Abosede Adejoke Ogunlana and Xiaoguang Bao
Chem. Commun., 2019,55, 11127-11130
DOI:
10.1039/C9CC05162G,
Communication
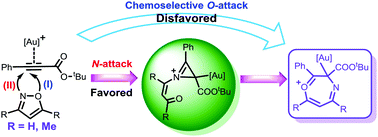
The detailed mechanisms of gold(I)-catalyzed annulations of propiolates with substituted and unsubstituted isoxazoles were investigated by DFT calculations. A unified rationale for the formation of the key seven-membered heterocyclic intermediate was proposed through initial chemoselective N-attack of isoxazole followed by sequential generation of an unprecedented 2H-azirine-containing intermediate, 6π electrocyclization and ring expansion. Subsequent substrate-dependent transformations were rationalized to generate the divergent products. The origins of the chemo- and regio-selectivities coupled with the factors responsible were addressed.