Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvent-free anhydrous Li+, Na+ and K+ salts of [B(3,5-(CF3)2C6H3)4]−, [BArF4]−. Improved synthesis and solid-state structures†
Antonio J.
Martínez-Martínez
 * and
Andrew S.
Weller
* and
Andrew S.
Weller
 *
*
Department of Chemistry, Chemistry Research Laboratories, Mansfield Road, University of Oxford, Oxford, OX1 3TA, UK. E-mail: antonio.martinez@chem.ox.ac.uk; andrew.weller@chem.ox.ac.uk
First published on 14th February 2019
Abstract
A modified, convenient, preparation of solvent-free, anhydrous, Li+, Na+ and K+ salts of the ubiquitous [BArF4]− anion is reported, that involves a simple additional recrystallisation step. Anhydrous Na[BArF4], K[BArF4], and [Li(H2O)][BArF4], were characterised by single-crystal X-ray diffraction.
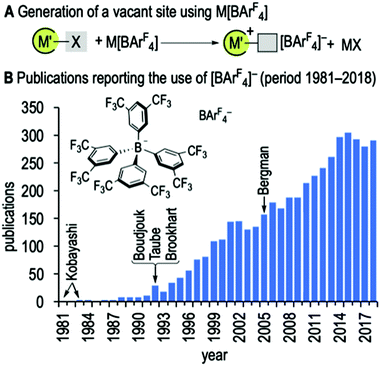
The use of weakly coordinating anions1 for the stabilisation of reactive low, or latent-low, coordinate cationic metal and main-group complexes now plays a central role in synthesis and catalysis,2 both in solution and in the solid-state.3 The anion [BArF4]− (ArF = 3,5-(CF3)2C6H3),4Fig. 1, enjoys particular utility amongst the small suite of common anions used, e.g. [B(C6F5)4]−,5 [Al(ORF)4]− (RF = fluoroalkyl),6 [B(3,5-Cl2C6H3)4]−,7 and [CB11X12]− (X = halogen)8 derivatives. A combination of synthetic accessibility, desirable properties of the resulting salts, i.e. solubility and crystallinity, and simple NMR-reporter groups, make [BArF4]− the go-to choice for many organometallic, main-group and catalytic applications. Very often such reactive species are generated by metathesis with the group 1 salts M[BArF4] (M = Li, Na, K), although alternative activating cations are also known, e.g. [(Et2O)2H][BArF4] (Taube and Brookhart)9 and [Ph3C][BArF4] (Boudjouk).10 The alkali salts have also been used as polymerisation initiators,11 in electrochemistry,12 as an additive in lithium ion batteries,13 in ionic liquids,14 and for the extraction of aqueous lanthanide ions.15 While considered to be non-interacting, under appropriate conditions [BArF4]− can coordinate to metal centres through its arene ring,16,3a or via metal⋯F–CF2 interactions.17 It can also undergo B–C bond cleavage.18
The synthesis and use, as a phase transfer catalyst,4 of the [BArF4]− anion was first reported by Kobayashi in 1981, followed by the preparation of hydrated [Na(H2O)3][BArF4].19 Brookhart subsequently reported the synthesis of Na[BArF4], by drying under vacuum and a cold CH2Cl2 wash.9a Both these preparations used the Grignard reagent 1,3,5-XMg(CF3)2C6H3 (X = Br, I). In 2005, Bergman described an alternative protocol that avoided the use of the Grignard/magnesium metal mixture20 for the preparation of anhydrous Na[BArF4],21 but required prolonged drying under vacuum over P2O5. Synthetic protocols have been reported for hydrated Li+ and K+ salts;22 or where the water content has not been reported.23 To date, the synthesis (Li+, K+) and structures24 (Li+, Na+, K+) of anhydrous M[BArF4] have not been reported in the open literature. Such anhydrous salts are of importance when using the [BArF4]− anion to access highly Lewis-acidic, and low-coordinate, complexes.25
We now detail here a robust multigram protocol to prepare solvent-free anhydrous Li+, Na+ and K+ salts of the [BArF4]− anion on ∼20 g scale in 60–70% yields, by adding a simple recrystallisation step of the crude product prior to drying under vacuum. As well as being synthetically expedient, this allows for the structures of solvent-free anhydrous Na+ and K+, and mono aquo Li+ salts of [BArF4]− to be determined.
