Direct growth of ordered PdCu and Co doped PdCu nanoparticles on graphene oxide based on a one-step hydrothermal method for ultrasensitive sensing of H2O2 in living cells†
Wu-Shuang
Bai
a,
Xin-Jin
Zhang
b and
Jian-Bin
Zheng
 *b
*b
aCollege of Food Science and Engineering, Shaanxi Provincial Key Laboratory of Electroanalytical Chemistry, Northwest University, Xi'an, Shaanxi 710069, China
bCollege of Chemistry & Materials Science, Shaanxi Provincial Key Laboratory of Electroanalytical Chemistry, Northwest University, Xi'an, Shaanxi 710069, China. E-mail: bws@nwu.edu.cn
First published on 22nd November 2018
Abstract
Ordered PdCu and Co doped PdCu nanoparticles have been synthesized on graphene oxide (GO). The obtained Co–PdCu/GO composites were used to fabricate a H2O2 electrochemical sensor which exhibits an ultralow LOD (limit of detection) of 1.2 nM and an extra broad linear range of 5 nM–5.774 mM, and has been used to detect H2O2 in living cells successfully.
Detection of reactive oxygen species (ROS) is very significant for clinical and biological studies because they can regulate DNA damage, protein synthesis and cell apoptosis which is closely related to various diseases such as cancer, diabetes, neurodegenerative Alzheimer's diseases, etc.1–3 As one of the most representative ROS, hydrogen peroxide (H2O2) can penetrate into other cellular compartments due to its long lifetime in living cells.4–6 Therefore, sensitive and fast detection of H2O2 released from living cells is of great significance in cell research and human pathology. Among the various detection methods, electrochemical sensors have attracted tremendous attention due to their simple fabrication, good sensitivity and selectivity.7,8 In addition, compared with enzyme base sensors, non-enzymatic sensors exhibit a wide range of applications and storage conditions. Therefore, how to improve the performance of non-enzymatic sensors is a key question for researchers. In recent years, nanotechnology has been developed rapidly. Nanomaterials, especially nanometals, have been used as catalysts for oxidation or reduction of H2O2 which provides an opportunity for the improvement of sensors.9–14 Commonly, Ag and Pt nanomaterials are most used due to their good catalytic properties, conductivity and easy synthesis.15–18 However, Ag can be oxidized easily which leads to poor stability and reproducibility. In addition, low abundance of Pt in nature and poor long-term duration also lead to the practical large-scale commercialization of electrochemical sensors.19,20 Compared with Ag and Pt, Pd is always a good choice due to its great potential in catalyzing the reduction of H2O2 and oxygen and oxidation of many small organic molecules which can be used in sensors and fuel cells.21–23 Therefore, great efforts have been focused on the development of Pd based nanocatalysts. One of the interesting strategies is to develop multi-metals with catalytic performance comparable to or even superior to that of a monometal. On one hand, the catalytic property will be improved due to the cooperation between every metal. On the other hand, the surface plasma band energy will be changed by the multi-metal interaction.24,25 Inspired by this, Pd based multi-metal nanomaterials exhibit great potential in electrochemical sensing and other catalytic fields, and synthesis of Pd based multi-metal nanomaterials with a desired morphology and composition tuning to further improve the properties is of significance. However, previous studies mainly focused on the synthesis of disordered nanomaterials which exhibited low stability in some special electrochemical environments and showed only a moderate improvement in activity. Compared with the disordered structures, the ordered intermetallic nanocrystals which have definite composition and structure will enhance the catalytic activities due to their strong electron interaction, the change in the bond length and electron configuration.26,27
In this regard, if Pd based multi-metal nanostructures with an ordered intermetallic phase can be engineered, it will extremely enhance the catalytic activity and stability which will be a significant opportunity for the improvement of sensors. In this work, controllable syntheses of PdCu nanoparticles and Co doped PdCu(Co–PdCu) nanoparticles with an ordered metallic structure were realized on the surface of graphene oxide (GO) based on a facile hydrothermal method. For the first time, the obtained ordered multi-metal materials were used for the fabrication of electrochemical sensors. The obtained sensor exhibits an ultralow LOD of 1.2 nM and an extra broad linear range of 5 nM–5.774 mM which can be used for the ultrasensitive detection of H2O2 released from living cells successfully. This work provides a new design strategy for creating an ordered multi-metallic nanostructure and a promising platform for fabrication of high-performance electrochemical sensors.
