Infrared heating of the top surface of a cyclonic spray chamber to improve the analytical performance of inductively coupled plasma optical emission spectrometry
Ahmed
Al Hejami
and
Diane
Beauchemin
 *
*
Queen's University, Department of Chemistry, Kingston, Ontario K7L 3N6, Canada. E-mail: diane.beauchemin@chem.queensu.ca; Fax: +1-613-533-6669; Tel: +1-613-533-2619
First published on 28th November 2018
Abstract
This study investigates how the figures of merit of inductively coupled plasma optical emission spectrometry can be improved by heating the top part of a cyclonic spray chamber. A ceramic beaded infrared (IR) rope heater was used to heat the top surface of a quartz baffled cyclonic spray chamber, side arm of the spray chamber, elbow connection to the torch and base of the torch up to 150 °C. A PFA nebulizer was utilized for all measurements. Optimum operating conditions were selected using multivariate optimizations for 21 elements. Upon IR heating, transport efficiency was significantly enhanced from 4.5% at room temperature to 15.7% at 150 °C. This improved sensitivity by 3.0 to 4.9 fold, depending on the element, and detection limit by 3.7 to 12 fold compared to those with the same sample introduction system at room temperature. The plasma robustness was also improved as assessed by Mg II 280.270/Mg I 285.213 nm ratios of 9 at room temperature and 11 at 150 °C. Accuracy was not jeopardized with IR heating, as similar results were obtained without and with IR heating for the elemental analyses of two certified reference materials of drinking and waste waters using a simple external calibration without internal standardization or matrix-matching.
Introduction
Pneumatic nebulization consisting of a spray chamber and pneumatic nebulizer is a traditional sample introduction system for inductively coupled plasma (ICP) optical emission spectrometry (OES) and mass spectrometry (MS). The main drawback of this system is its low sample transport efficiency (typically less than 5%), which is why it is considered as the weakest link of ICP spectrometry. However, it is still a standard system for routine multi-elemental analysis by ICP-based techniques because of its simplicity, stability and relatively low cost.1–4 Different systems have been used to enhance sample transport efficiency, for instance ultrasonic nebulization (USN)5 and direct injection nebulisation,6 but they have limited applications because of associated matrix effects and clogging issues, respectively.Pre-evaporation of the analyte aerosol, without removing the solvent (usually water), prior to its introduction into the plasma is an alternative approach to significantly improve analytical performance of ICP spectrometry by enhancing sample transport efficiency.7–9 Preserving water vapour has many advantages. Water acts as a load buffer in the plasma, which helps to minimize matrix effects.10 Moreover, it is the main source of hydrogen, which can facilitate energy transfer between the surrounding plasma and the central channel.11 In addition, converting the sample aerosol into vapour greatly decreases the background noise associated with desolvation and vaporization of sample droplets in the plasma.12
Greenfield and Smith13 were the first to perform pre-evaporation, for the determination of trace elements in oil, organic compounds and blood. They heated a spray chamber by convective heating, thereby achieving total consumption of microliter samples, which improved the sensitivity of ICPOES by up to one order of magnitude. Peters and Beauchemin14,15 installed a pre-evaporation tube (PET) between the spray chamber and ICP torch, which was wrapped with heating tape and maintained at 400 °C. This improved sensitivity and detection limit for ICPMS, as a result of the reduction of noise arising from the decrease in average droplet size of the sample aerosol entering the plasma. Replacing the whole desolvation system of a USN system by a PET (400 °C) wrapped with heating tape also improved sensitivity, detection limit and plasma robustness for ICPOES.16 Switching from a heating tape to infrared (IR) heating further improved the analytical performance achieved with the same USN system.17 The combination of a PET with a multimode sample introduction system (MSIS) also improved the analytical figures of merit of ICPOES.18
The so-called heated torch integrated sample introduction system (hTISIS), which consists of a pneumatic micro-nebuliser and single-pass spray chamber, has been used to improve sensitivity of ICPOES19,20 and ICPMS8 by increasing sample transport efficiency. A variety of samples have been analysed by the hTISIS including environmental, fuel, biological and environmental samples.21–28 However, the hTISIS operates at very low sample uptake rate (i.e. 20 μL min−1), which limits the analysis speed and consequently the sample throughput. Moreover, the micro-nebuliser precludes the applications of the hTISIS to samples with high salt content because of the ensuing blockage problems. A pneumatic nebulization in programmable temperature spray chamber (PN-PTSC) system combined with chemical vapour generation, where the spray chamber was heated to 40–60 °C significantly improved ICPOES sensitivity and detection limit without degradation in plasma thermal characteristics.29–32
Because IR heating provides uniform and more efficient heating as well as better short-term stability, shorter washout time and less carry-over than convective heating with heating tape,33–35 which was nonetheless used in most of the above applications, it was employed to enhance transport efficiency for PN. Using ceramic block IR heaters to heat a non-commercial flip single-pass spray chamber improved the analytical performance of ICPOES although the sample uptake rate was an order of magnitude less than that used for PN at room temperature.9 Sensitivity, detection limit and plasma robustness were also significantly improved for ICPOES using a new IR-heated modified cyclonic (MC) spray chamber with an IR lamp heater inserted in its centre.36 The bulky block IR heater was replaced by a more flexible ceramic beaded rope IR heater, which provides both IR and convective heating.7 The later was utilized to heat the entire spray chambers,37 the torch base for the MC,36 as well as a PET for USN7 and MSIS38 systems.