Following a modified Kobayashi synthesis, Li+, Na+ and K+ salts of [BArF4]− (Fig. 2) were prepared from 1,3,5-BrMg(CF3)2C6H3/BF3 followed by treatment with the aqueous alkali metal carbonate of choice (M2CO3, M = Li+, Na+ and K+). Extraction into diethyl ether gave the corresponding crude [M(solvent)x][BArF4] (solvent = H2O and/or Et2O). Our key improvement is a subsequent double recrystallisation step to yield the corresponding pure solvent-complexes in high yield. [Li(solvent)x][BArF4] was successively recrystallised from undried diethyl ether/n-pentane and then undried fluorobenzene/n-pentane at −23 °C to give [Li(H2O)4][BArF4]22a as determined by NMR spectroscopy and single-crystal X-ray diffraction (ESI). Anhydrous Li[BArF4] 1 was then conveniently obtained as a highly hygroscopic off-white solid (64% yield) after drying under dynamic vacuum (10−2 mbar) at 80 °C for 72 h. Shorter drying times (24 h) gave [Li(H2O)][BArF4] 4. Using [Li(H2O)4]+ is crucial, as any bound ether results in decomposition on drying. Anhydrous Na[BArF4], 2 (68%, white solid), and K[BArF4], 3 (58%, off-white solid), were obtained from drying the pure THF solvates [M(THF)6][BArF4] under vacuum (80 °C, 48 h).22c [M(THF)6][BArF4] were themselves isolated by two consecutive recrystallisations of crude [M(solvent)x][BArF4] from THF/CH2Cl2. These two recrystallisation steps ensure high purity of the final anhydrous salts. These procedures routinely yield ∼20 g of anhydrous hydroscopic Li+, Na+ and K+ salts of [BArF4]−. This method also works for the Bergman synthesis, by recrystalising crude [Na(solvent)x][BArF4] prior to drying, to give anhydrous Na[BArF4] (12.3 g isolated yield, 58%).
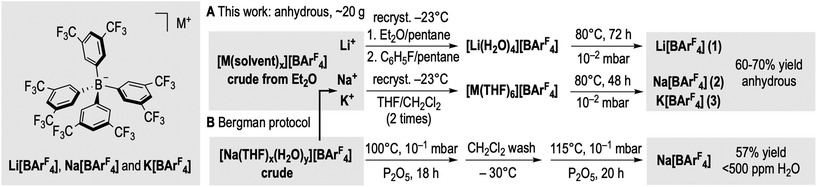 | ||
| Fig. 2 Preparation and isolation of solvent-free anhydrous Li+, Na+ and K+ salts of [BArF4]− and comparison with the Bergman synthesis. | ||
The [BArF4]− salts 1–4 were fully characterised in solution using multinuclear 1H, 11B, 13C and 19F NMR spectroscopy in THF-d8 (298 K) and ESI-MS, and these data are consistent with previously reported examples (ESI†).21,22a,23 Additionally, in the 7Li NMR spectrum of 1 a single resonance is observed at δ −0.54, which shifts to δ −0.38 in 4. The H2O ligand in complex 4 is observed at δ 4.02 as a sharp singlet (2 H) in the 1H NMR spectrum. While elemental analysis did not suggest the presence of water in 1–3, following Bergman's procedure, (η5-C5H5)2ZrMe2 was used to determine H2O content, using 1H NMR spectroscopy to measure the thus formed oxo-bridged complex (and CH4).21 No evidence of residual H2O was observed in any of the dried salts. Importantly, this 1H NMR titration method confirmed the presence of one molecule of H2O in 4.