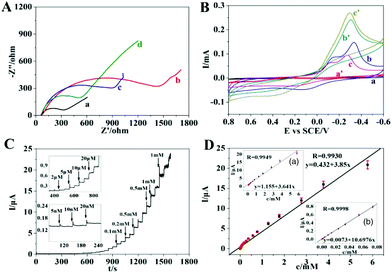
Fig. 1 shows morphology and structure characterization of obtained PdCu/GO and Co–PdCu/GO. Ordered PdCu nanoparticles with spherical shape are dispersed on the surface of GO which exhibit an average diameter of ∼7 nm (Fig. 1A and C). According to the elemental mapping results (Fig. S1, ESI†) and EDS pattern (Fig. S2, ESI†), it can be found that PdCu particles are synthesized successfully and the weight percentage of Pd and Cu is consistent with the experimental dose. Compared with PdCu particles, Co–PdCu particles show a very different ordered morphology (Fig. 1B and D). The particles are dispersed uniformly on the surface of GO, and they exhibit an average diameter of ∼9 nm which is bigger than that of PdCu particles. Likewise, the elemental mapping results (Fig. S3, ESI†) and EDS pattern (Fig. S4, ESI†) also indicate that Co–PdCu particles are synthesized successfully and the weight percentage of Pd, Cu and Co is consistent with the experimental dose. The X-ray diffraction (XRD) patterns of PdCu/GO (Fig. 1E a) and Co–PdCu/GO (Fig. 1E b) show the representative diffraction peaks at 40.8 and 47.6° which are indexed to the (111) and (200) reflections (JCPDS 48-1551). Compared with PdCu/GO, Co–PdCu/GO exhibits a similar XRD pattern except for a weak peak shift (Fig. 1E b). In addition, FTIR studies were also performed to further investigate the obtained materials (Fig. 1F). It can be seen that GO exhibits various typical functional groups (Fig. 1F a). The broad peak at about 3400 cm−1 represents the –OH stretching. The two peaks at 1729 cm−1 and 1603 cm−1 are due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, water –OH bending and C
O stretching, water –OH bending and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching. The three peaks located at 1400, 1250 and 1052 cm−1 are attributed to epoxide C–O–C or phenolic C–O–H stretching and C–O stretching. Compared with GO, PdCu/GO (Fig. 1F b) and Co–PdCu/GO (Fig. 1F c) all exhibit similar FTIR spectra with a lower peak intensity which is due to the reduction of GO. Compared with PdCu/GO (Fig. 1F b), Co–PdCu/GO (Fig. 1F c) exhibits almost the same spectrum. However, there are some weak peak shifts appearing which may be caused by the interaction between Co and PdCu. Based on the previous research studies, the synthesis of ordered PdCu and Co–PdCu particles may be divided into three steps:28 intermediate formation, nucleation and growth. First, the intermediates are formed based on the combination between metal precursors and amino of OA. Second, the intermediates will be reduced by AA and the crystal nuclei appear. Finally, the nuclei will aggregate into bigger particles. In addition, according to the TEM results of the comparison experiment (Fig. S5, ESI†), PdCu particles are dispersed randomly on the surface of GO when the experiment is processed in water–OA phases. This is perhaps because AA and FeCl3 will be dissolved more easily in water, and Pd(acac)2 and Cu(acac)2 will be dissolved in OA which will be separated from AA and FeCl3. According to the results, AA and FeCl3 may also be the protective agent in the synthesis of the ordered nanostructure. The detailed synthetic mechanism will be researched in our further work. Electrochemical research studies of the obtained materials were performed. According to the EIS results shown in Fig. 2A, it can be seen that GO/GCE exhibits the largest charge transfer resistance (Rct) which is due to the semi-conductivity of GO (Fig. 2A b). PdCu/GO/GCE exhibits lower Rct which is due to the conductivity of PdCu (Fig. 2A c). Compared with GO/GCE and PdCu/GO/GCE, Co–PdCu/GO/GCE has the lowest Rct (Fig. 2A d). This may be because of the fact that the doping of Co will improve the charge transfer between the three kinds of metal which is beneficial for electrochemical sensing. In contrast, although