In the previous study performed in this laboratory,37 different spray chambers were entirely heated with IR rope heaters to achieve total sample consumption at low sample uptake rate (i.e. 0.1 mL min−1), which may affect the analysis time. The aim of the present work was to improve the analytical performance of ICPOES at a sample uptake rate close to 1 mL min−1 by using the IR rope heater to heat only the top of a quartz baffled cyclonic (BC) spray chamber along with its side arm, the elbow connection to the torch and the torch base. The outlet of the BC spray chamber in this work, which is normally used with ICPMS, is bent so as to connect to a horizontal torch, in contrast to those used previously, whose outlet is vertical so as to connect to a vertical torch. The resulting sensitivity, detection limit, precision and plasma robustness were compared with those obtained at room temperature with the same system and with previous IR-heated BC systems with vertical outlets. Certified reference materials of drinking and waste water were then analysed to verify the accuracy of the optimized system.
Experimental
Instrumentation
All measurements were made using a lateral view ARCOS ICPOES instrument (SPECTRO Analytical Instruments, Kleve, Germany) equipped with a Fassel-type torch (SCP Science, Baie d'Urfé, QC, Canada), a 50 mL quartz baffled cyclonic spray chamber and PFA nebulizer (both ESI Elemental Scientific, Omaha, NE, USA). To avoid melting by IR heating, the Teflon adapter outlet of the spray chamber (Fig. 1) was replaced by a 12 mm/5 mm female quartz joint (by JRV Scientific Glass, Montreal, QC, Canada). The top surface of the spray chamber was heated by a ceramic beaded IR rope heater along with its side arm (7 cm long), elbow glass connection to the torch (7 cm long) and torch base (7 cm long, Fig. 2). In total, the aerosol travelled a 21 cm IR-heated path between the spray chamber and the torch injector. The IR heating temperature was controlled by a PL512 Mantle-Minder temperature controller (GLAS-COL Apparatus Company, Terre Haute, USA) connected to a thermocouple, which was inserted between the surface of the spray chamber and the IR rope heater.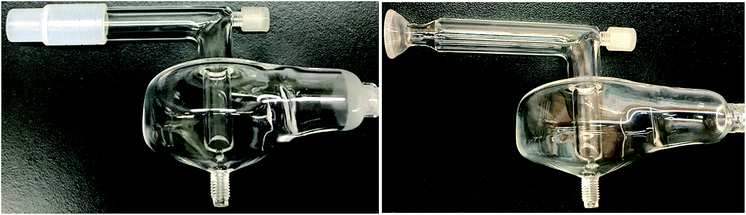 | ||
| Fig. 1 Quartz baffled cyclonic spray chamber with Teflon adapter outlet (left) and 12 mm/5 mm female quartz joint (right). | ||
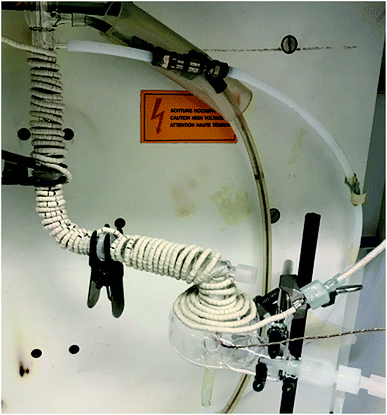 | ||
| Fig. 2 Experimental set-up for IR-heated quartz baffled cyclonic spray chamber using ceramic beaded IR rope heater to heat from the top of the spray chamber to the base of the torch. | ||
Chemicals
Individual elemental standard solutions of 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 (SCP Science) were diluted to prepare all multi-elemental standard solutions in 2% v/v HNO3. Doubly deionized water 18 MΩ cm (Arium Pro UV/DI System, Sartorius Stedim Biotech, Goettingen, Germany) and sub-boiled HNO3 were used to prepare all standard solutions. HNO3 (ACS grade; Fisher Scientific, Ottawa, Canada) was purified using a DST-1000 sub-boiling distillation system (Savillex, Minnetonka, USA). Two certified reference materials of water, namely drinking water EP-L3 and waste water EU-L-3 (both SCP Science) were directly analysed to verify method accuracy.
