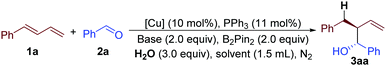Cu-Catalyzed highly selective reductive functionalization of 1,3-diene using H2O as a stoichiometric hydrogen atom donor†
Qifan
Li
a,
Xiaoyang
Jiao
a,
Mimi
Xing
a,
Penglin
Zhang
a,
Qian
Zhao
a and
Chun
Zhang
 *ab
*ab
aDepartment of Chemistry, Tianjin Key Laboratory of Molecular Optoelectronic Science, School of Sciences, Institute of Molecular Plus, Tianjin University, Weijin Rd. 92, Tianjin 300072, China. E-mail: chunzhang@tju.edu.cn
bState Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China
First published on 26th June 2019
Abstract
A copper-catalyzed highly regio- and diastereo-selective reductive reaction of terminal 1,3-diene with water and aldehyde has been developed. This chemistry afforded a product containing a terminal alkenyl group, which is a versatile kind of precursor for organic synthesis, with the scope for various substrates. The present reaction system could realize the catalytic transfer of hydrogen to diene using water as a stoichiometric H atom donor. In this transformation, B2Pin2, a mild and practical kind of reductant was used as the mediator. The reaction pathway of this practical strategy was illustrated by a control experiment.
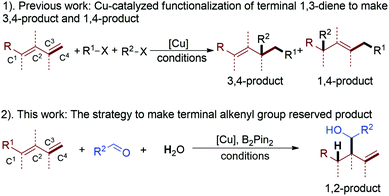
Developing new organic transformation strategies to preserve an active functional group is important for the synthesis of drugs, natural products and functional material molecules.1 Among a lot of versatile starting compounds, 1,3-diene has caught more and more attention, because the difunctionalization of a conjugated diene with high regio- and diastereo-selectivity is an important type of transformation, which allows the controlled formation of multiple complex isomeric products from a simple precursor.2,3 In recent years, copper-catalyzed reaction systems have been extensively applied in such reactions, owing to the low price of copper-catalysts, ambient reaction conditions or practical reaction protocols.4 With such a powerful strategy, the terminal diene can be converted into a 1,4- or 3,4-functionalized product with good selectivity (1, Scheme 1). Compared with the above synthetic strategy, we have developed a novel copper-catalyzed B2Pin2 mediated highly regio- and diastereo-selective functionalization of terminal 1,3-diene to afford a 1,2-functionalized product (2, Scheme 1).
In contrast to other metal reagents, the organic boron-reagent is much more stable, and practical as it reacts under ambient conditions, so the preparation of this kind of compound has been studied intensely.5 Among these methods, the hydroboration of dienes affords important boron reagents, which are powerful intermediates in many transformations.6 However, some air or moisture sensitive reagents and catalysts, such as Ni(cod)2 or HBPin, are usually used for these reactions. The present transformation results in the formation of an allyl boron intermediate with good regioselectivity using a copper-salt/H2O/B2Pin2 system, which makes this method more practical. Importantly, examples of the catalytic transfer hydrogenation of simple alkenes using H2O as the stoichiometric H atom donor show that it is a sustainable strategy for organic synthesis.7 Our work exploits a new way of using H2O as a safe and cost-efficient hydrogen atom source for a highly selective catalytic reductive transformation process.
Our study commenced with the copper-catalyzed reaction of (E)-buta-1,3-dien-1-ylbenzene (1a), benzaldehyde (2a), B2Pin2 and H2O. Interestingly, this practical one-pot reaction afforded 2-benzyl-1-phenylbut-3-en-1-ol (3aa) with 76% yield and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity (entry 1, Table 1 and ESI†). The efficiency of this reaction decreased when other kinds of bases were used, such as NaOt-Bu, KOt-Bu, or LiOMe (entries 1–4, Table 1 and ESI†). We then surveyed the effect of different solvents. Compared with THF, 1,4-dioxane induced low yield and selectivity, but toluene and DCE afforded much better results (entries 5 to 7, Table 1). In particular, when DCE was chosen as a solvent, the product could be obtained with 93% yield and better than 20
1 diastereoselectivity (entry 1, Table 1 and ESI†). The efficiency of this reaction decreased when other kinds of bases were used, such as NaOt-Bu, KOt-Bu, or LiOMe (entries 1–4, Table 1 and ESI†). We then surveyed the effect of different solvents. Compared with THF, 1,4-dioxane induced low yield and selectivity, but toluene and DCE afforded much better results (entries 5 to 7, Table 1). In particular, when DCE was chosen as a solvent, the product could be obtained with 93% yield and better than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity (entry 7, Table 1). Further studies indicated that different kinds of copper-salts affected the result of this reaction (entries 8 to 10, Table 1). Among them, Cu(OTf)2 was the best choice. The data for entries 11 and 12 proved that this reaction did not proceed without a copper catalyst or B2Pin2 (Table 1).