Co–PdCu/GO/GCE exhibits a larger Rct than bare GCE (Fig. 2A a) due to the semi-conductivity of GO, there is little difference between them in the presence of a CoPdCu alloy. In addition, electrode effective surface areas of bare GCE, GO/GCE and Co–PdCu/GCE were also researched (Fig. S6, ESI†). According to the Randles–Sevcik equation (ip = (2.69 × 105)n3/2AeffD1/2Cv1/2), compared with the bare GCE (Fig. S6A and B, ESI†) and GO/GCE (Fig. S6C and D, ESI†), Co–PdCu/GCE exhibits the largest effective surface area (Fig. S6E and F,† ESI†). Then GO/GCE, PdCu/GCE and Co–PdCu/GCE have been used for the electro-catalysis of H2O2. According to the CV results (Fig. 2B), it can be seen that GO/GCE exhibits no current peak in the absence or presence of H2O2 which indicates that GO has no catalytic properties towards H2O2 (Fig. 2B a and a′). However, the reduction peak current of PdCu/GCE has increased by about 0.1 mA at −0.3 V (Fig. 2B b and b′) while it has increased by about 0.2 mA at −0.28 V for Co–PdCu/GCE (Fig. 2B c and c′) in the absence and presence of 5 mM H2O2. In addition, after adding H2O2, a weaker oxidation peak also appeared at about 0.4 V. This means that PdCu has good catalytic properties for H2O2 reduction, and compared with PdCu, Co–PdCu exhibits better catalytic properties. This may be due to the following two reasons: 1 compared with bimetal, trimetal will improve the charge transfer between the three kinds of metals which is beneficial for electrochemical sensing. 2 Reduction of H2O2 can also be catalyzed by single Co, and Co–PdCu will exhibit better catalytic properties due to the synergistic effect between Co and PdCu. Then Co–PdCu/GCE was used to study the relationship between peak current and H2O2 concentration and a good linear relationship is found between the peak current and H2O2 concentration at a range from 0 to 5 mM (Fig. S7, ESI†). In addition, according to the CV results of Co–PdCu/GCE at different scan rates (Fig. S8, ESI†), it can be seen that the peak current shows linear dependence on the square root of the scan rate which indicates that the apparent electron diffusion of Co–PdCu/GCE can be controlled in a process of H2O2 catalysis (based on the Randles–Sevcik equation). In addition, the catalytic properties of Co–PdCu nanomaterials with different contents of Co were researched (Co–PdCu nanomaterials were synthesized using different doses of Co(acac)2). According to the CV results (Fig. S9, ESI†), it can be seen that when 6.4 mg Co(acac)2 are used for the preparation, the fabricated Co–PdCu/GCE exhibits the highest peak current, which means that the Co–PdCu nanomaterials synthesized by 6.4 mg Co(acac)2 exhibit the best catalytic properties. In this work, a typical amperometric method is used for the sensing of H2O2. In order to minimize the background noise and avoid signal interference, a potential choice has been researched before detection (Fig. S10, ESI†). It can be seen that compared with the results tested under oxidation potentials, the signals under reduction potentials exhibit the higher intensity. In addition, among various reduction potentials, considering the signal intensity, background noise and possible interference in cell detection, 0 V is the best choice. According to the amperometric response of Co–PdCu/GCE (Fig. 2C and D), it can be seen that the obtained Co–PdCu/GCE exhibits a fast current response after injection of H2O2 with a LOD of ∼1.2 nM, and the modified electrode exhibits a good linear range of 5 nM–5.774 mM with the linear regression equation of I (μA) = 3.85C (mM) + 0.432 (R = 0.9930). In addition, it also exhibits a good linear range of 5 nM–73.89 μM with the linear regression equation of I (μA) = 10.6976C (mM) + 0.0073 (R = 0.9998) (inset b in Fig. 2D) and a linear range of 73.89 μM–5.774 mM with the regression equation of I (μA) = 3.641C (mM) + 1.155 (R = 0.9949) (inset a in Fig. 2D). Before the detection of
