Open Access Article
Open Access ArticleFerri- and ferro-magnetism in CaMnMReO6 double double perovskites of late transition metals M = Co and Ni†‡
Elena
Solana-Madruga
 a,
Yu
Sun
a,
Ángel M.
Arévalo-López
a,
Yu
Sun
a,
Ángel M.
Arévalo-López
 b and
J. Paul
Attfield
b and
J. Paul
Attfield
 *a
*a
aCentre for Science at Extreme Conditions (CSEC) and School of Chemistry, The University of Edinburgh, EH9 3FD, UK. E-mail: j.p.attfield@ed.ac.uk
bUniv. Lille, CNRS, Centrale Lille, ENSCL, Univ. Artois, UMR 8181 – UCCS – Unité de Catalyse et Chimie du Solide, F-59000 Lille, France
First published on 6th February 2019
Abstract
Two new double double perovskites of ideal compositions CaMnMReO6 (M = Co, Ni) are reported. The M = Co material has refined composition CaMn0.7Co1.3ReO6 and orders ferrimagnetically below TC = 188 K with a relatively large saturated magnetisation of 4.5 μB. The M = Ni product, CaMn1.2Ni0.8ReO6, is a remarkable example of a ferromagnetic oxide with four distinct spin sublattices all collinearly ordered below TC = 152 K.
The versatility of the perovskite structure1–5 has led to the synthesis and study of many perovskite materials exhibiting a wide range of useful properties. 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ordering of cations at the A and B site cations in the basic ABO3 perovskite structure in layered, columnar or rock-salt arrangements6 leads to AA′B2O67,8 or A2BB′O69 double perovskites (DPv) but combinations of cation order in both sublattices are uncommon. These double double (or doubly ordered) perovskites usually combine layered and rock-salt motifs of A and B cations respectively.10–13 A new double double perovskite (DDPv) type was recently reported to combine a columnar arrangement of A-site cations with the rock-salt order of B and B′ cations for RMnMnSbO6 (R = rare earth).14,15 This structure crystallizes with the tetragonal P42/n space group and has five independent cation sites according to the composition AA′0.5A′′0.5BB′O6; A (Wyckoff position 4e), A′ (2a) and A′′ (2b) sites with ten-fold, tetrahedral and square planar coordinations, respectively, and octahedral sites for B (4c) and B′ (4d) cations. CaMnMReO6 double double perovskites with the same structure were subsequently reported for M = Mn and Fe. These are found to have different charge distributions and magnetic properties, as CaMnFe3+Re5+O6 is ferrimagnetic with Curie temperature TC = 500 K, while CaMnMn2+Re6+O6 has a mix of ferro- and antiferro-magnetic spin sublattices ordered below 120 K.16 To discover the cation and charge distributions for late first row transition metal ions, which have not previously been incorporated into double double perovskites, we report here the syntheses, structure and properties of CaMnMReO6 double double perovskites with M = Co and Ni, which are discovered to have ferromagnetic order over 3 or 4 spin sublattices.
1 ordering of cations at the A and B site cations in the basic ABO3 perovskite structure in layered, columnar or rock-salt arrangements6 leads to AA′B2O67,8 or A2BB′O69 double perovskites (DPv) but combinations of cation order in both sublattices are uncommon. These double double (or doubly ordered) perovskites usually combine layered and rock-salt motifs of A and B cations respectively.10–13 A new double double perovskite (DDPv) type was recently reported to combine a columnar arrangement of A-site cations with the rock-salt order of B and B′ cations for RMnMnSbO6 (R = rare earth).14,15 This structure crystallizes with the tetragonal P42/n space group and has five independent cation sites according to the composition AA′0.5A′′0.5BB′O6; A (Wyckoff position 4e), A′ (2a) and A′′ (2b) sites with ten-fold, tetrahedral and square planar coordinations, respectively, and octahedral sites for B (4c) and B′ (4d) cations. CaMnMReO6 double double perovskites with the same structure were subsequently reported for M = Mn and Fe. These are found to have different charge distributions and magnetic properties, as CaMnFe3+Re5+O6 is ferrimagnetic with Curie temperature TC = 500 K, while CaMnMn2+Re6+O6 has a mix of ferro- and antiferro-magnetic spin sublattices ordered below 120 K.16 To discover the cation and charge distributions for late first row transition metal ions, which have not previously been incorporated into double double perovskites, we report here the syntheses, structure and properties of CaMnMReO6 double double perovskites with M = Co and Ni, which are discovered to have ferromagnetic order over 3 or 4 spin sublattices.