1 diastereoselectivity (entry 7, Table 1). Further studies indicated that different kinds of copper-salts affected the result of this reaction (entries 8 to 10, Table 1). Among them, Cu(OTf)2 was the best choice. The data for entries 11 and 12 proved that this reaction did not proceed without a copper catalyst or B2Pin2 (Table 1).
| Entry | Solvent | Base | [Cu] | Yieldb (%) | dr |
|---|---|---|---|---|---|
| a 1a (0.25 mmol), 2a (0.5 mmol), B2Pin2 (0.5 mmol), base (0.5 mmol), cat (10 mol%), PPh3 (11 mol%), H2O (3.0 equiv.), solvent (1.5 ml). b Isolated yield, diastereomers are inseparable, dr was determined by 1H-NMR. c Without B2Pin2. | |||||
| 1 | THF | LiOt-Bu | Cu(OTf)2 | 76 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | THF | NaOt-Bu | Cu(OTf)2 | 52 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | THF | KOt-Bu | Cu(OTf)2 | 69 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | THF | LiOMe | Cu(OTf)2 | 66 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 1,4-Dioxane | LiOt-Bu | Cu(OTf)2 | 64 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Toluene | LiOt-Bu | Cu(OTf)2 | 80 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | DCE | LiOt-Bu | Cu(OTf)2 | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | DCE | LiOt-Bu | Cu(acac)2 | 76 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | DCE | LiOt-Bu | CuCI | 78 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | DCE | LiOt-Bu | CuOAc | 23 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11c | DCE | LiOt-Bu | Cu(OTf)2 | 0 | — |
| 12 | DCE | LiOt-Bu | None | 0 | — |
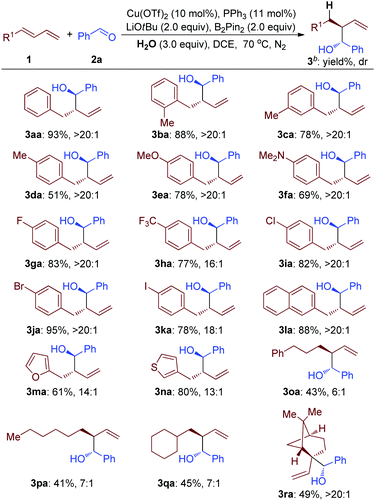
Using H2O as a hydrogen atom donor and B2Pin2 as a mediator for this reductive transformation, the substrate scope of terminal 1,3-diene was investigated (Table 2). In general, modest to good yields and diastereoselectivity using either electron-rich (MeO– and Me2N–), or electron-deficient (F–, CF3– and Cl–) aryl substituted 1,3-diene were observed (3aa to 3ka, Table 2). And, substituents at the ortho-position of the arene group did not affect the efficiency (3ba, Table 2). Notably, when bromo-substituted aromatic 1,3-diene was employed as a substrate, the corresponding product was afforded in 95% yield with great selectivity, which could be used for further transformation (3ja, Table 2). The iodo-substituted aromatic 1,3-diene still worked, but with lower efficiency (3ka, Table 2). To our delight, the naphthyl, furyl and thienyl groups survived under these reductive reaction conditions (3la to 3na, Table 2). Importantly, alkyl 1,3-diene was smoothly transformed into the corresponding products (3oa to 3ra, Table 2). The 1,2-disubstituted 1,3-diene still worked well, giving a product containing a quaternary carbon atom (3ra, Table 2).
