and abrasion resistant, which make them suitable for practical application.| Substrates | Adhesion | Wear index [mg] | Hardness |
|---|---|---|---|
| SS-PVA-g-DBA | 5B | 15 | 5H |
| SS-PVA-g-DBA-MPEG | 5B | 16 | 5H |
| SS-PVA-g-DBA-SHS | 5B | 18 | 5H |
| SS-PVA-g-DBA-PFO | 5B | 14 | 5H |
| SS-PVA-g-DBA-MPEG/PFO | 5B | 15 | 5H |
Conclusions
In conclusion, a biomimetic polymer, PVA-g-DBA, could be used as a versatile platform for robust antifouling coatings. By using a simple grafting-to approach, isocyanate-terminated MPEG, SHS, PFO and MPEG/PFO were tethered separately onto the PVA-g-DBA-coated surfaces via an effective urethane bond formation reaction to fabricate mussel-inspired antifouling coatings, utilizing the hydroxyl groups remaining in the PVA-g-DBA coating. The presence of MPEG, SHS, PFO and MPEG/PFO could obviously alter the hydrophobicity/hydrophilicity of the surfaces and significantly improve the antifouling efficiency of the resulting coatings to resist protein adsorption and bacterial adhesion. The antifouling efficiency was dependent on the coating components. Among them, SHS and MPEG/PFO grafted coatings showed the best antifouling efficacy, due to the highest hydrophilicity and amphiphilic nature, respectively. Meanwhile, the grafting process does not alter the mechanical properties of the original SS-PVA-g-DBA coating. All grafted coatings are mechanically robust, highly adhesive and abrasion resistant. Taking into account the robust substrate-independent PVA-g-DBA coating and the flexibility of grafting monomers, this property enables the catechol-functionalized PVA-based biomimetic polymer to be used as a venue for not only antifouling coatings but also other functionalized coatings for use in industrial and biomedical areas.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Science Foundation of China (51603223) and the Shandong Provincial Natural Science Foundation of China (ZR2015EQ008).Notes and references
- J. L. Dalsin, L. Lin, S. Tosatti, J. Vörös, M. Textor and P. B. Messersmith, Langmuir, 2005, 21, 640 CrossRef CAS PubMed.
- I. Banerjee, R. C. Pangule and R. S. Kane, Adv. Mater., 2011, 23, 690 CrossRef CAS PubMed.
- X. Fan, L. Lin, J. L. Dalsin and P. B. Messersmith, J. Am. Chem. Soc., 2005, 127, 15843 CrossRef CAS PubMed.
- X. Khoo, P. Hamilton, G. A. O'Toole, B. D. Snyder, D. J. Kenan and M. W. Grinstaff, J. Am. Chem. Soc., 2009, 131, 10992 CrossRef CAS PubMed.
- A. Charlot, V. Sciannamea, S. Lenoir, E. Faure, R. Jerome, C. Jerome, C. Van De Weerdt, J. Martial, C. Archambeau, N. Willet, A. S. Duwez, C. A. Fustin and C. Detrembleur, J. Mater. Chem., 2009, 19, 4117 RSC.
- C. He, C. Cheng, S. Q. Nie, L. R. Wang, C. X. Nie, S. D. Sun and C. S. Zhao, J. Mater. Chem. B, 2016, 4, 6143 RSC.
- Y. Xia, C. Cheng, R. Wang, C. Nie, J. Deng and C. Zhao, J. Mater. Chem. B, 2015, 3, 9295 RSC.
- C. Cheng, A. He, C. Nie, Y. Xia, C. He, L. Ma and C. Zhao, J. Mater. Chem. B, 2015, 3, 4170 RSC.
- S. Yuan, D. Wan, B. Liang, S. O. Pehkonen, Y. P. Ting, K. G. Neoh and E. T. Kang, Langmuir, 2011, 27, 2761 CrossRef CAS PubMed.
- M. Lejars, A. Margaillan and C. Bressy, Chem. Rev., 2012, 112, 4347 CrossRef CAS PubMed.
- H. Lee, K. D. Lee, K. B. Pyo, S. Y. Park and H. Lee, Langmuir, 2010, 26, 3790 CrossRef CAS PubMed.
- M. K. Chaudhury and G. M. Whitesides, Science, 1992, 255, 1230 CAS.
- R. Hofer, M. Textor and N. D. Spencer, Langmuir, 2001, 17, 4014 CrossRef CAS.
- H. Ma, O. Acton, D. O. Hutchins, N. Cernetic and A. K. Y. Jen, Phys. Chem. Chem. Phys., 2012, 14, 14110 RSC.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426 CrossRef CAS PubMed.
- Q. Lin, D. Gourdon, C. Sun, N. Holten-Andersen, T. H. Anderson, J. H. Waite and J. N. Israelachvili, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 3782 CrossRef CAS PubMed.
- M. Liu, G. Zeng, K. Wang, Q. Wan, L. Tao, X. Zhang and Y. Wei, Nanoscale, 2016, 8, 16819 RSC.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057 CrossRef CAS PubMed.
- C. Liu, J. Lee, J. Ma and M. Elimelech, Environ. Sci. Technol., 2017, 51, 2161 CrossRef CAS PubMed.
- C. J. Huang, L. C. Wang, J. J. Shyue and Y. C. Chang, Langmuir, 2014, 30, 12638 CrossRef CAS PubMed.
- H. Wu, J. M. Ang, J. Kong, C. Zhao, Y. Du and X. Lu, RSC Adv., 2016, 6, 103390 RSC.
- C. C. CartilageChang, K. W. Kolewe, Y. Li, I. Kosif, B. D. Freeman, K. R. Carter, J. D. Schiffman and T. Emrick, Adv. Mater. Interfaces, 2016, 3, 1500521 CrossRef PubMed.