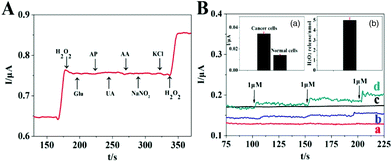
H2O2 released from living cells, anti-interference and reproducibility experiments were performed to research the anti-interference properties and reproducibility of Co–PdCu/GCE. According to Table S1,† it can be seen that the modified electrode exhibits good reproducibility. According to the amperometric response of Co–PdCu/GCE upon the addition of 10 μM H2O2, 5 μM AP (acetaminophen), 5 μM AA, 5 μM UA (uric acid), 5 μM Glu (glucose), 5 μM NaNO2 and 5 μM KCl (Fig. 3A), it can be seen that the Co–PdCu/GCE exhibits good anti-interference ability in the presence of AA, UA, AP, Glu, KCl and NaNO2. According to the amperometric responses of Co–PdCu/GCE under different conditions (Fig. 3B), it can be seen that no current change is observed in the situation of cancer cells with catalase which is due to the rapid disintegration of H2O2 in the presence of catalase. In contrast, Co–PdCu/GCE exhibits an obvious current change in PC-12 and normal cell solution. This indicates the successful stimulation of AA. In addition, compared with PC-12 cells, the current response is lower in normal cell solution in the presence of the same concentration of AA which indicates that cancer cells release a higher amount of H2O2 (inset a). This is consistent with an earlier report.29 This may be because the peroxidases in cancer cells have been destroyed. According to the standard curve (inset b, Fig. 2D), the current of about 34 nA in cancer cells roughly corresponds to H2O2 concentrations of 2.496 μM. The cancer cell solution is 2 mL, corresponding to 4.99 nmol H2O2 release from cancer cells (inset b). The cell number is 4 × 107, corresponding to 125 amol H2O2 release from single PC-12 cells, as reported earlier.30
C stretching. The three peaks located at 1400, 1250 and 1052 cm−1 are attributed to epoxide C–O–C or phenolic C–O–H stretching and C–O stretching. Compared with GO, PdCu/GO (Fig. 1F b) and Co–PdCu/GO (Fig. 1F c) all exhibit similar FTIR spectra with a lower peak intensity which is due to the reduction of GO. Compared with PdCu/GO (Fig. 1F b), Co–PdCu/GO (Fig. 1F c) exhibits almost the same spectrum. However, there are some weak peak shifts appearing which may be caused by the interaction between Co and PdCu. Based on the previous research studies, the synthesis of ordered PdCu and Co–PdCu particles may be divided into three steps:28 intermediate formation, nucleation and growth. First, the intermediates are formed based on the combination between metal precursors and amino of OA. Second, the intermediates will be reduced by AA and the crystal nuclei appear. Finally, the nuclei will aggregate into bigger particles. In addition, according to the TEM results of the comparison experiment (Fig. S5, ESI†), PdCu particles are dispersed randomly on the surface of GO when the experiment is processed in water–OA phases. This is perhaps because AA and FeCl3 will be dissolved more easily in water, and Pd(acac)2 and Cu(acac)2 will be dissolved in OA which will be separated from AA and FeCl3. According to the results, AA and FeCl3 may also be the protective agent in the synthesis of the ordered nanostructure. The detailed synthetic mechanism will be researched in our further work. Electrochemical research studies of the obtained materials were performed. According to the EIS results shown in Fig. 2A, it can be seen that GO/GCE exhibits the largest charge transfer resistance (Rct) which is due to the semi-conductivity of GO (Fig. 2A b). PdCu/GO/GCE exhibits lower Rct which is due to the conductivity of PdCu (Fig. 2A c). Compared with GO/GCE and PdCu/GO/GCE, Co–PdCu/GO/GCE has the lowest Rct (Fig. 2A d). This may be because of the fact that the doping of Co will improve the charge transfer between the three kinds of metal which is beneficial for electrochemical sensing. In contrast, although