000 mg L−1 (SCP Science) were diluted to prepare all multi-elemental standard solutions in 2% v/v HNO3. Doubly deionized water 18 MΩ cm (Arium Pro UV/DI System, Sartorius Stedim Biotech, Goettingen, Germany) and sub-boiled HNO3 were used to prepare all standard solutions. HNO3 (ACS grade; Fisher Scientific, Ottawa, Canada) was purified using a DST-1000 sub-boiling distillation system (Savillex, Minnetonka, USA). Two certified reference materials of water, namely drinking water EP-L3 and waste water EU-L-3 (both SCP Science) were directly analysed to verify method accuracy.
Measurements of transport efficiency and washout time
The transport efficiency was measured in triplicate as the ratio of the mass of a 5 mg L−1 multi-elemental standard solution trapped by dry silica gel (in a 5 mL micropipette tip placed at the outlet of the spray chamber) over that aspirated.36,39 The washout time, defined as the time taken for the steady-state signal from a 5 mg L−1 multi-elemental standard solution to return to baseline, was measured for the 14 emission lines that did not saturate the detector.Optimization
The ICPOES instrumental operating parameters are listed in Table 1, along with those used in previous works36,37 on IR heating of cyclonic spray chambers. The R.F. power was set at 1.6 kW to prevent plasma extinction while the transport efficiency was increased upon IR heating of the sample aerosol. The plasma gas flow rate and observation height were selected based on previous optimizations carried out on the same instrument.9 The sample uptake rate was set at 0.9 mL min−1 for a good sample throughput. A 5 mg L−1 multi-elemental standard solution in 2% v/v HNO3 along with the corresponding blank were used to perform multivariate optimization of the remaining parameters. A face-centred central composite experimental design7,9,36–38 was utilized to optimize of the nebulizer gas flow rate (in the range of 0.6–1.2 L min−1), the auxiliary gas flow rate (in the range of 0.6–2 L min−1) and the IR heating temperature (in the range of 20–200 °C) as the goal was to find the best compromise parameters providing improved sensitivity for all analytes and robust plasma conditions. Plasma robustness was assessed by measuring the ionic to atomic emission intensity ratio of Mg II (280.270 nm)/Mg I (285.213 nm). Plasma excitation under robust plasma conditions (when the Mg II/Mg I ratio is at least 10) is not remarkably affected by small changes in operating conditions and/or matrix effect.40–42| Parameter | Present work | Previous work37 | Previous work36 | ||
|---|---|---|---|---|---|
| RT | IR rope | IR rope | IR block | IR lamp in the baffle | |
| Nebulizer | PFA nebulizer | SeaSpray nebulizer | Burgener nebulizer | ||
| Baffled cyclonic spray chamber design | With bent outlet | With straight outlet | With straight outlet and enlarged baffle | ||
| R.F. power (kW) | 1.6 | 1.6 | 1.7 | 1.7 | 1.7 |
| Plasma gas flow rate (L min−1) | 14.5 | 14.5 | 14.5 | 14.5 | 14.5 |
| Auxiliary gas flow rate (L min−1) | 1 | 1.2 | 1 | 1.8 | 2 |
| Nebulizer gas flow rate (L min−1) | 1 | 0.9 | 0.85 | 0.9 | 0.6 |
