Bimodal detection of carbon dioxide using fluorescent molecular aggregates†
Rakesh K.
Mishra
 *ab,
Samiyappan
Vijayakumar
*ab,
Samiyappan
Vijayakumar
 a,
Arindam
Mal
a,
Arindam
Mal
 ac,
Varsha
Karunakaran
ac,
Varsha
Karunakaran
 cd,
Jith C.
Janardhanan
cd,
Jith C.
Janardhanan
 a,
Kaustabh Kumar
Maiti
a,
Kaustabh Kumar
Maiti
 cd,
Vakayil K.
Praveen
cd,
Vakayil K.
Praveen
 *ac and
Ayyappanpillai
Ajayaghosh
*ac and
Ayyappanpillai
Ajayaghosh
 *ac
*ac
aPhotosciences and Photonics Section, Chemical Sciences and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Thiruvananthapuram – 695019, India. E-mail: vkpraveen@niist.res.in; aajayaghosh@niist.res.in
bDepartment of Sciences and Humanities, National Institute of Technology, Uttarakhand (NITUK), Srinagar (Garhwal) – 246174, India. E-mail: rkmishra@nit.uk.ac.in
cAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad – 201002, India
dOrganic Chemistry Section, Chemical Sciences and Technology Division, CSIR-NIIST, Thiruvananthapuram – 695019, India
First published on 24th April 2019
Abstract
Cyano-substituted p-phenylenevinylene (R-1) aggregates exhibiting fluorescence and Raman spectroscopic responses towards CO2 are described. The aggregation-induced emission (AIE) as well as the aggregation-enhanced Raman scattering (AERS) of R-1 in aqueous conditions was reduced in the presence of a small amount of CO2, which enabled its easy and fast bimodal detection in different analytical samples.
The capture, storage, release and conversion of CO2 are issues of high priority research.1,2 In this context, the easy and fast detection of CO2 is an important objective. Hence, researchers are looking for simple and effective sensors/receptors for CO2.3–5 For this purpose, optical detection based on self-assembled fluorescent molecules is considered to be viable owing to its high sensitivity, fast response time, low cost, visual signal transduction, real-time in situ responses, and compatibility for in vivo analysis.3 A possible approach to realize such sensors depends on the analyte-triggered self-assembly or disassembly of a given molecular probe, producing a turn-on/off fluorescence response.6 Moreover, the combination of more than one detection modality is preferred in order to increase the selectivity and sensitivity of the probe.3,6 For instance, fluorescent probes that are Raman-active can be used as bimodal sensors.7 This is particularly relevant considering the fact that many of the reported CO2 sensors rely on single detection modality and often utilize more than one receptor components.5
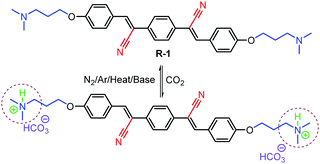
Herein, we report the fluorescence as well as Raman-assisted sensing of CO2 using a cyano-substituted8p-phenylenevinylene9 molecule bearing terminal tertiary amine moieties (Scheme 1). The receptor R-1 was synthesized as per Scheme S1 (ESI†). All intermediates and final compounds obtained were characterized using FT-IR, 1H, and 13C NMR techniques and mass spectrometry (Fig. S1–S8, ESI†).
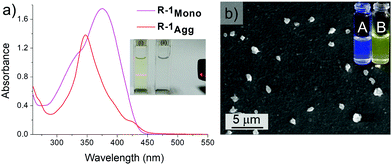
The receptor R-1 was highly soluble in THF and DMSO and formed aggregates (R-1Agg) in an aqueous medium, as indicated by the observed Tyndall effect (Fig. 1a, inset). The UV/Vis spectrum of R-1Agg (1 × 10−4 M) in the aqueous medium shows a narrow blue-shifted band (λmax = 348 nm) in comparison to the broad spectrum in DMSO (λmax = 375 nm), where it mainly exists as molecularly dissolved species (Fig. 1a). The aggregation of R-1 was also marked by the origin of an intense yellow emission (λem = 550 nm) owing to the AIE properties of the chromophoric unit (Fig. 1b, inset).10 On the contrary, the R-1 monomer in DMSO displayed a blue-shifted weak emission at 465 nm, τ = 5.84 ps (Fig. S9a and Table S1, ESI†). The red-shift and structureless features of emission in aqueous conditions indicated the formation of excimer-like species on aggregation, as evident from the longer average lifetime value (τ = 5.67 ns).11 Systematic aggregation studies were carried out in a DMSO-water mixture using UV/Vis and fluorescence spectroscopies, revealing that R-1 starts to aggregate in a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 water–DMSO mixture. The maximum fluorescence intensity was observed in a 99
30 water–DMSO mixture. The maximum fluorescence intensity was observed in a 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water–DMSO mixture (Fig. S9 (ESI†) and Fig. 1b, inset). The scanning electron microscopy (SEM) images of a drop cast sample of R-1Agg (1 × 10−4 M, water/DMSO, 99/1 v/v) over a silicon wafer show particle-like morphology (Fig. 1b).