Samples of ideal composition CaMnCoReO6 and CaMnNiReO6 have been treated under high pressure and high temperature conditions using a Walker-type multi anvil apparatus. A stoichiometric mixture of CaMnO3 (obtained by heating a pellet of CaCO3 and MnO in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at 1473 K for 24 h under an O2 flow with intermediate grinding and repelletising), ReO2 and MO (M = Co, Ni) oxides, were packed in a Pt capsule and treated at 10 GPa and 1373 K (for M = Co) or 1473 K (M = Ni) during 20 minutes. The samples were quenched and slowly depressurized to ambient conditions. These synthesis temperatures were found to be optimal for production of the DDPv type phases, as syntheses at 1423–1473 K for M = Co lead to secondary Re phases, while products made at 1373–1423 K for M = Ni contained unreacted ReO2 and NiO. However secondary phases were present for both materials and were not eliminated in repeated syntheses. Laboratory X-ray powder diffraction (XPD) patterns were collected using a D2 Bruker diffractometer. Magnetic susceptibilities (ZFC-FC under an applied magnetic field of 1000 Oe) and magnetization-field loops up to 7 T were measured using an MPMS SQUID magnetometer. Neutron powder diffraction (NPD) patterns collected at room temperature and 1.5 K on the WISH beamline at the ISIS facility were used to determine crystal and magnetic structures.
1 ratio at 1473 K for 24 h under an O2 flow with intermediate grinding and repelletising), ReO2 and MO (M = Co, Ni) oxides, were packed in a Pt capsule and treated at 10 GPa and 1373 K (for M = Co) or 1473 K (M = Ni) during 20 minutes. The samples were quenched and slowly depressurized to ambient conditions. These synthesis temperatures were found to be optimal for production of the DDPv type phases, as syntheses at 1423–1473 K for M = Co lead to secondary Re phases, while products made at 1373–1423 K for M = Ni contained unreacted ReO2 and NiO. However secondary phases were present for both materials and were not eliminated in repeated syntheses. Laboratory X-ray powder diffraction (XPD) patterns were collected using a D2 Bruker diffractometer. Magnetic susceptibilities (ZFC-FC under an applied magnetic field of 1000 Oe) and magnetization-field loops up to 7 T were measured using an MPMS SQUID magnetometer. Neutron powder diffraction (NPD) patterns collected at room temperature and 1.5 K on the WISH beamline at the ISIS facility were used to determine crystal and magnetic structures.
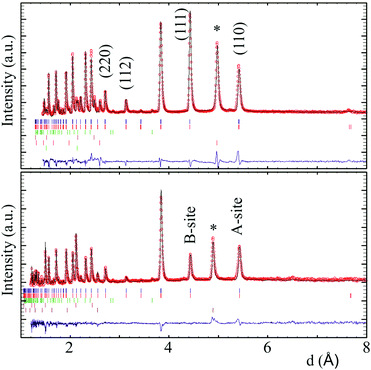
Fits to XPD (Fig. S1, ESI‡) and NPD data showed that both CaMnMReO6 (M = Co, Ni) samples adopt the P42/n DDPv structure. XPD patterns reveal traces of secondary ReO2 (1.7 and 3.0 weight% respectively for M = Co, Ni). The NPD patterns (Fig. 1 and ESI‡) show the presence of a rocksalt type monoxide impurity (8.0 and 17.1% respectively for M = Co, Ni). In both cases the cation distribution in the latter refined to composition M0.67Mn0.33O, suggesting that high pressure stabilises a rocksalt-related M2MnO3 phase, but possible long range cation ordering was not detected in this study. Table 1 summarizes the refined DDPv structures of the CaMnMReO6 (M = Co, Ni) compounds.