Co–PdCu/GO/GCE exhibits a larger Rct than bare GCE (Fig. 2A a) due to the semi-conductivity of GO, there is little difference between them in the presence of a CoPdCu alloy. In addition, electrode effective surface areas of bare GCE, GO/GCE and Co–PdCu/GCE were also researched (Fig. S6, ESI†). According to the Randles–Sevcik equation (ip = (2.69 × 105)n3/2AeffD1/2Cv1/2), compared with the bare GCE (Fig. S6A and B, ESI†) and GO/GCE (Fig. S6C and D, ESI†), Co–PdCu/GCE exhibits the largest effective surface area (Fig. S6E and F,† ESI†). Then GO/GCE, PdCu/GCE and Co–PdCu/GCE have been used for the electro-catalysis of H2O2. According to the CV results (Fig. 2B), it can be seen that GO/GCE exhibits no current peak in the absence or presence of H2O2 which indicates that GO has no catalytic properties towards H2O2 (Fig. 2B a and a′). However, the reduction peak current of PdCu/GCE has increased by about 0.1 mA at −0.3 V (Fig. 2B b and b′) while it has increased by about 0.2 mA at −0.28 V for Co–PdCu/GCE (Fig. 2B c and c′) in the absence and presence of 5 mM H2O2. In addition, after adding H2O2, a weaker oxidation peak also appeared at about 0.4 V. This means that PdCu has good catalytic properties for H2O2 reduction, and compared with PdCu, Co–PdCu exhibits better catalytic properties. This may be due to the following two reasons: 1 compared with bimetal, trimetal will improve the charge transfer between the three kinds of metals which is beneficial for electrochemical sensing. 2 Reduction of H2O2 can also be catalyzed by single Co, and Co–PdCu will exhibit better catalytic properties due to the synergistic effect between Co and PdCu. Then Co–PdCu/GCE was used to study the relationship between peak current and H2O2 concentration and a good linear relationship is found between the peak current and H2O2 concentration at a range from 0 to 5 mM (Fig. S7, ESI†). In addition, according to the CV results of Co–PdCu/GCE at different scan rates (Fig. S8, ESI†), it can be seen that the peak current shows linear dependence on the square root of the scan rate which indicates that the apparent electron diffusion of Co–PdCu/GCE can be controlled in a process of H2O2 catalysis (based on the Randles–Sevcik equation). In addition, the catalytic properties of Co–PdCu nanomaterials with different contents of Co were researched (Co–PdCu nanomaterials were synthesized using different doses of Co(acac)2). According to the CV results (Fig. S9, ESI†), it can be seen that when 6.4 mg Co(acac)2 are used for the preparation, the fabricated Co–PdCu/GCE exhibits the highest peak current, which means that the Co–PdCu nanomaterials synthesized by 6.4 mg Co(acac)2 exhibit the best catalytic properties. In this work, a typical amperometric method is used for the sensing of H2O2. In order to minimize the background noise and avoid signal interference, a potential choice has been researched before detection (Fig. S10, ESI†). It can be seen that compared with the results tested under oxidation potentials, the signals under reduction potentials exhibit the higher intensity. In addition, among various reduction potentials, considering the signal intensity, background noise and possible interference in cell detection, 0 V is the best choice. According to the amperometric response of Co–PdCu/GCE (Fig. 2C and D), it can be seen that the obtained Co–PdCu/GCE exhibits a fast current response after injection of H2O2 with a LOD of ∼1.2 nM, and the modified electrode exhibits a good linear range of 5 nM–5.774 mM with the linear regression equation of I (μA) = 3.85C (mM) + 0.432 (R = 0.9930). In addition, it also exhibits a good linear range of 5 nM–73.89 μM with the linear regression equation of I (μA) = 10.6976C (mM) + 0.0073 (R = 0.9998) (inset b in Fig. 2D) and a linear range of 73.89 μM–5.774 mM with the regression equation of I (μA) = 3.641C (mM) + 1.155 (R = 0.9949) (inset a in Fig. 2D). Before the detection of