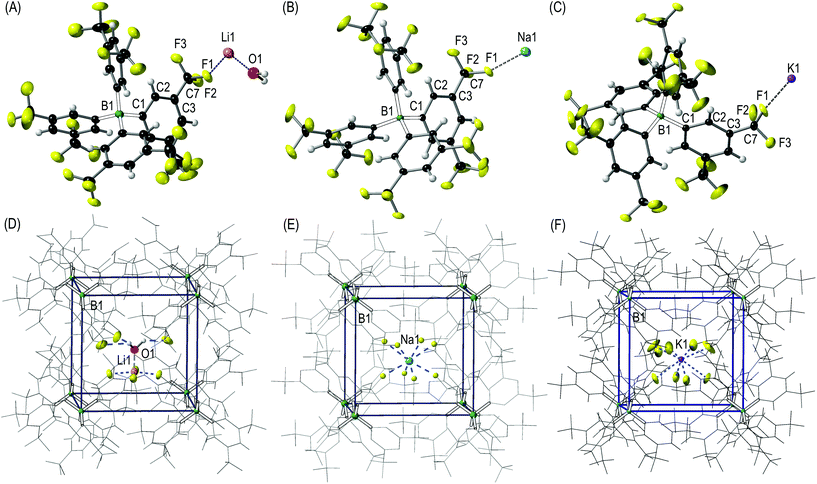
Single-crystals of solvent-free anhydrous Na[BArF4] 2 and K[BArF4] 3 suitable for X-ray diffraction studies were obtained by slow diffusion of dry n-pentane into a solution of the corresponding anhydrous salt in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dry C6H5F/CH2Cl2 (Fig. 3). For Li[BArF4] 1, although isolated in bulk in its anhydrous form, its highly hygroscopic nature meant that adventitious moisture present during the recrystallisation process routinely resulted in single crystals of [Li(H2O)][BArF4] 4 being isolated (Fig. 3A). Complexes 2, 3, and 4 crystallise in the tetragonal space group P4/n as contact ion-pairs (Fig. 3A–C). The contents of asymmetric units include 1/4 of the alkali metal and [BArF4]− anion (S4 symmetry), with an additional quarter of one molecule of water for 4. The [BArF4]− anions dictate the microenvironment around the [Li(H2O)]+, Na+ and K+ cations, and eight [BArF4]− anions encapsulate each alkali metal cation through CF3⋯alkali metal interactions forming an essentially cubic environment (Fig. 3D–F). The alkali metal sits in a pseudo body centred position, displaced towards one face of the cube. The Li+ ion in 4 has a square pyramidal geometry, interacting with CF3 groups from four distinct [BArF4]− anions [Li⋯F, 1.995(7)–2.045(4) Å] with the water molecule [Li–O, 1.842(8) Å] in the apical position (Fig. 3D). Additional CF3⋯H2O interactions are also present in 4 (F⋯H, 1.854(5)–2.09(1) Å). In 2 and 3 the Na+ and K+ centres, respectively, interact with a total of eight CF3 groups from the [BArF4]− anions that surround each cation (Fig. 3E and F) through M⋯F interactions. The increase in the ionic radius,26 in the series Li < Na < K, translates into correspondingly longer M⋯F distances [Li: 1.995(7)–2.045(4); Na: 2.473(2)–2.631(3); and K: 2.72(3)–2.74(2) Å].
1 mixture of dry C6H5F/CH2Cl2 (Fig. 3). For Li[BArF4] 1, although isolated in bulk in its anhydrous form, its highly hygroscopic nature meant that adventitious moisture present during the recrystallisation process routinely resulted in single crystals of [Li(H2O)][BArF4] 4 being isolated (Fig. 3A). Complexes 2, 3, and 4 crystallise in the tetragonal space group P4/n as contact ion-pairs (Fig. 3A–C). The contents of asymmetric units include 1/4 of the alkali metal and [BArF4]− anion (S4 symmetry), with an additional quarter of one molecule of water for 4. The [BArF4]− anions dictate the microenvironment around the [Li(H2O)]+, Na+ and K+ cations, and eight [BArF4]− anions encapsulate each alkali metal cation through CF3⋯alkali metal interactions forming an essentially cubic environment (Fig. 3D–F). The alkali metal sits in a pseudo body centred position, displaced towards one face of the cube. The Li+ ion in 4 has a square pyramidal geometry, interacting with CF3 groups from four distinct [BArF4]− anions [Li⋯F, 1.995(7)–2.045(4) Å] with the water molecule [Li–O, 1.842(8) Å] in the apical position (Fig. 3D). Additional CF3⋯H2O interactions are also present in 4 (F⋯H, 1.854(5)–2.09(1) Å). In 2 and 3 the Na+ and K+ centres, respectively, interact with a total of eight CF3 groups from the [BArF4]− anions that surround each cation (Fig. 3E and F) through M⋯F interactions. The increase in the ionic radius,26 in the series Li < Na < K, translates into correspondingly longer M⋯F distances [Li: 1.995(7)–2.045(4); Na: 2.473(2)–2.631(3); and K: 2.72(3)–2.74(2) Å].