| Sheath gas flow rate (L min−1) | 0 | 0 | 0.4 | 0.5 | 0 |
| Plasma observation height (mm) | 10 | 10 | 10 | 10 | 10 |
| Sample uptake rate (mL min−1) | 0.9 | 0.9 | 0.12 | 0.3 | 1 |
| IR temperature (°C) | — | 150 | 200 | 200 | 150 |
Data analysis
The Smart Analyzer Vision software (SPECTRO Analytical Instruments) was utilized to select emission (atomic and ionic) lines free from potential spectroscopic interference for 21 elements. Minitab Release 17 statistical software (Minitab Inc., State College, PA, USA) was used to carry out all experimental designs for multivariate optimizations. All data processing, starting with blank subtraction, was done using Microsoft Excel 2013. Detection limit was computed as three times the standard deviation corresponding to the average emission signal intensity of 10 consecutive blanks divided by the calibration slope (i.e., sensitivity).Results and discussion
Sensitivity, detection limit and precision
As reported in the literature,9,17,36,37 sensitivity and detection limit were significantly improved (according to a paired Student's t-test at the 95% confidence level) upon IR heating the quartz BC spray chamber compared to those with the same system at room temperature (Tables 2 and 3). The improvement in sensitivity and detection limit for BC(IR) with bent outlet used in this work was higher than that obtained with the previous BC (IR rope and block)37 with straight outlet and MC(IR)36 (Tables 2 and 3). The average (n = 26) factors of improvement in sensitivity and detection limit with the present set-up were 3.7 ± 0.6 and 7.6 ± 2.6, respectively. Moreover, the noise associated with droplet desolvation and vaporization12 was clearly reduced with IR heating because a greater improvement in detection limit than in sensitivity was achieved, as was also previously observed with other IR-heated spray chambers36,37 and USN-PET(IR).17| Analyte, nm | BC (bent outlet; IR rope) | BC (straight outlet)37 | MC (straight outlet; IR lamp)36 | |
|---|---|---|---|---|
| IR rope | IR block | |||
| Al II 167.078 | 4.9 | |||
| As I 189.042 | 4.0 | 2.4 | 1.6 | |
| Be II 313.107 | 3.9 | 2.6 | 2.3 | |
| Be I 234.861 | 3.1 | 2.1 | 1.8 | |
| Cd II 226.502 | 4.3 | 3.4 | 2.3 | 2.8 |
| Cd I 228.802 | 3.0 | 2.5 | 1.9 | |
| Ce II 413.765 | 3.2 | 3.6 | 3.5 | |
| Co II 228.616 | 3.9 | 2.8 | 1.9 | 2.6 |
| Cr II 267.716 | 3.8 | 4.8 | 3.4 | 2.4 |
| Cr I 302.156 | 3.0 | 3.1 | 2.4 | |
| Cu II 224.700 | 3.4 | 2.2 | 2.1 | 2.6 |
| Fe II 238.204 | 3.8 | 2.8 | 2.1 | 2.5 |
| K I 766.491 | 3.0 | 3.1 | 2.5 | |
| La II 333.749 | 3.4 | 4.0 | 3.6 | 2.1 |
| Mg II 280.270 | 3.7 | 2.5 | ||
| Mg I 285.213 | 3.1 | 2.4 | 2.1 | |
| Mn II 257.611 | 3.7 | 3.0 | 2.4 | 2.4 |
| Ni II 221.648 | 4.1 | 3.7 | 2.5 | |
| Pb II 220.353 | 4.0 | 3.7 | 2.2 | 2.7 |
| P I 178.287 | 4.3 | |||
| S I 182.034 | 4.3 | 2.6 | 1.7 | 3.2 |
| Se I 204.050 | 4.5 | 3.3 | 2.1 | 2.9 |
| Sr II 421.552 | 3.2 | 2.9 | 2.7 | |
| V II 292.402 | 3.5 | 3.9 | 3.3 | 2.3 |
| Zn II 206.200 | 4.6 | 3.1 | ||
| Zn I 213.856 | 3.2 | |||
| Element, nm | BC (bent outlet) | DPa (RT)37 | BC (straight outlet)37 | DP (RT)36 | MC (straight outlet; IR lamp)36 | ||
|---|---|---|---|---|---|---|---|