| Site | x | y | z | Mn/M occ | BVS |
|---|---|---|---|---|---|
| A′ (2a) | 0.75 | 0.75 | 0.75 | 0.692/0.308(1) | 1.9 |
| 0.892/0.108(1) | 1.9 | ||||
| A′′ (2b) | 0.25 | 0.25 | 0.75 | 0.612/0.388(1) | 1.7 |
| 0.788/0.212(1) | 1.6 | ||||
| Ca (4e) | 0.25 | 0.75 | 0.779(1) | 2.0 | |
| 0.783(1) | |||||
| B (4c) | 0 | 0.5 | 0.5 | 0.040/0.960(1) | 2.0 |
| 0.366/0.634(1) | 1.7 | ||||
| Re (4d) | 0 | 0 | 0.5 | 5.9 | |
| O1 (8g) | −0.055(2) | 0.556(2) | 0.239(1) | ||
| −0.049(1) | 0.559(2) | 0.24(1) | |||
| O2 (8g) | −0.237(2) | −0.049(1) | 0.571(1) | ||
| −0.240(3) | −0.049(1) | 0.566(1) | |||
| O3 (8g) | −0.263(1) | 0.061(1) | −0.0298(5) | ||
| −0.265(2) | 0.055(1) | −0.029(1) | |||
The varied neutron scattering lengths for the metals in these compounds (4.70, −3.73, 2.49, 10.3, 9.2 fm for Ca, Mn, Co, Ni and Re respectively)17 provide a high degree of contrast enabling cation mixing to be investigated across the five metal sites in the AA′0.5A′′0.5BB′O6 structure. The A = Ca (ten-fold) and B′ = Re (octahedral) sites were found to be fully occupied in both materials within experimental error in the NPD refinements. CaMnCoReO6 has 96% Co at the octahedral B site, but 30–40% of Co substitutes for Mn at the A′ (tetrahedral) and A′′ (square planar) sites. This leads to an overall Co-rich composition CaMn0.7Co1.3ReO6 relative to the ideal formula. CaMnNiReO6 has a mix of 37% Mn and 63% Ni at the B site but with 10–20% Ni substituting at the A′ and A′′ sites, leading to an overall Ni-poor composition CaMn1.2Ni0.8ReO6. This is consistent with the higher proportion of observed M2MnO3 secondary phase than in the M = Co sample. Apparent lack of Ca in the products may be because this has formed amorphous phases, or crystalline products below limits of XPD detection. XPD data were fitted using the NPD structural models, and with M/Re disorder at the B-sites refined. Small antisite occupancies of 3.4(1) and 2.5(1)% for M = Co and Ni respectively show that the high charge contrast between (Mn/Co/Ni)2+ and Re6+ leads to a high degree of B-site ordering.
The cation oxidation states at each atomic position have been estimated from BVS calculations,18 as shown in Table 1, confirming that the formal charge distributions are Ca2+A′2+0.5A′′2+0.5B2+Re6+O6 in both cases with A′, A′′, and B sites occupied by mixtures of Mn2+ and Co2+/Ni2+. This disorder reflects the similarity of ionic radii between the divalent transition metal cations. However, no substitutions at the Ca and Re sites were found in either material.
Magnetic susceptibility and field dependent magnetization measurements are depicted in Fig. 2. CaMnCoReO6 and CaMnNiReO6 show ferrimagnetic behaviour below TC = 188 and 152 K respectively. A fit of the Curie–Weiss law to the high temperature inverse susceptibilities (shown in ESI‡) results in Weiss constants θ = 188 and 151 K respectively, indicative of ferromagnetic exchange interactions for both compounds. The effective paramagnetic moments are 5.8 and 4.9 μB f.u.−1 for M = Co and Ni respectively. Predicted values are 6.9 and 7.2 μB f.u.−1 for the refined compositions CaMn0.7Co1.3ReO6 and CaMn1.2Ni0.8ReO6, assuming all cations are in high spin states. Both experimental values lie below the predicted values which most likely reflects the presence of impurity contributions to the susceptibilities. Low temperature M–H loops in Fig. 2 show that both materials have substantial saturated magnetic moments, of 4.5 and 1.8 μB f.u.−1 for M = Co and Ni respectively, indicating that they have ferri- or ferro-magnetic orders at low temperature. Their coercivities are small and the M = Co hysteresis loop shows a narrowing near H = 0 indicating that competing ferro- and antiferro-magnetic exchange interactions are present.