1 water–DMSO mixture (Fig. S9 (ESI†) and Fig. 1b, inset). The scanning electron microscopy (SEM) images of a drop cast sample of R-1Agg (1 × 10−4 M, water/DMSO, 99/1 v/v) over a silicon wafer show particle-like morphology (Fig. 1b).
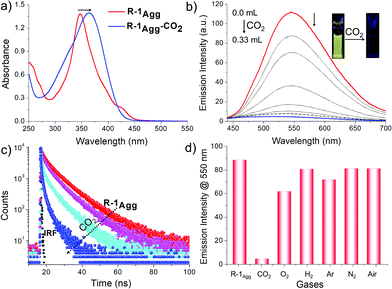
Having these results in hand, we tested the CO2 sensing ability of R-1Agg (1 × 10−4 M, water/DMSO, 99/1 v/v) on purging CO2 into R-1Agg while monitoring the corresponding changes in its absorption and emission properties. It was observed that in the presence of CO2, the sharp absorption band at 348 nm of R-1Agg broadened and red-shifted to 364 nm (Fig. 2a). Interestingly, gradual quenching in the emission of R-1Agg was observed on purging of CO2 (Fig. 2b and Fig. S10, ESI†). Fluorescence titration experiments revealed that the yellow emission of the aggregates reduced by 25-fold with 0.33 mL of CO2. Fig. 2c shows the fluorescence decay profiles of R-1Agg (probed at 550 nm) on purging with CO2. R-1Agg exhibited a triexponential decay with an average lifetime of 5.67 ns, whereas in the presence of CO2, a faster decay with an average lifetime of 0.33 ns was observed (Table S1, ESI†). The changes in absorption, steady state and time-resolved emission characteristics indicated the transformation of R-1Agg into a new-type of species having monomer-like absorption and emission features.
This observation was also supported by dynamic light scattering (DLS) studies. The average particle diameter of R-1Agg (1 × 10−4 M) as measured by DLS was found to be 156 ± 10 nm. On purging a small amount of CO2, the size of the aggregates gradually decreased; this was inferred from the reduction in the average particle diameter to 102 ± 10 nm, which was further reduced to 83 ± 5 nm with excess (0.33 mL) of CO2 (Fig. S11, ESI†). Furthermore, a reduction in the Tyndall effect of R-1Agg in the presence of CO2 (0.33 mL) was also noticed (Fig. S11c, ESI†). The results of the fluorescence titration studies, size distribution, Tyndall effect and CO2 chemistry with amine moieties in an aqueous medium suggested that R-1Agg was protonated in the presence of CO2 gas, leading to a change in the molecular character from hydrophobic to hydrophilic (Scheme 1). Thus, the large R-1Agg was converted into a mixture of monomers and smaller aggregates of the positively charged R-1.12 These processes were indicated by the simultaneous red shift of the absorption maximum and the reduction in the AIE property of the fluorophores; this was corroborated by the pH-dependent fluorescence titration studies (Table S2 and Fig. S12, ESI†), yielding a pKa value for R-1 as 6.98 (±0.007).
To further evaluate the selectivity of R-1Agg towards CO2, we carried out fluorescence titration experiments with different gases in excess amounts (3.0 mL) including N2, O2, Ar, H2, and air under identical conditions (Fig. 2d). It was clearly observed that CO2 produced the most significant effect on the fluorescence properties of R-1Agg. The experimental results also indicated that the detection of CO2 using R-1Agg experiences less interference from other common gases present in air. In addition, the binding of CO2 to R-1Agg was found to be reversible (Scheme 1). The less emissive R-1Agg–CO2 adduct was readily converted back into highly fluorescent R-1Agg by purging N2/Ar or by adding a small amount of K2CO3 at room temperature. As shown in Fig. S13 (ESI†), on repeated purging of CO2 and addition of 0.1 mM K2CO3 solution, the emission of R-1Agg exhibits multiple cycles of reversible on–off changes.