- J. Cui, Y. Ju, K. Liang, H. Ejima, S. Lorcher, K. T. Gause, J. J. Richardson and F. Caruso, Soft Matter, 2014, 10, 2656 RSC.
- Y. Li, Y. Su, X. Zhao, X. He, R. Zhang, J. Zhao, X. Fan and Z. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 5548 CAS.
- R. Zhang, Y. Su, L. Zhou, T. Zhou, X. Zhao, Y. Li, Y. Liu and Z. Jiang, RSC Adv., 2016, 6, 32863 RSC.
- J. Ren, P. Han, H. Wei and L. Jia, ACS Appl. Mater. Interfaces, 2014, 6, 3829 CAS.
- G. R. Alas, R. Agarwal, D. M. Collard and A. J. García, Acta Biomater., 2017, 1, 108 CrossRef PubMed.
- C. Rodriguez-Emmenegger, C. M. Preuss, B. Yameen, O. Pop-Georgievski, M. Bachmann, J. O. Mueller, M. Bruns, A. S. Goldmann, M. Bastmeyer and C. Barner-Kowollik, Adv. Mater., 2013, 25, 6123 CrossRef CAS PubMed.
- Z. Wang and H. Zuilhof, J. Mater. Chem. A, 2016, 4, 2408 CAS.
- T. Kang, D. X. Oh, J. Heo, H. K. Lee, S. Choy, C. J. Hawker and D. S. Hwang, ACS Appl. Mater. Interfaces, 2015, 7, 24656 CAS.
- T. Kang, X. Banquy, J. Heo, C. Lim, N. A. Lynd, P. Lundberg, D. X. Oh, H. K. Lee, Y. K. Hong, D. S. Hwang, J. H. Waite, J. N. Israelachvili and C. J. Hawker, ACS Nano, 2016, 10, 930 CrossRef CAS PubMed.
- L. Li, B. Yan, L. Zhang, Y. Tian and H. Zeng, Chem. Commun., 2015, 51, 15780 RSC.
- Y. S. Choi, H. Kang, D. G. Kim, S. H. Cha and J. C. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 21297 CAS.
- L. Li, B. Yan, J. Yang, L. Chen and H. Zeng, Adv. Mater., 2015, 27, 1294 CrossRef CAS PubMed.
- H. Wei, L. Han, J. Ren and L. Jia, ACS Appl. Mater. Interfaces, 2013, 5, 12571 CAS.
- J. Y. Park, J. S. Kim and Y. S. Nam, Carbohydr. Polym., 2013, 97, 753 CrossRef CAS PubMed.
- R. Wang, X. Song, T. Xiang, Q. Liu, B. Su, W. Zhao and C. Zhao, Carbohydr. Polym., 2017, 168, 310 CrossRef CAS PubMed.
- H. Ye, Y. Xia, Z. Liu, R. Huang, R. Su, W. Qi, L. Wang and Z. He, J. Mater. Chem. B, 2016, 4, 4084 RSC.
- W. Cheng, C. Yang, X. Ding, A. C. Engler, J. L. Hedrick and Y. Y. Yang, Biomacromolecules, 2015, 16, 1967 CrossRef CAS PubMed.
- H. O. Ham, S. H. Park, J. W. Kurutz, I. G. Szleifer and P. B. Messersmith, J. Am. Chem. Soc., 2013, 135, 13015 CrossRef CAS PubMed.
- Q. Sun, H. Li, C. Xian, Y. Yang, Y. Song and P. Cong, Appl. Surf. Sci., 2015, 344, 17 CrossRef CAS.
- Â. Serrano, S. Zürcher, S. Tosatti and N. D. Spencer, Macromol. Rapid Commun., 2016, 37, 622 CrossRef PubMed.
- L. Wang, G. Li, Y. Lin, Z. Zhang, Z. Chen and S. Wu, Polym. Chem., 2016, 7, 4964 RSC.
- J. Zhong, H. Ji, J. Duan, H. Tu and A. Zhang, Colloids Surf., B, 2016, 140, 254 CrossRef CAS PubMed.
- H. Chen, X. Li, Y. Zhao, J. Li, J. Chen, P. Yang, M. F. Maitz and N. Huang, Appl. Surf. Sci., 2015, 347, 169 CrossRef CAS.
- Y. K. Gong, L. P. Liu and P. B. Messersmith, Macromol. Biosci., 2012, 12, 979 CrossRef CAS PubMed.
- Y. Mu and X. Wan, Macromol. Rapid Commun., 2016, 37, 545 CrossRef CAS PubMed.
- Y. Mu, Z. Wu, Y. Ma, J. Zheng, W. Zhang, Z. Sun, X. Wang, D. Pei, L. Li, W. Jiang, J. Hou and X. Wan, J. Mater. Chem. B, 2017, 5, 1742 RSC.
- L. Q. Xu, D. Pranantyo, K. G. Neoh, E. T. Kang, S. L. M. Teo and G. D. Fu, Polym. Chem., 2016, 7, 493 RSC.
- L. Q. Xu, D. Pranantyo, J. B. Liu, K. G. Neoh, E. T. Kang, Y. X. Ng, S. Lay-Ming Teo and G. D. Fu, RSC Adv., 2014, 4, 32335 RSC.
- S. Lowe, N. M. O'Brien-Simpson and L. A. Connal, Polym. Chem., 2015, 6, 198 RSC.
- Q. Wei, T. Becherer, R. C. Mutihac, P. L. Noeske, F. Paulus, R. Haag and I. Grunwald, Biomacromolecules, 2014, 15, 3061 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7tb02400b |
| This journal is © The Royal Society of Chemistry 2018 |