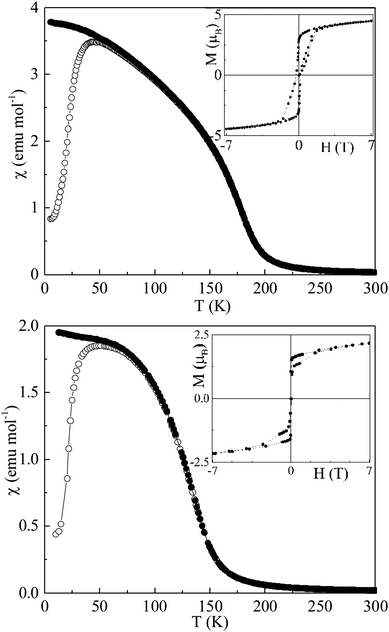 | ||
| Fig. 2 Magnetic susceptibilities of CaMnCoReO6 (top) and CaMnNiReO6 (bottom) with insets showing magnetization-field loops at 7 and 2 K respectively. | ||
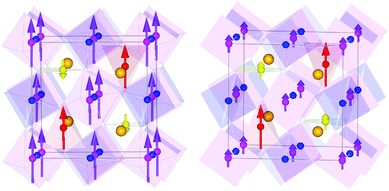
Magnetic diffraction peaks observed in the 1.5 K NPD patterns of CaMnCoReO6 and CaMnNiReO6 are indexed by propagation vector [0 0 0]. Rietveld fits (Rmag = 5.54% and 5.83% respectively) yield the magnetic structures shown in Fig. 3. Both CaMnCoReO6 and CaMnNiReO6 show a simultaneous order of A and B sublattices with spins along the z axis. All of the magnetic moments were refined independently, converging to a net ferrimagnetic arrangement of A and B sublattices for CaMnCoReO6 and their ferromagnetic alignment for CaMnNiReO6, as summarised in Table 2. Other magnetic modes were not consistent with the data. See ESI‡ for further details of the magnetic symmetry analysis. An additional magnetic diffraction peak was assigned and fitted as the (½½½) peak of the M0.67Mn0.33O impurity phases. This peak is also observed in the 300 K pattern of CaMnNiReO6. The high ordering temperature is consistent with the Néel temperatures of NiO (TN = 523 K) and MnO (TN = 122 K),19 so a transition near 390 K may be interpolated for Ni0.67Mn0.33O. An equivalent interpolation based on TN = 291 K for CoO predicts spin ordering around 235 K for Co0.67Mn0.33O, consistent with the observed appearance of magnetic neutron diffraction peaks between 300 and 1.5 K.
| M | M sat | μ(A′) | μ(A′′) | μ(B) | μ(B′) | μ |
|---|---|---|---|---|---|---|
| Co | 4.5 | 2.9(1) | −1.3(1) | 3.27(3) | 0.3(1) | 4.4 |
| Ni | 1.8 | 3.0(1) | 1.2(2) | 1.2(1) | 0.2(1) | 3.5 |
It is notable that all four magnetic sublattices in these two double double perovskites order ferromagnetically. The Re moments are very small, consistent with those in other Re6+ oxides,16 but refine to be parallel to the other B sublattice spins whereas those in CaMnFe3+Re5+O6 are antiparallel due to spin polarized conduction that leads to a high Curie temperature of 500 K. The TC's below 200 K and parallel alignment of B = Co, Ni and B’ = Re spins in the CaMnMReO6 (M = Co, Ni) materials indicate that they are insulating ferromagnets, although we have not yet obtained well-sintered ceramic pellets for direct conductivity measurements to confirm this. Ordered tetrahedral A′ site moments are larger than those at square planar A′′ sites in both materials, reflecting the greater degree of cation disorder at the latter sites. B site moments are relatively large (3.3 μB) for the M = Co material which has almost no cation disorder at this site, whereas substantial Mn/Ni mixing leads to a much smaller ordered moment of 1.2 μB for M = Ni. The reduction of the site moments compared to ideal 2S values reflects magnetic disorder resulting from a complex mix of antiferro- and ferro-magnetic exchange interactions between the different sites and cation dn configurations. This may be sensitive to the precise compositions of the studied samples, both of which are off-stoichiometric as noted above.
CaMnCoReO6 is a ferrimagnet overall as the ferromagnetic A′′ sublattice spins are antiparallel to those of the A′, B and B′ sublattices. The net moment of 4.4 μB for M = Co predicted from the NPD results is in good agreement with the observed net magnetization from the hysteresis loop (4.5 μB at 7 K). The refined spin structure of CaMnNiReO6 is remarkable as all four ferromagnetic sublattices are parallel so this material is a rare example of a ferromagnetic (and likely insulating) oxide. The observed magnetization is below the predicted value from the refined NPD moments, most likely reflecting the presence of the antiferromagnetic Ni0.67Mn0.33O impurity phase. Ferrimagnetism could also account for the smaller saturated moment, but attempts to refine such models against the neutron data always resulted in the ferromagnetic spin structure shown.