| RT | IR rope | IR rope | IR block | ||||
| a Scott double-pass spray chamber used as a reference in previous works. | |||||||
| Al II 167.078 | 0.6 | 0.09 | |||||
| As I 189.042 | 20 | 2 | 10 | 3 | 3 | ||
| Be II 313.107 | 1 | 0.1 | 0.3 | 0.1 | 0.09 | ||
| Be I 234.861 | 0.6 | 0.08 | 0.2 | 0.2 | 0.09 | ||
| Cd II 226.502 | 3 | 0.5 | 1 | 0.3 | 0.4 | 7 | 1 |
| Cd I 228.802 | 3 | 0.8 | 2 | 0.6 | 0.6 | ||
| Ce II 413.765 | 306 | 70 | 116 | 72 | 39 | ||
| Co II 228.616 | 7 | 1 | 3 | 1 | 1 | 10 | 2 |
| Cr II 267.716 | 11 | 1 | 10 | 2 | 1 | 12 | 6 |
| Cr I 302.156 | 394 | 43 | 126 | 48 | 31 | ||
| Cu II 224.700 | 17 | 3 | 13 | 9 | 2 | 19 | 8 |
| Fe II 238.204 | 6 | 1 | 3 | 0.7 | 0.5 | 6 | 2 |
| K I 766.491 | 429 | 115 | 234 | 85 | 56 | ||
| La II 333.749 | 24 | 2 | 10 | 2 | 2 | 39 | 12 |
| Mg II 280.270 | 0.6 | 0.09 | 0.9 | 0.2 | |||
| Mg I 285.123 | 6 | 1 | 3 | 1 | 0.5 | ||
| Mn II 257.611 | 2 | 0.2 | 0.8 | 0.3 | 0.2 | 3 | 0.6 |
| Ni II 221.648 | 9 | 1 | 3 | 1 | 0.6 | ||
| Pb II 220.353 | 46 | 5 | 29 | 7 | 5 | 81 | 22 |
| P I 177.495 | 29 | 5 | |||||
| S I 182.034 | 37 | 4 | 19 | 15 | 5 | 43 | 8 |
| Se I 204.050 | 111 | 12 | 45 | 9 | 9 | 118 | 29 |
| Sr II 421.552 | 3 | 0.7 | 0.8 | 0.8 | 0.3 | ||
| V II 292.402 | 12 | 1 | 6 | 2 | 2 | 15 | 6 |
| Zn II 206.200 | 3 | 0.3 | 3 | 0.7 | |||
| Zn I 213.856 | 2 | 0.5 | |||||
The improvement in sensitivity is consistent with the significant enhancement in transport efficiency by 3.5 fold upon IR heating, from 4.5% (±0.1) at room temperature to 15.7% (±0.5) with IR heating. The latter is slightly better than the 11.7% (±0.4) transport efficiency with MC(IR)36 but less than the essentially 100% achieved when a whole BC was heated with IR rope.37 Yet, despite the lower transport efficiency than in the latter case, similar detection limits were achieved in this work. This suggests that pre-evaporation of the whole aerosol, including the primary aerosol, with IR heating of a whole BC chamber, led to an aerosol with a broader size distribution, which translated into a noisier signal in the ICP than when mostly pre-evaporating the tertiary aerosol, as performed in the present work.
The percent relative standard deviation (% RSD) was used to assess the instrumental precision and is summarized in Table 4. The average % RSD for the partially heated BC was 1.0 ± 0.3 and 2.7 ± 0.4 at room temperature and with IR heating, respectively. Precision was systematically degraded upon IR heating with a ceramic beaded IR rope heater as reported previously.37 Nonetheless, consistent with the observed detection limits, the precisions of BC(IR) with bent outlet and BC(IR rope) with straight outlet are comparable, whereas the precisions of BC(IR block) and MC(IR) (both with straight outlet) are better than that of BC(IR) with bent outlet according to a paired Student's t-test at the 95% confidence level. This likely stems from the IR rope heater providing both convective and IR heating,7 whereas mostly IR heating is generated by the IR block heater9,17,37 and the IR lamp heater.36 Furthermore, heating the top of the spray chamber likely induced temperature gradients in the spray chamber.