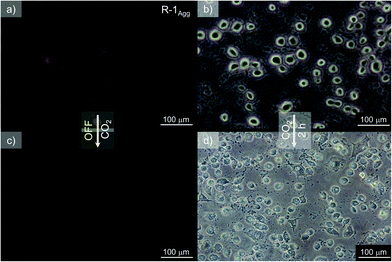
Fluorescence microscopy imaging was carried out to explore the capability of R-1Agg as a probe for monitoring the variation of external CO2 concentration inside living cells. For this purpose, lung adenocarcinoma cells A549 were incubated with 100 μM of R-1Agg for 1 h under normal atmospheric conditions (0.038% CO2) at 25 °C as we observed that the above-mentioned concentration gave optimum fluorescence intensity with minimal cytotoxicity around 3 h (Fig. S14, ESI†). Also, initial localization of R-1Agg was observed inside the nucleus in 1 h (Fig. S15, ESI†), but it eventually spread throughout the cells. As shown in Fig. 3, a yellow fluorescence signal can be observed, confirming the internalization of the aggregates inside the intercellular milieu. Thereafter, the cells were kept in an incubation chamber with 5% CO2 for 2 h, and the fluorescence was monitored against time. The emission intensity of the yellow fluorescence was reduced and almost no fluorescence was observed, while the sample cells kept under ambient conditions still showed the corresponding emission (Fig. S16, ESI†). These observations suggest that the increase in external CO2 concentration causes a reduction in the fluorescence emission intensity, thus revealing the capability of R-1Agg to detect CO2 variations in cellular conditions. Even though R-1 showed slight toxicity after a higher incubation time, the probe could efficiently sense CO2 inside the cellular environment.
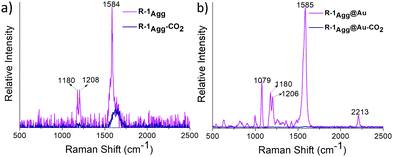
The aggregation-enhanced Raman scattering (AERS) of R-1Agg was exploited as an additional modality for chemosensing purposes.7,13 First, the Raman spectrum of R-1Agg (4 × 10−4 M, water/THF, 99/1 v/v) was recorded with a Raman microscope using 633 nm laser excitation.13 The spectra were obtained using a CCD detector with an integration time of 0.5 s and 10 accumulations. A tiny droplet of R-1Agg was placed on a glass slide to obtain the corresponding Raman fingerprint, which was observed with a high signal-to-noise ratio. The Raman spectrum of R-1Agg shows two peaks at 1584 cm−1 (strong, C–C aromatic ring chain vibrations) and 1180–1208 cm−1 (weak, C–O–C asymmetric vibrations). Furthermore, the droplets examined after purging the aggregate solution with CO2 showed a reduction in the peak intensity to almost baseline; thus, by monitoring the Raman-active peaks of R-1Agg, it was possible to detect CO2 gas (Fig. 4a).
Along with the inherent AERS phenomenon, we also looked for surface-enhanced Raman scattering (SERS) due to its enhanced sensitivity compared to normal Raman scattering. The Raman spectrum of R-1Agg was recorded using spherical gold nanoparticles (Au-NPs) as the SERS substrate. R-1Agg was mixed with as-prepared Au-NPs (size ca. 40 nm) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio (v/v), and the mixture was incubated for 10 min to obtain maximum adsorption. The SERS spectra were recorded as described above, and ca. 25-fold enhancement in the intensity was observed when compared with normal Raman intensity obtained for R-1Agg (Fig. 4). In comparison to the spectrum obtained from AERS, the SERS-assisted spectrum showed well-resolved peaks along with a unique signal at 2213 cm−1 resulting from the –CN group, which can be monitored easily for the CO2 sensing experiment.
9 ratio (v/v), and the mixture was incubated for 10 min to obtain maximum adsorption. The SERS spectra were recorded as described above, and ca. 25-fold enhancement in the intensity was observed when compared with normal Raman intensity obtained for R-1Agg (Fig. 4). In comparison to the spectrum obtained from AERS, the SERS-assisted spectrum showed well-resolved peaks along with a unique signal at 2213 cm−1 resulting from the –CN group, which can be monitored easily for the CO2 sensing experiment.
In conclusion, the cyano-substituted p-phenylenevinylene derivative R-1 reported in this study underwent aggregation in an aqueous medium, exhibiting AIE and AERS phenomena, which were used for the detection of CO2 gas among different specimens of neutral gases. The AIE-based CO2 assay described here is useful for detecting CO2 in biological specimens. The novelty of the present system is that a combination of fluorescence and AERS or SERS can be used for sensing CO2. However, for ratiometric sensing and in vivo applications, further improvement over the present design is required and such studies are in progress.