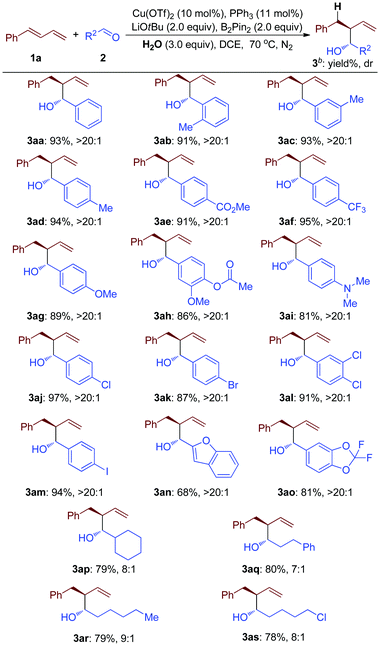
The substrate scope of the Cu-catalyzed B2Pin2 mediated highly selective reductive transformation was further expanded to a variety of substituted aldehydes (2) (Table 3). These results indicated that aryl aldehyde with both electron-donating and electron-withdrawing groups proceeded well with good yields and great diastereoselectivity (3aa to 3ap, Table 3). Substrate groups at the para-, meta-, and ortho-positions of the arene ring did not affect the efficiency (3ab to 3ad, Table 3). Notably, even when bromo-substituted or iodo-substituted arylaldehyde was employed as a starting material, good results were afforded (3ak and 3am, Table 3). These products could be used for a further coupling reaction to make complicated molecules. Moreover, under these ambient reaction conditions, the substrates with hetero-cycle groups could be converted into the desired products with good efficiency, which demonstrated the good substrate tolerance of this method (3an and 3ao, Table 3). Furthermore, the substrate scope was expanded to alkyl aldehydes, affording good yields and moderate diastereoselectivity (3ap to 3as, Table 3).
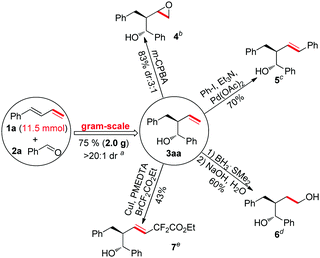
To test the feasibility of a large-scale reaction, the reaction of (E)-buta-1,3-dien-1-ylbenzene (1a) (11.5 mmol) and benzaldehyde (2a) (23 mmol) was investigated. The reaction afforded 2.0 g of 3aa (75% yield) with great diastereoselectivity (a, Scheme 2). The product of this chemistry, which installed a terminal alkenyl group, is a kind of versatile intermediate for chemical synthesis. As shown in Scheme 2, the product 3aa went through an epoxidation reaction,8 a Heck reaction,9 a hydroboration–oxidation reaction10 and a radical coupling reaction11 to afford the desired complex products smoothly (b, c, d and e, Scheme 2).
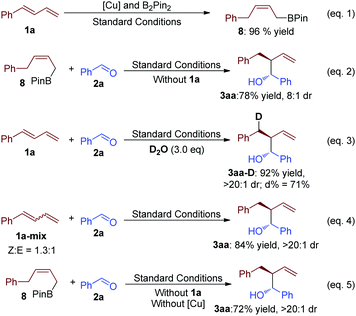
To investigate the mechanism of this transformation, control experiments were designed. Firstly, without benzaldehyde (2a), compound 8 was separated with 96% yield (eqn (1), Scheme 3). This result suggested that 8 should be the key intermediate of the present transformation. Furthermore, when it was put under similar standard conditions, the desired product 3aa was afforded with good yield (eqn (2), Scheme 3). A slight difference in the reaction system might affect the diastereoselectivity and yield, which exhibited the advantage of this one pot-reaction strategy. The reaction of 1a and 2a was tested in the presence of D2O, and the D-labeled product could be detected (eqn (3), Scheme 3). This result proved that H2O is the stoichiometric H atom donor of the Cu-catalyzed B2Pin2 mediated highly selective reductive transformation. Importantly, when a mixed Z and E version of buta-1,3-dien-1-ylbenzene was selected as the starting material, this chemistry afforded a similar result to the example of 3aa from Table 1 (eqn (4), Scheme 3). This data suggests that the isomerization process of the allyl copper intermediate might be involved in this transformation. Furthermore, without a copper-catalyst, 8 reacted with 2a afforded 3aa with good yield and diastereoselectivity (eqn (5), Scheme 3). This result suggests that copper did not participate in this step of the reaction.