| Analyte, nm | BC (bent outlet) | BC (straight outlet)37 | MC (straight outlet; IR lamp)36 | ||
|---|---|---|---|---|---|
| RT | IR rope | IR rope | IR block | ||
| Al II 167.078 | 1.1 | 2.6 | |||
| As I 189.042 | 0.6 | 3.4 | 2.8 | 1.9 | |
| Be II 313.107 | 1.4 | 3.2 | 3.2 | 2.9 | |
| Be I 234.861 | 1.2 | 3.1 | 3.1 | 2.7 | |
| Cd II 226.502 | 1.0 | 2.1 | 2.0 | 2.4 | 0.8 |
| Cd I 228.802 | 0.9 | 2.1 | 1.7 | 2.2 | |
| Ce II 413.765 | 1.2 | 3.1 | 2.7 | 2.1 | |
| Co II 228.616 | 1.0 | 2.2 | 1.8 | 2.4 | 0.9 |
| Cr II 267.716 | 1.0 | 3.1 | 2.5 | 2.0 | 0.6 |
| Cr I 302.156 | 0.5 | 2.8 | 3.4 | 2.0 | |
| Cu II 224.700 | 1.0 | 2.4 | 3.3 | 2.0 | 0.6 |
| Fe II 238.204 | 0.9 | 2.3 | 1.9 | 2.4 | 0.7 |
| K I 766.491 | 0.6 | 2.9 | 2.9 | 2.0 | |
| La II 333.749 | 0.9 | 3.3 | 2.9 | 2.2 | 0.7 |
| Mg II 280.270 | 1.4 | 3.0 | 0.9 | ||
| Mg I 285.213 | 0.9 | 2.2 | 2.6 | 2.2 | |
| Mn II 257.611 | 1.4 | 2.5 | 1.7 | 2.3 | 0.9 |
| Ni II 221.648 | 1.1 | 3.0 | 2.0 | 2.1 | |
| Pb II 220.353 | 0.8 | 2.8 | 2.9 | 1.9 | 0.9 |
| P I 178.287 | 1.0 | 2.8 | |||
| S I 182.034 | 0.7 | 2.9 | 3.8 | 2.3 | 0.4 |
| Se I 204.050 | 1.4 | 2.8 | 3.8 | 2.0 | 0.5 |
| Sr II 421.552 | 1.3 | 3.0 | 3.5 | 2.7 | |
| V II 292.402 | 0.9 | 3.3 | 2.5 | 2.2 | 0.7 |
| Zn II 206.200 | 1.2 | 1.8 | 0.8 | ||
| Zn I 213.856 | 0.9 | 2.2 | |||
The average (n = 14 elements) washout time was 17 ± 4 and 21 ± 5 for BC(RT) and BC(IR), respectively. Although the sample uptake rate was identical in both cases, the washout time with IR heating was significantly longer than that without heating according to a paired Student's t test at the 95% confidence level. This is likely because of the temperature gradient induced by IR heating the top of the spray chamber, as well as the convective heating from the ceramic beads in contact with the spray chamber, which may favour analyte deposition on the walls during evaporation.
Plasma robustness
The Mg II/Mg I ratio (n = 7) was 9.4 ± 0.1 and 11.1 ± 0.2 without and with IR heating, respectively. According to a Student's t-test at the 95% confidence level, IR heating significantly improved plasma robustness because increasing water vapour loading improves plasma excitation conditions.11,20 A ratio larger than 10 was also reported with IR-heated spray chambers37 and USN-PET(IR)17 on the same ICPOES instrument as well as with hTISIS.20Analysis of water certified reference materials
Two certified reference materials of water were analysed using a simple external calibration without internal standardization or matrix-matching to assess the accuracy with the IR-heated BC spray chamber. The results, summarized in Table 5, fall within the tolerance limits of the certified values. Hence, the IR-heated spray chamber is suitable as a sample introduction system for ICPOES.| Analyte | CRM drinking water EP-L-3 | CRM waste water EU-L-3 | ||||
|---|---|---|---|---|---|---|
| Certified ± tolerance limit | BC(RT) | BC(IR) | Certified ± tolerance limit | BC(RT) | BC(IR) | |
| Al | 100 ± 10 | 97.5 ± 4.2 | 107 ± 13 | 6.28 ± 1.50 | 7.51 ± 0.14 | 6.79 ± 0.31 |
| As | 10.6 ± 1.8 | 10.59 ± 0.02 | 10.4 ± 0.2 | 8.40 ± 0.98 | 7.71 ± 0.07 | 7.43 ± 0.09 |
| Cd | 1.97 ± 0.22 | 1.98 ± 0.02 | 1.89 ± 0.07 | 2.28 ± 0.42 | 2.24 ± 0.03 | 2.09 ± 0.01 |
| Co | 9.75 ± 1.22 | 10.08 ± 0.05 | 9.84 ± 0.23 | 8.25 ± 0.63 | 8.07 ± 0.09 | 7.78 ± 0.04 |