H2O2 released from living cells, anti-interference and reproducibility experiments were performed to research the anti-interference properties and reproducibility of Co–PdCu/GCE. According to Table S1,† it can be seen that the modified electrode exhibits good reproducibility. According to the amperometric response of Co–PdCu/GCE upon the addition of 10 μM H2O2, 5 μM AP (acetaminophen), 5 μM AA, 5 μM UA (uric acid), 5 μM Glu (glucose), 5 μM NaNO2 and 5 μM KCl (Fig. 3A), it can be seen that the Co–PdCu/GCE exhibits good anti-interference ability in the presence of AA, UA, AP, Glu, KCl and NaNO2. According to the amperometric responses of Co–PdCu/GCE under different conditions (Fig. 3B), it can be seen that no current change is observed in the situation of cancer cells with catalase which is due to the rapid disintegration of H2O2 in the presence of catalase. In contrast, Co–PdCu/GCE exhibits an obvious current change in PC-12 and normal cell solution. This indicates the successful stimulation of AA. In addition, compared with PC-12 cells, the current response is lower in normal cell solution in the presence of the same concentration of AA which indicates that cancer cells release a higher amount of H2O2 (inset a). This is consistent with an earlier report.29 This may be because the peroxidases in cancer cells have been destroyed. According to the standard curve (inset b, Fig. 2D), the current of about 34 nA in cancer cells roughly corresponds to H2O2 concentrations of 2.496 μM. The cancer cell solution is 2 mL, corresponding to 4.99 nmol H2O2 release from cancer cells (inset b). The cell number is 4 × 107, corresponding to 125 amol H2O2 release from single PC-12 cells, as reported earlier.30
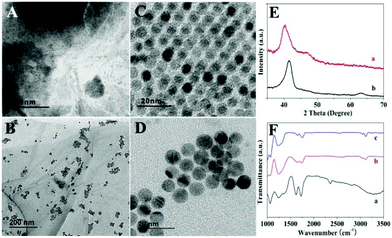 | ||
| Fig. 1 TEM patterns of PdCu/GO (A, C) and Co–PdCu/GO (B, D). XRD patterns (E) of PdCu/GO (a) and Co–PdCu/GO (b). (F) FTIR spectra of GO (a), PdCu/GO (b) and Co–PdCu/GO (c). | ||
Conclusions
To summarize, we have successfully created ordered nanostructures with interesting intermetallic phase tuning for boosting the electrochemical sensing of H2O2 released from living cells. The obtained PdCu and Co–PdCu nanoparticles with ordered morphologies were grown on the surface of GO based on a one-step hydrothermal method. Then ordered PdCu and Co–PdCu nanoparticles were used for electrochemical catalysis of H2O2 respectively. Compared with PdCu, Co–PdCu exhibits better charge transfer and catalytic properties which may be due to the interaction between Co and PdCu. Finally, ordered Co–PdCu nanomaterials were used for the fabrication of the H2O2 electrochemical sensor which exhibits an extra low LOD, broad linear range and good anti-interference ability, and can be used for sensing of H2O2 released from living cells successfully. This work provides a new design strategy for creating an ordered multi-metallic nanostructure with excellent electrochemical catalysis and sensing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support of this project by the National Science Fund of China (no. 21275116, 21575113), the Specialized Research Fund for the Doctoral Program of Higher Education (no. 20126101110013), the Natural Science Fund of Shaanxi Province (2018JQ2029) and the Scientific Research Foundation of Shaanxi Provincial Key Laboratory (13JS097, 13JS098, 14JS094, 15JS100). In addition, the cells which were used in experiments were provided by Xi'an Medical University (Xi'an, Shaanxi, PR China).Notes and references
- C. Amatore, S. Arbault, M. Guille and F. Lemaitre, Chem. Rev., 2008, 108, 2585 CrossRef CAS PubMed.
- D. Trachootham, J. Alexandre and P. Huang, Nat. Rev. Drug Discovery, 2009, 8, 579 CrossRef CAS PubMed.
- M. Hara-Chikuma, H. Satooka and S. Watanabe, Nat. Commun., 2015, 6, 1 Search PubMed.
- M. A. O'Connell, J. R. Lewis and A. J. Wain, Chem. Commun., 2015, 51, 10314 RSC.