R. K. M. is thankful to DST, Govt. of India, for an INSPIRE Faculty Award (IFA-12 CH-50). V. K. P. and K. K. M. acknowledge CSIR mission mode project, nano-biosensor and microfluidics for healthcare (HCP-0012) and fast-track translation project, cellular sensor (MLP0027), Government of India for financial support. A. A. is grateful to the DST-SERB, Govt. of India, for a J. C. Bose National Fellowship (SB/S2/JCB-11/2014).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) F. A. Rahman, M. M. A. Aziz, R. Saidur, W. A. W. A. Bakar, M. R. Hainin, R. Putrajaya and N. A. Hassan, Renewable Sustainable Energy Rev., 2017, 71, 112–126 CrossRef; (b) T. R. Anderson, E. Hawkins and P. D. Jones, Endeavour, 2016, 40, 178–187 CrossRef PubMed; (c) D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- (a) S. Zeng, X. Zhang, L. Bai, X. Zhang, H. Wang, J. Wang, D. Bao, M. Li, X. Liu and S. Zhang, Chem. Rev., 2017, 117, 9625–9673 CrossRef CAS PubMed; (b) H. Liu, S. Lin, Y. Feng and P. Theato, Polym. Chem., 2017, 8, 12–23 RSC; (c) A. Darabi, P. G. Jessop and M. F. Cunningham, Chem. Soc. Rev., 2016, 45, 4391–4436 RSC; (d) P. G. Jessop, S. M. Mercera and D. J. Heldebrant, Energy Environ. Sci., 2012, 5, 7240–7253 RSC; (e) T. Yu, R. Cristiano and R. G. Weiss, Chem. Soc. Rev., 2010, 39, 1435–1447 RSC; (f) D. M. Rudkevich and H. Xu, Chem. Commun., 2005, 2651–2659 RSC.
- X. Zhou, S. Lee, Z. Xu and J. Yoon, Chem. Rev., 2015, 115, 7944–8000 CrossRef CAS PubMed.
- (a) Y. Ma, M. Cametti, Z. Džolić and S. Jiang, J. Mater. Chem. C, 2018, 6, 9232–9237 RSC; (b) D. S. Philips, S. Ghosh, K. V. Sudheesh, C. H. Suresh and A. Ajayaghosh, Chem. − Eur. J., 2017, 23, 17973–17980 CrossRef CAS; (c) X. Zhang, S. Lee, Y. Liu, M. Lee, J. Yin, J. L. Sessler and J. Yoon, Sci. Rep., 2014, 4, 4593 CrossRef PubMed; (d) W. Hong, Y. Chen, X. Feng, Y. Yan, X. Hu, B. Zhao, F. Zhang, D. Zhang, Z. Xu and Y. Lai, Chem. Commun., 2013, 49, 8229–8231 RSC; (e) T. Tian, X. Chen, H. Li, Y. Wang, L. Guo and L. Jiang, Analyst, 2013, 138, 991–994 RSC; (f) Q. Xu, S. Lee, Y. Cho, M. H. Kim, J. Bouffard and J. Yoon, J. Am. Chem. Soc., 2013, 135, 17751–17754 CrossRef CAS PubMed; (g) M. Ishida, P. Kim, J. Choi, J. Yoon, D. Kim and J. L. Sessler, Chem. Commun., 2013, 49, 6950–6952 RSC; (h) L. Q. Xu, B. Zhang, M. Sun, L. Hong, K.-G. Neoh, E.-T. Kang and G. D. Fu, J. Mater. Chem. A, 2013, 1, 1207–1212 RSC.
- (a) Z. Guo, N. R. Song, J. H. Moon, M. Kim, E. J. Jun, J. Choi, J. Y. Lee, C. W. Bielawski, J. L. Sessler and J. Yoon, J. Am. Chem. Soc., 2012, 134, 17846–17849 CrossRef CAS PubMed; (b) R. Ali, T. Lang, S. M. Saleh, R. J. Meier and O. S. Wolfbeis, Anal. Chem., 2011, 83, 2846–2851 CrossRef CAS PubMed; (c) Y. Liu, Y. Tang, N. N. Barashkov, I. S. Irgibaeva, J. W. Y. Lam, R. Hu, D. Birimzhanova, Y. Yu and B. Z. Tang, J. Am. Chem. Soc., 2010, 132, 13951–13953 CrossRef CAS PubMed; (d) T. Gunnlaugsson, P. E. Kruger, P. Jensen, F. M. Pfeffer and G. M. Hussey, Tetrahedron Lett., 2003, 44, 8909–8913 CrossRef CAS; (e) E. M. Hampe and D. M. Rudkevich, Chem. Commun., 2002, 1450–1451 RSC.