| Cr | 12.7 ± 1.0 | 13.3 ± 0.1 | 13.5 ± 0.3 | 6.26 ± 1.39 | 6.08 ± 0.10 | 6.04 ± 0.06 |
| Fe | 27.9 ± 3.7 | 27.95 ± 0.02 | 28.8 ± 0.6 | 5.80 ± 0.76 | 6.33 ± 0.09 | 6.38 ± 0.06 |
| K | 404 ± 42 | 411 ± 1 | 405 ± 5 | 207 ± 39 | 210 ± 2 | 205 ± 1 |
| Mg | 45.8 ± 4.3 | 47.2 ± 0.4 | 46.7 ± 0.6 | 93.8 ± 18.5 | 103.0 ± 0.9 | 102 ± 1 |
| Mn | 5.85 ± 0.58 | 5.82 ± 0.01 | 5.71 ± 0.11 | 12.2 ± 1.5 | 12.0 ± 0.1 | 11.9 ± 0.1 |
| Ni | 19.9 ± 2.0 | 20.5 ± 0.1 | 20.1 ± 0.5 | 8.34 ± 1.03 | 8.22 ± 0.09 | 7.96 ± 0.05 |
| Sr | 141 ± 10 | 141 ± 1 | 134.0 ± 0.4 | 14.0 ± 3.7 | 14.2 ± 0.08 | 13.1 ± 0.07 |
| V | 13.6 ± 1.1 | 14.4 ± 0.1 | 14.5 ± 0.2 | 4.95 ± 0.61 | 4.98 ± 0.08 | 4.80 ± 0.04 |
| Zn | 42.5 ± 3.6 | 43.00 ± 0.02 | 44.7 ± 1.2 | 3.05 ± 1.79 | 2.75 ± 0.04 | 2.67 ± 0.02 |
Conclusions
Infrared heating of the top surface of a cyclonic spray chamber improves sensitivity, detection limit and plasma robustness in ICPOES. The increased transport efficiency and reduction in noise lead to, in general, 3–12 fold improvement in sensitivity and detection limit compared to those obtained at room temperature at the same sample uptake rate. Moreover, accuracy was not jeopardized with IR heating, as very similar results were obtained with and without IR heating for the multi-elemental analysis of waters with a simple external calibration without internal standardization or matrix-matching. Future work will include using the optimized system to improve the analytical performance of ICPMS.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge Anglo American Plc for the donation of a SPECTRO ARCOS ICPOES instrument. This research was conducted as part of the Engineered Nickel Catalysts for Electrochemical Clean Energy project administered from Queen's University and supported by Grant No. RGPNM 477963-2015 under the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Frontiers Program. AAH also thanks the School of Graduate Studies and Research of Queen's University for a graduate award.References
- J. Mora, S. Maestre, V. Hernandis and J. L. Todoli, Trends Anal. Chem., 2003, 22, 123–132 CrossRef CAS.
- N. H. Bings, J. O. Orlandini von Niessen and J. N. Schaper, Spectrochim. Acta, Part B, 2014, 100, 14–37 CrossRef CAS.
- G. L. Donati, R. S. Amais and C. B. Williams, J. Anal. At. Spectrom., 2017, 32, 1283–1296 RSC.
- R. F. Browner and A. W. Boorn, Anal. Chem., 1984, 56, 786A–798A CrossRef CAS.
- J. Borkowska-Burnecka, A. Lesniewicz and W. Zyrnicki, Spectrochim. Acta, Part B, 2006, 61, 579–587 CrossRef.
- J. A. McLean, H. Zhang and A. Montaser, Anal. Chem., 1998, 70, 1012–1020 CrossRef CAS.
- T. K. Anderlini and D. Beauchemin, J. Anal. At. Spectrom., 2018, 33, 127–134 RSC.
- M. Grotti, F. Soggia and J. L. Todoli, Analyst, 2008, 133, 1388–1394 RSC.
- Y. Makonnen, J. Burgener and D. Beauchemin, J. Anal. At. Spectrom., 2015, 30, 214–224 RSC.
- I. Novotny, J. C. Farinas, W. Jia-Liang, E. Poussel and J. M. Mermet, Spectrochim. Acta, Part B, 1996, 51, 1517–1526 CrossRef.
- S. E. Long and R. F. Browner, Spectrochim. Acta, Part B, 1988, 43, 1461–1471 CrossRef.
- J. W. Olesik and J. C. Fister III, Spectrochim. Acta, Part B, 1991, 46, 851–868 CrossRef.
- S. Greenfield and P. B. Smith, Anal. Chim. Acta, 1972, 59, 341–348 CrossRef CAS.