- M. Baghayeri and H. Veisi, Biosens. Bioelectron., 2015, 74, 190 CrossRef CAS.
- D. A. Capdevila, W. A. Marmisollé and F. Tomasina, Chem. Sci., 2015, 6, 705 RSC.
- M. Y. Hua, H. C. Chen and C. K. Chuang, Biomaterials, 2011, 32, 4885 CrossRef CAS PubMed.
- J. J. Zhang, Y. G. Liu, L. P. Jiang and J. J. Zhu, Electrochem. Commun., 2008, 10, 355 CrossRef CAS.
- X. Y. Lang, H. Y. Fu and C. Hou, Nat. Commun., 2013, 4, 2169 CrossRef PubMed.
- A. Deep, S. K. Bhardwaj and A. K. Paul, Biosens. Bioelectron., 2015, 65, 226 CrossRef CAS PubMed.
- R. Tel-Vered, J. S. Kahn and I. Willner, Small, 2016, 12, 51 CrossRef CAS PubMed.
- C. M. Yu, L. Wang, W. B. Li, C. Zhu, N. Bao and H. Y. Gu, Sens. Actuators, B, 2015, 211, 17 CrossRef CAS.
- M. R. Zhang, X. Q. Chen and G. B. Pan, Sens. Actuators, B, 2017, 240, 142 CrossRef CAS.
- K. Wang, Q. Liu, X. Y. Wu, Q. M. Guan and H. N. Li, Talanta, 2010, 82, 372 CrossRef CAS PubMed.
- L. Li, Y. Zhang and L. Zhang, Anal. Chem., 2016, 88, 5369 CrossRef CAS PubMed.
- Y. Huang, Y. E. Miao and S. Ji, ACS Appl. Mater. Interfaces, 2014, 6, 12449 CrossRef CAS PubMed.
- Y. Xu and B. Zhang, Chem. Soc. Rev., 2014, 43, 2439 RSC.
- W. Chen, S. Cai and Q. Q. Ren, Analyst, 2012, 137, 49 RSC.
- J. Wang, K. Wang, F. B. Wang and X. H. Xia, Nat. Commun., 2014, 5, 5285 CrossRef CAS PubMed.
- J. Zhang, Z. Zhao, Z. Xia and L. Dai, Nat. Nanotechnol., 2015, 10, 444 CrossRef CAS PubMed.
- Z. Fan, X. Huang, C. Tan and H. Zhang, Chem. Sci., 2015, 6, 95 RSC.
- T. D. Thanh, J. Balamurugan and S. H. Lee, Biosens. Bioelectron., 2016, 85, 669 CrossRef CAS PubMed.
- Y. Lu, Y. Jiang and X. Gao, J. Am. Chem. Soc., 2014, 136, 11687 CrossRef CAS PubMed.
- M. J. Cheng and W. A. Goddard, J. Am. Chem. Soc., 2015, 137, 13224 CrossRef CAS PubMed.
- S. Ohkoshi, A. Namai and M. Yoshikiyo, Angew. Chem., Int. Ed., 2016, 55, 11403 CrossRef CAS PubMed.
- D. Wang, H. L. Xin, R. Hovden, H. Wang and Y. Yu, Nat. Mater., 2013, 12, 81 CrossRef CAS PubMed.
- K. A. Kuttiyiel, K. Sasaki and D. Su, Nat. Commun., 2014, 5, 5185 CrossRef CAS PubMed.
- Z. Q. Niu, Q. Peng, H. P. Rong and Y. D. Li, Angew. Chem., Int. Ed., 2011, 123, 6439 CrossRef.
- M. H. Naveen, N. G. Gurudatt and H. B. Noh, Adv. Funct. Mater., 2016, 26, 1590 CrossRef CAS.
- Y. Zhang, X. Bai and X. Wang, Anal. Chem., 2014, 86, 9459 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, SEM elemental mapping and EDS of PdCu/GO and Co-PdCu/GO, TEM of PdCu/GO obtained by comparison experiment, various CV results of modified electrodes and potential choice. See DOI: 10.1039/c8an01875h |
| This journal is © The Royal Society of Chemistry 2019 |