In conclusion, solvent-free anhydrous Li[BArF4], Na[BArF4] and K[BArF4] has been prepared in multigram scale following a revised protocol, that rests upon a simple recrystallisation step. Our method stands by its simplicity to obtain the Li+, Na+ and K+ salts of the [BArF4]− anion and high purity. This allows for the characterisation of these salts by single-crystal X-ray diffraction. Given the importance of these salts in synthesis and catalysis we hope the community finds these improvements useful.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
EPSRC EP/M024210/1 and SCG Chemicals Co. Ltd. Radleys UK for the laboratory equipment used in the synthetic protocols.References
- (a) S. H. Strauss, Chem. Rev., 1993, 93, 927–942 CrossRef CAS; (b) C. A. Reed, Acc. Chem. Res., 1998, 31, 133–139 CrossRef CAS; (c) I. Krossing and I. Raabe, Angew. Chem., Int. Ed., 2004, 43, 2066–2090 CrossRef CAS PubMed.
- (a) W. Beck and K. Suenkel, Chem. Rev., 1988, 88, 1405–1421 CrossRef CAS; (b) T. A. Engesser, M. R. Lichtenthaler, M. Schleep and I. Krossing, Chem. Soc. Rev., 2016, 45, 789–899 RSC; (c) S. D. Pike, M. R. Crimmin and A. B. Chaplin, Chem. Commun., 2017, 53, 3615–3633 RSC; (d) I. M. Riddlestone, A. Kraft, J. Schaefer and I. Krossing, Angew. Chem., Int. Ed., 2018, 57, 13982–14024 CrossRef CAS PubMed.
- (a) S. D. Pike, F. M. Chadwick, N. H. Rees, M. P. Scott, A. S. Weller, T. Krämer and S. A. Macgregor, J. Am. Chem. Soc., 2015, 137, 820–833 CrossRef CAS PubMed; (b) A. J. Martínez-Martínez, B. E. Tegner, A. I. McKay, A. J. Bukvic, N. H. Rees, G. J. Tizzard, S. J. Coles, M. R. Warren, S. A. Macgregor and A. S. Weller, J. Am. Chem. Soc., 2018, 140, 14958–14970 CrossRef PubMed.
- H. Kobayashi, T. Sonoda, H. Iwamoto and M. Yoshimura, Chem. Lett., 1981, 10, 579–580 CrossRef.
- (a) A. G. Massey and A. J. Park, J. Organomet. Chem., 1964, 2, 245–250 CrossRef CAS; (b) E. Martin, D. L. Hughes and S. J. Lancaster, Inorg. Chim. Acta, 2010, 363, 275–278 CrossRef CAS.
- (a) S. M. Ivanova, B. G. Nolan, Y. Kobayashi, S. M. Miller, O. P. Anderson and S. H. Strauss, Chem. – Eur. J., 2001, 7, 503–510 CrossRef CAS PubMed; (b) I. Krossing, Chem. – Eur. J., 2001, 7, 490–502 CrossRef CAS PubMed; (c) I. Krossing and A. Reisinger, Eur. J. Inorg. Chem., 2005, 2005, 1979–1989 CrossRef.
- A. B. Chaplin and A. S. Weller, Eur. J. Inorg. Chem., 2010, 2010, 5124–5128 CrossRef.
- C. Douvris and J. Michl, Chem. Rev., 2013, 113, PR179–PR233 CrossRef PubMed.
- (a) M. Brookhart, B. Grant and A. F. Volpe, Organometallics, 1992, 11, 3920–3922 CrossRef CAS; (b) R. Taube and S. Wache, J. Organomet. Chem., 1992, 428, 431–442 CrossRef CAS.
- S. R. Bahr and P. Boudjouk, J. Org. Chem., 1992, 57, 5545–5547 CrossRef CAS.