- (a) P. Anees, V. K. Praveen, K. K. Kartha and A. Ajayaghosh, Comprehensive Supramolecular Chemistry II, Elsevier, 2017, pp. 297–320 Search PubMed; (b) D. Zhai, W. Xu, L. Zhang and Y.-T. Chang, Chem. Soc. Rev., 2014, 43, 2402–2411 RSC.
- (a) M. M. Joseph, N. Narayanan, J. B. Nair, V. Karunakaran, A. N. Ramya, P. T. Sujai, G. Saranya, J. S. Arya, V. M. Vijayan and K. K. Maiti, Biomaterials, 2018, 181, 140–181 CrossRef CAS PubMed; (b) D. L. Akins, Nanomater. Nanotechnol., 2014, 4 DOI:10.5772/58403.
- (a) M. Martínez-Abadía, R. Giménez and M. B. Ros, Adv. Mater., 2018, 30, 1704161 CrossRef PubMed; (b) T. Noguchi, B. Roy, D. Yoshihara, J. Sakamoto, T. Yamamoto and S. Shinkai, Angew. Chem., Int. Ed., 2016, 128, 5802–5806 CrossRef; (c) F. Aparicio, S. Cherumukkil, A. Ajayaghosh and L. Sánchez, Langmuir, 2016, 32, 284–289 CrossRef CAS PubMed; (d) L. Zhu and Y. Zhao, J. Mater. Chem. C, 2013, 1, 1059–1065 RSC; (e) B.-k. An, J. Gierschner and S. Y. Park, Acc. Chem. Res., 2012, 45, 544–554 CrossRef CAS PubMed.
- (a) S. Yagai, S. Okamura, Y. Nakano, M. Yamauchi, K. Kishikawa, T. Karatsu, A. Kitamura, A. Ueno, D. Kuzuhara, H. Yamada, T. Seki and H. Ito, Nat. Commun., 2014, 5, 4013 CrossRef CAS PubMed; (b) V. K. Praveen, C. Ranjith, E. Bandini, A. Ajayaghosh and N. Armaroli, Chem. Soc. Rev., 2014, 43, 4222–4242 RSC; (c) L. Maggini and D. Bonifazi, Chem. Soc. Rev., 2012, 41, 211–241 RSC; (d) A. Ajayaghosh and V. K. Praveen, Acc. Chem. Res., 2007, 40, 644–656 CrossRef CAS PubMed.
- (a) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed; (b) X. Ma, R. Sun, J. Cheng, J. Liu, F. Gou, H. Xiang and X. Zhou, J. Chem. Educ., 2016, 93, 345–350 CrossRef CAS.
- (a) S.-J. Yoon, J. W. Chung, J. Gierschner, K. S. Kim, M.-G. Choi, D. Kim and S. Y. Park, J. Am. Chem. Soc., 2010, 132, 13675–13683 CrossRef CAS PubMed; (b) S. S. Babu, V. K. Praveen, S. Prasanthkumar and A. Ajayaghosh, Chem. – Eur. J., 2008, 14, 9577–9584 CrossRef CAS PubMed; (c) J. Gierschner, M. Ehni, H.-J. Egelhaaf, B. Milián-Medina, D. Beljonne, H. Benmansour and G. C. Bazan, J. Chem. Phys., 2005, 123, 144914 CrossRef PubMed.
- (a) P. D. Vaidya and E. Y. Kenig, Chem. Eng. Technol., 2007, 30, 1467–1474 CrossRef CAS; (b) M. Caplow, J. Am. Chem. Soc., 1968, 90, 6795–6803 CrossRef CAS.
- (a) N. Narayanan, V. Karunakaran, W. Paul, K. Venugopal, K. Sujathan and K. K. Maiti, Biosens. Bioelectron., 2015, 70, 145–152 CrossRef CAS PubMed; (b) A. N. Ramya, A. Samanta, N. Nisha, Y. T. Chang and K. K. Maiti, Nanomedicine, 2015, 10, 561–571 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis procedures and characterization data of the compounds, and additional figures. See DOI: 10.1039/c9cc01564g |
| This journal is © The Royal Society of Chemistry 2019 |