In conclusion, two new members of the double double perovskite family, of ideal compositions CaMnCoReO6 and CaMnNiReO6, have been synthesized under high pressure and high temperature conditions. They retain the cation ordering pattern of CaMnFeReO6, but with more substantial Mn/M cation mixing across three of the five available cation sites leading to non-stoichiometric CaMn0.7Co1.3ReO6 and CaMn1.2Ni0.8ReO6 compositions. Ferromagnetic ordering within all of their spin sublattices occurs at a single magnetic transition, unlike previous studied M = Mn and Fe analogues where two transitions were observed. CaMnCoReO6 orders ferrimagnetically below TC = 188 K with a relatively large saturated magnetization of 4.5 μB. CaMnNiReO6 is a remarkable example of a ferromagnetic oxide with four distinct spin sublattices all collinearly ordered below TC = 152 K.
We thank EPSRC for support, and STFC for provision of access to ISIS and Dr P. Manuel for assistance with data collection.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. D. Megaw, Proc. Phys. Soc., 1946, 58, 326 CrossRef.
- C. J. Howard and H. T. Stokes, Acta Crystallogr., Sect. B: Struct. Sci., 1998, 54, 782–789 CrossRef.
- C. J. Howard, B. J. Kennedy and P. M. Woodward, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 463–471 CrossRef.
- M. Anderson, K. Greenwood, G. Taylor and K. Poeppelmeier, Prog. Solid State Chem., 1993, 22, 197–233 CrossRef CAS.
- R. H. Mitchell, Perovskites: Modern and ancient, Almaz Press, Ontario, Canada, 2002 Search PubMed.
- G. King and P. M. Woodward, J. Mater. Chem., 2010, 20, 5785–5797 RSC.
- K. Lienenweber and J. Parise, J. Solid State Chem., 1995, 114, 277–281 CrossRef.
- A. Aimi, D. Mori, K. Hiraki, T. Takahashi, Y. J. Shan, Y. Shirako, J. Zhou and Y. Inaguma, Chem. Mater., 2014, 26, 2601–2608 CrossRef CAS.
- S. Vasala and M. Karppinen, Prog. Solid State Chem., 2015, 43, 1–36 CrossRef CAS.
- M. C. Knapp and P. M. Woodward, J. Solid State Chem., 2006, 179, 1076–1085 CrossRef CAS.
- G. King, S. Thimmaiah, A. Dwivedi and P. M. Woodward, Chem. Mater., 2007, 19, 6451–6458 CrossRef CAS.
- P. Zuo, H. Klein, C. Darie and C. V. Colin, J. Magn. Magn. Mater., 2018, 485, 48–51 CrossRef.
- C. De and A. Sundaresan, Phys. Rev. B, 2018, 97, 214418 CrossRef.
- E. Solana-Madruga, A. M. Arévalo-López, A. J. Dos santos-García, E. Urones-Garrote, D. Avila-Brande, R. Sáez-Puche and J. P. Attfield, Angew. Chem., Int. Ed., 2016, 55, 9340–9344 CrossRef CAS PubMed.
- E. Solana-Madruga, A. M. Arévalo-López, A. J. Dos santos-García, C. Ritter, C. Cascales, R. Sáez-Puche and J. P. Attfield, Phys. Rev. B, 2018, 97, 134408 CrossRef.
- G. M. McNally, A. M. Arévalo-López, P. Kearins, F. Orlandi, P. Manuel and J. P. Attfield, Chem. Mater., 2017, 29, 8870–8874 CrossRef CAS.
- V. F. Sears, Neutron News, 1992, 3, 26–37 CrossRef.
- J. P. Attfield, Solid State Sci., 2006, 8, 861–867 CrossRef CAS.
- W. L. Roth, Phys. Rev., 1958, 110, 1333–1341 CrossRef CAS.
Footnotes |
| † Data that support the findings of this study have been deposited at https://datashare.is.ed.ac.uk/handle/10283/838. |
| ‡ Electronic supplementary information (ESI) available: Supporting figures and tables. See DOI: 10.1039/c8cc09612k |
| This journal is © The Royal Society of Chemistry 2019 |