- C.-T. Chang, C.-L. Chen, Y.-H. Liu, S.-M. Peng, P.-T. Chou and S.-T. Liu, Inorg. Chem., 2006, 45, 7590–7592 CrossRef CAS PubMed.
- F. Barrière, N. Camire, W. E. Geiger, U. T. Mueller-Westerhoff and R. Sanders, J. Am. Chem. Soc., 2002, 124, 7262–7263 CrossRef.
- F. Kita, H. Sakata, S. Sinomoto, A. Kawakami, H. Kamizori, T. Sonoda, H. Nagashima, J. Nie, N. V. Pavlenko and Y. L. Yagupolskii, J. Power Sources, 2000, 90, 27–32 CrossRef CAS.
- A. Bösmann, G. Franciò, E. Janssen, M. Solinas, W. Leitner and P. Wasserscheid, Angew. Chem., Int. Ed., 2001, 40, 2697–2699 CrossRef.
- H. Suzuki, H. Naganawa and S. Tachimori, Phys. Chem. Chem. Phys., 2003, 5, 726–733 RSC.
- (a) J. Powell, A. Lough and T. Saeed, J. Chem. Soc., Dalton Trans., 1997, 4137–4138 RSC; (b) T. M. Douglas, E. Molinos, S. K. Brayshaw and A. S. Weller, Organometallics, 2007, 26, 463–465 CrossRef CAS.
- (a) P. Holze, T. Corona, N. Frank, B. Braun-Cula, C. Herwig, A. Company and C. Limberg, Angew. Chem., Int. Ed., 2017, 56, 2307–2311 CrossRef CAS PubMed; (b) M. Everett, A. Jolleys, W. Levason, D. Pugh and G. Reid, Chem. Comm., 2014, 50, 5843–5846 RSC; (c) J. B. Smith, S. H. Kerr, P. S. White and A. J. M. Miller, Organometallics, 2017, 36, 3094–3103 CrossRef CAS.
- (a) W. V. Konze, B. L. Scott and G. J. Kubas, Chem. Commun., 1999, 1807–1808 RSC; (b) H. Salem, L. J. W. Shimon, G. Leitus, L. Weiner and D. Milstein, Organometallics, 2008, 27, 2293–2299 CrossRef CAS.
- H. Nishida, N. Takada, M. Yoshimura, T. Sonoda and H. Kobayashi, Bull. Chem. Soc. Jpn., 1984, 57, 2600–2604 CrossRef CAS.
- J. L. Leazer, R. Cvetovich, F.-R. Tsay, U. Dolling, T. Vickery and D. Bachert, J. Org. Chem., 2003, 68, 3695–3698 CrossRef CAS PubMed.
- N. A. Yakelis and R. G. Bergman, Organometallics, 2005, 24, 3579–3581 CrossRef CAS PubMed.
- (a) J. H. Golden, P. F. Mutolo, E. B. Lobkovsky and F. J. DiSalvo, Inorg. Chem., 1994, 33, 5374–5375 CrossRef CAS; (b) M. R. Kita and A. J. M. Miller, J. Am. Chem. Soc., 2014, 136, 14519–14529 CrossRef CAS PubMed; (c) L. Carreras, L. Rovira, M. Vaquero, I. Mon, E. Martin, J. Benet-Buchholz and A. Vidal-Ferran, RSC Adv., 2017, 7, 32833–32841 RSC.
- W. E. Buschmann, J. S. Miller, K. Bowman-James and C. N. Miller, Inorg. Synth., 2002, 33, 85 Search PubMed.
- The structure of solvent-free Na[BArF4] has been deposited as a private comunication to the Cambridge Structural Database (VEGDAP, DOI:10.5517/ccdc.csd.cc1ptr7h). Detailed synthesis and structural discussion were not reported.
- A. D. Piascik, R. Li, H. J. Wilkinson, J. C. Green and A. E. Ashley, J. Am. Chem. Soc., 2018, 140, 10691–10694 CrossRef CAS PubMed.
- M. Rahm, R. Hoffmann and N. W. Ashcroft, Chem. – Eur. J., 2016, 22, 14625–14632 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, characterisation, NMR and selected crystallographic X-ray data. CCDC 1886445–1886447. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9dt00235a |
| This journal is © The Royal Society of Chemistry 2019 |