- G. R. Peters and D. Beauchemin, Anal. Chem., 1993, 65, 97–103 CrossRef CAS.
- G. R. Peters and D. Beauchemin, Spectrochim. Acta, Part B, 1993, 48, 1481–1494 CrossRef.
- A. Asfaw and D. Beauchemin, Spectrochim. Acta, Part B, 2010, 65, 376–384 CrossRef.
- A. Asfaw, W. R. MacFarlane and D. Beauchemin, J. Anal. At. Spectrom., 2012, 27, 1254–1263 RSC.
- A. Asfaw and D. Beauchemin, J. Anal. At. Spectrom., 2012, 27, 80–91 RSC.
- R. Sanchez, J. L. Todoli, C. P. Lienemann and J. M. Mermet, J. Anal. At. Spectrom., 2009, 24, 1382–1388 RSC.
- E. Paredes, M. Grotti, J. M. Mermet and J. L. Todoli, J. Anal. At. Spectrom., 2009, 24, 903–910 RSC.
- F. Ardini, M. Grotti, R. Sanchez and J. L. Todoli, J. Anal. At. Spectrom., 2012, 27, 1400–1404 RSC.
- R. Sanchez, J. L. Todoli, C. P. Lienemann and J. M. Mermet, J. Anal. At. Spectrom., 2010, 25, 1888–1894 RSC.
- R. Sanchez, J. L. Todoli, C. P. Lienemann and J. M. Mermet, J. Anal. At. Spectrom., 2012, 27, 937–945 RSC.
- C. Sanchez, C. P. Lienemann and J. L. Todoli, Spectrochim. Acta, Part B, 2016, 115, 16–22 CrossRef CAS.
- C. Sanchez, C. P. Lienemann and J. L. Todoli, Spectrochim. Acta, Part B, 2016, 124, 99–108 CrossRef CAS.
- M. Grotti, F. Ardini and J. L. Todoli, Anal. Chim. Acta, 2013, 767, 14–20 CrossRef CAS.
- A. Canabate, E. Garcia-Ruiz, M. Resano and J. L. Todoli, J. Anal. At. Spectrom., 2017, 32, 78–87 RSC.
- A. Canabate, E. Garcia-Ruiz, M. Resano and J. L. Todoli, J. Anal. At. Spectrom., 2017, 32, 1916–1924 RSC.
- K. Jankowski, J. Giersz and M. Paprocka, Microchem. J., 2014, 113, 17–22 CrossRef CAS.
- J. Giersz and K. Jankowski, Microchem. J., 2016, 124, 1–8 CrossRef CAS.
- J. Giersz, M. Bartosiak and K. Jankowski, Talanta, 2017, 167, 279–285 CrossRef CAS.
- J. Giersz, M. Bartosiak and K. Jankowski, J. Anal. At. Spectrom., 2017, 32, 1885–1892 RSC.
- K. O. Douglass, N. Fitzgerald, B. J. Ingebrethsen and J. F. Tyson, Spectrochim. Acta, Part B, 2004, 59, 261–270 CrossRef.
- L. Gras, J. Mora, J. L. Todoli, A. Canals and V. Hernandis, Spectrochim. Acta, Part B, 1999, 54, 1321–1333 CrossRef.
- A. R. Eastgate, R. C. Fry and G. H. Gower, J. Anal. At. Spectrom., 1993, 8, 305–308 RSC.
- A. Al Hejami and D. Beauchemin, J. Anal. At. Spectrom., 2018, 33, 738–744 RSC.
- A. Al Hejami and D. Beauchemin, J. Anal. At. Spectrom., 2018, 33, 2008–2014 RSC.
- T. K. Anderlini and D. Beauchemin, J. Anal. At. Spectrom., 2018, 33, 1068–1075 RSC.
- R. P. Lamsal, G. Jerkiewicz and D. Beauchemin, Anal. Chem., 2016, 88, 10552–10558 CrossRef CAS.
- J. M. Mermet, Anal. Chim. Acta, 1991, 250, 85–94 CrossRef CAS.
- A. Fernandez, M. Murillo, N. Carrion and J. M. Mermet, J. Anal. At. Spectrom., 1994, 9, 217–221 RSC.
- J. L. Todoli and J. M. Mermet, J. Anal. At. Spectrom., 1998, 13, 727–734 RSC.
| This journal is © The Royal Society of Chemistry 2019 |