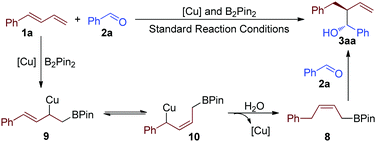
On the basis of the above control experiments, a proposed mechanism for the highly selective reductive reaction is illustrated in Scheme 4. The first step of this reaction is the formation of the tautomeric copper complexes 9 and 10.4c,f,12 The intermediate 10 reacted with water to give intermediate 8, which was proved by the control experiment.4f Further reaction of 8 with benzaldehyde (2a) afforded product 3aa.13
In summary, we have demonstrated a novel Cu-catalyzed B2Pin2 mediated highly selective reductive functionalization of 1,3-diene using H2O as the hydrogen donor. This practical chemistry afforded the terminal alkenyl group containing product with various substrate scopes, a useful building block for use in organic synthesis. Furthermore, this method supported gram-scale preparation without diminished diastereoselectivity. Further studies into synthetic applications are ongoing in our laboratory.
We acknowledge financial support from Tianjin University, the National Science Foundation of China (No. 21801181), the “1000-Youth Talents Plan” and the State Key Laboratory of Elemento-Organic Chemistry (Nankai University). We acknowledge Prof. Ning Jiao (Peking University), Prof. Zhuangzhi Shi (Nanjing University) and Prof. Jun-An Ma (Tianjin University) for helpful discussions.
Conflicts of interest
The authors declare no competing financial interest.Notes and references
- (a) Transition Metals for Organic Synthesis, Building Block and Fine Chemicals, ed. M. Beller and C. Bolm, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed; (b) Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and A. de Meijere, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed.
- For selected reviews on 1,3-dienes as building blocks in recent 10 years, see: (a) M. Büschleb, S. Dorich, S. Hanessian, D. Tao, K. B. Schenthal and L. E. Overman, Angew. Chem., Int. Ed., 2016, 55, 4156 CrossRef PubMed; (b) M. M. Heravi, T. Ahmadi, M. Ghavidel, B. Heidari and H. Hamidi, RSC Adv., 2015, 5, 101999 RSC; (c) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Rev., 2015, 115, 5301 CrossRef CAS PubMed; (d) E. McNeill and T. Ritter, Acc. Chem. Res., 2015, 48, 2330 CrossRef CAS PubMed; (e) Y. Zhu, R. G. Cornwall, H. Du, B. Zhao and Y. Shi, Acc. Chem. Res., 2014, 47, 3665 CrossRef CAS PubMed.
- For some selected examples on 1,3-dienes as building blocks in recent 10 years, see: (a) X.-H. Yang, R. T. Davison, S.-Z. Nie, F. A. Cruz, T. M. McGinnis and V. M. Dong, J. Am. Chem. Soc., 2019, 141, 3006 CrossRef CAS PubMed; (b) X.-H. Yang, R. T. Davison and V. M. Dong, J. Am. Chem. Soc., 2018, 140, 10443 CrossRef CAS PubMed; (c) L. Cheng, M.-M. Li, L.-J. Xiao, J.-H. Xie and Q.-L. Zhou, J. Am. Chem. Soc., 2018, 140, 11627 CrossRef CAS PubMed; (d) M.-S. Wu, T. Fan, S.-S. Chen, Z.-Y. Han and L.-Z. Gong, Org. Lett., 2018, 20, 2485 CrossRef CAS PubMed; (e) X.-H. Yang and V. M. Dong, J. Am. Chem. Soc., 2017, 139, 1774 CrossRef CAS PubMed; (f) J. S. Marcum, C. C. Roberts, R. S. Manan, T. N. Cervarich and S. J. Meek, J. Am. Chem. Soc., 2017, 139, 15580 CrossRef CAS PubMed; (g) S.-S. Chen, M.-S. Wu and Z.-Y. Han, Angew. Chem., Int. Ed., 2017, 56, 6641 CrossRef CAS PubMed; (h) Z.-L. Tao, A. Adili, H.-C. Shen, Z.-Y. Han and L.-Z. Gong, Angew. Chem., Int. Ed., 2016, 55, 4322 CrossRef CAS PubMed; (i) Y. Liu, Y. Xie, H. Wang and H. Huang, J. Am. Chem. Soc., 2016, 138, 4314 CrossRef CAS PubMed; (j) K. D. Nguyen, D. Herkommer and M. J. Krische, J. Am. Chem. Soc., 2016, 138, 14210 CrossRef CAS PubMed; (k) C. C. Roberts, D. M. Matías, M. J. Goldfogel and S. J. Meek, J. Am. Chem. Soc., 2015, 137, 6488 CrossRef CAS PubMed; (l) V. Saini, M. O’Dair and M. S. Sigman, J. Am. Chem. Soc., 2015, 137, 608 CrossRef CAS PubMed; (m) X. Wu, H.-C. Lin, M.-L. Li, L.-L. Li, Z.-Y. Han and L.-Z. Gong, J. Am. Chem. Soc., 2015, 137, 13476 CrossRef CAS PubMed; (n) M. J. Goldfogel, C. C. Roberts and S. J. Meek, J. Am. Chem. Soc., 2014, 136, 6227 CrossRef CAS PubMed; (o) B. J. Stokes, L. Liao, A. Me. de Andrade, Q. Wang and M. S. Sigman, Org. Lett., 2014, 16, 4666 CrossRef CAS PubMed; (p) B. Y. Park, T. P. Montgomery, V. J. Garza and M. J. Krische, J. Am. Chem. Soc., 2013, 135, 16320 CrossRef CAS PubMed; (q) L. Liao, R. Jana, K. B. Urkalan and M. S. Sigman, J. Am. Chem. Soc., 2011, 133, 5784 CrossRef CAS PubMed; (r) C. H. Schuster, B. Li and J. P. Morken, Angew. Chem., Int. Ed., 2011, 50, 7906 CrossRef CAS PubMed; (s) L. Liao and M. S. Sigman, J. Am. Chem. Soc., 2010, 132, 10209 CrossRef CAS PubMed.
- (a) C. Li, R. Y. Liu, L. T. Jesikiewicz, Y. Yang, P. Liu and S. L. Buchwald, J. Am. Chem. Soc., 2019, 141, 5062 CrossRef CAS PubMed; (b) Y. Huang, S. Torker, X. Li, J. D. Pozo and A. H. Hoveyda, Angew. Chem., Int. Ed., 2019, 58, 2685 CrossRef CAS PubMed; (c) K. B. Smith, Y. Huang and M. K. Brown, Angew. Chem., Int. Ed., 2018, 57, 6146 CrossRef CAS PubMed; (d) Y. Liu, D. Fiorito and C. Mazet, Chem. Sci., 2018, 9, 5284 RSC; (e) Y.-Y. Gui, N. Hu, X.-W. Chen, L.-L. Liao, T. Ju, J.-H. Ye, Z. Zhang, J. Li and D.-G. Yu, J. Am. Chem. Soc., 2017, 139, 17011 CrossRef CAS PubMed; (f) S. R. Sardini and M. K. Brown, J. Am. Chem. Soc., 2017, 139, 9823 CrossRef CAS PubMed; (g) T. Iwasaki, R. Shimizu, R. Imanishi, H. Kuniyasu and N. Kambe, Angew. Chem., Int. Ed., 2015, 54, 9347 CrossRef CAS PubMed.
- D. G. Hall, Boronic Acids, Wiley-VCH, Weinheim, 2nd edn, 2011 Search PubMed.
- (a) K. Duvvuri, K. R. Dewese, M. M. Parsutkar, S. M. Jing, M. M. Mehta, J. C. Gallucci and T. V. RajanBabu, J. Am. Chem. Soc., 2019, 141, 7365 CrossRef CAS PubMed; (b) M. E.-Viguri, S. E. Neale, N. T. Coles, S. A. Macgregor and R. L. Webster, J. Am. Chem. Soc., 2019, 141, 572 CrossRef PubMed; (c) D. Fiorito and C. Mazet, ACS Catal., 2018, 8, 9382 CrossRef CAS; (d) S. Gao, M. Wang and M. Chen, Org. Lett., 2018, 20, 7921 CrossRef CAS PubMed; (e) M. Morimoto, T. Miura and M. Murakami, Angew. Chem., Int. Ed., 2015, 54, 12659 CrossRef CAS PubMed; (f) R. J. Ely and J. P. Morken, Org. Synth., 2011, 88, 342 CrossRef CAS; (g) R. E. Kyne, M. C. Ryan, L. T. Kliman and J. P. Morken, Org. Lett., 2010, 12, 3796 CrossRef CAS PubMed; (h) R. J. Ely and J. P. Morken, J. Am. Chem. Soc., 2010, 132, 2534 CrossRef CAS PubMed; (i) J. Y. Wu, B. Moreau and T. Ritter, J. Am. Chem. Soc., 2009, 131, 12915 CrossRef CAS PubMed; (j) Y. Matsumoto and T. Hayashi, Tetrahedron Lett., 1991, 32, 3387 CrossRef CAS.
- (a) S. P. Cummings, T.-N. Le, G. E. Fernandez, L. G. Quiambao and B. J. Stokes, J. Am. Chem. Soc., 2016, 138, 6107 CrossRef CAS PubMed; (b) Y.-T. Xia, X.-T. Sun, L. Zhang, K. Luo and L. Wu, Chem. – Eur. J., 2016, 22, 17151 CrossRef CAS PubMed; (c) A. G. Campaña, R. E. Estévez, N. Fuentes, R. Robles, J. M. Cuerva, E. Buñuel, D. Cárdenas and J. E. Oltra, Org. Lett., 2007, 9, 2195 CrossRef PubMed; (d) J. M. Cuerva, A. G. Campana, J. Justicia, A. Rosales, J. L. Oller-Lopez, R. Robles, D. J. Cardenas, E. Bunuel and J. E. Oltra, Angew. Chem., Int. Ed., 2006, 45, 5522 CrossRef CAS PubMed; (e) D. A. Spiegel, K. B. Wiberg, L. N. Schacherer, M. R. Medeiros and J. L. Wood, J. Am. Chem. Soc., 2005, 127, 12513 CrossRef CAS PubMed.
- F. Benfatti, G. Cardillo, L. Gentilucci and A. Tolomelli, Eur. J. Org. Chem., 2007, 3199 CrossRef CAS.
- K. L. Bolduc, S. D. Larsen and D. H. Sherman, Chem. Commun., 2012, 48, 6414 RSC.
- L. Huang, Y. Cai, C. Zheng, L.-X. Dai and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 10545 CrossRef CAS PubMed.
- X. Wang, S. Zhao, J. Liu, D. Zhu, M. Guo, X. Tang and G. Wang, Org. Lett., 2017, 19, 4187 CrossRef CAS PubMed.
- (a) J. Hu, Y. Zhao and Z. Shi, Nat. Catal., 2018, 1, 860 CrossRef CAS; (b) P. Gao, C. Yuan, Y. Zhao and Z. Shi, Chem, 2018, 4, 2201 CrossRef CAS; (c) D. Li, Y. Park and J. Yun, Org. Lett., 2018, 20, 7526 CrossRef CAS PubMed; (d) K. B. Smith and M. K. Brown, J. Am. Chem. Soc., 2017, 139(23), 7721 CrossRef CAS PubMed; (e) L. Jiang, P. Cao, M. Wang, B. Chen, B. Wang and J. Liao, Angew. Chem., Int. Ed., 2016, 55, 13854 CrossRef CAS PubMed; (f) K. Semba, M. Shinomiya, T. Fujihara, J. Terao and Y. Tsuji, Chem. – Eur. J., 2013, 19, 7125 CrossRef CAS PubMed.
- (a) T. Miura, J. Nakahashi, T. Sasatsu and M. Murakami, Angew. Chem., Int. Ed., 2019, 58, 1138 CrossRef CAS PubMed; (b) H. E. Burks, L. T. Kliman and J. P. Morken, J. Am. Chem. Soc., 2009, 131, 9134 CrossRef CAS PubMed; (c) L. T. Kliman, S. N. Mlynarski, G. E. Ferris and J. P. Morken, Angew. Chem., Int. Ed., 2012, 51, 521 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc04011k |
| This journal is © The Royal Society of Chemistry 2019 |