Open Access Article
Open Access ArticleSuppression of the environmental risks of lead in cropland soil using biomass ash and its modified product†
Hongyu
Liu
ab,
Zhuangwei
Zhou
a,
Yujie
Zhang
*a,
Ningyi
Chen
ab,
Jingyan
Kang
a,
Guijian
Liu
b,
Narayan S.
Hosmane
c and
Aiguo
Wu
 *a
*a
aCixi Institute of Biomedical Engineering, CAS Key Laboratory of Magnetic Materials and Devices & Key Laboratory of Additive Manufacturing Materials of Zhejiang Province, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, China. E-mail: aiguo@nimte.ac.cn; zhangyujie@nimte.ac.cn; Fax: +86 574 86685163; Tel: +86 574 86685039
bUniversity of Science and Technology of China, Hefei 230026, China
cDepartment of Chemistry and Biochemistry, Northern Illinois University, DeKalb, IL 60115, USA
First published on 8th April 2019
Abstract
In this work, modified biomass ash (MA), obtained through the hydrothermal treatment technique with biomass ash (BA) and alkaline phosphate as raw materials, was used as a useful soil amendment to reduce the environmental risk of lead and was compared with raw ash. In order to confirm the composition changes from BA to MA, the materials before and after modification were characterized by using transmission electron microscopy (TEM), energy dispersive spectroscopy (EDS), Fourier transform infrared (FT-IR) spectroscopy, and X-ray diffraction (XRD). Subsequently, the suppression of the environmental risks of lead in the contaminated cropland soil by MA and BA was systematically investigated through pH evaluation, toxicity-extraction, and fractional analysis. The results show that needle-like hydroxyapatite nanoparticles were generated during the modification process of BA. After incubation with 5% BA and 5% MA for 60 days, the pH of soil samples increased from 5.46 to 7.20 and 6.83, respectively; the lead concentration by TCLP extraction decreased by about 52.6% and 95.2%, respectively. And the content of lead bound to RES increased from 36.28% to 49.09% and 59.58%, respectively. MA showed higher immobilization efficiency, better immobilization stability, and less disturbance to the soil pH than BA, and it can suppress the environmental risk of lead below the standard toxicity level. These results undoubtedly demonstrate that BA has great potential in the practical application of remediation of lead contaminated soil, and its modified product may become a useful amendment to suppress and/or eliminate the high environmental risk of lead by transforming industrial waste into useful nanomaterials for green environmental chemistry.
1. Introduction
Lead (Pb) is a heavy metal element with potent neurotoxicity and even low levels of exposure can have a profound and long-lasting effect on human health. The Centers for Disease Control and Prevention defines that the blood lead level (BLL) is 5 μg dL−1 in order to protect human health, particularly for the safety of children.1 In previous research, the results showed that bioavailable lead concentration in soil is positively correlated with human BLL.2 It is urgent to suppress and/or eliminate the environmental risk of lead in contaminated soils, such as in farmland, post-industrial environments or urban soils. For these soils, the major pathways for exposure of humans to lead include consumption of contaminated food and inhalation of soil particles.3 The most widely used way to suppress the environmental risk of lead in soils is in situ remediation by utilizing the chemical immobilization method, in which the new materials added into soil (soil amendments) can transform heavy metals from the available state to a stable fixed state.4–6 Consequently, this transformation decreases the mobility and bioavailability of toxic heavy metals with available state and high concentration. Nonetheless, the current protocols for in situ remediation are severely restricted by the large area of soil, large demand and high price of amendments.7–9Biomass resources are extremely abundant in agricultural producing countries like China, India, Bangladesh, Brazil, US, Cambodia, Vietnam, Myanmar, and Southeast Asia.10 Biomass has been widely used for energy production all over the world and is considered as an environmentally safe way for energy access; during the process a solid by-product is produced, namely biomass ash (BA).11–13 The utilization of biomass ash has become an important issue for the sustainable development of biomass energy.14 Biomass ash is low cost and environmentally friendly, and is often used to improve the soil fertility during agricultural production. It also has good functional application in the adsorption of pollutants in water.15,16 In this work, the biomass ash from burning wheat stem, maize straw, groundnut shell, cotton stalk and other agricultural residues was modified and used as a new passivation material for lead contaminated soil, which can provide scientific guidance for the selection of soil amendments. The results of this investigation are presented in detail.
2. Materials and methods
2.1. Materials
Biomass ash was obtained from Anhui Guozhen Biomass Power Co., Ltd., China. Modified biomass ash (MA) was prepared by using a hydrothermal reaction with biomass ash and alkaline phosphate as raw materials. In order to simulate natural mineralization, the specific preparation process was as follows: first, the total content of Ca in BA was measured by using X-Ray Fluorescence spectra (XRF) analysis (S1 in the ESI†). Subsequently, 5 g biomass ash and a calculated amount of disodium hydrogen phosphate were dispersed into 40 mL Milli-Q water to ensure that the molar ratio of Ca/P in the mixed solution was 1.67. The mixture was stirred evenly, stored for 1 h, and then poured into a 50 mL hydrothermal reactor. The sealed reactor was heated to 200 °C in an air drying oven and maintained for 20 h. Finally, the solid residues were filtered, washed by using Milli-Q water and dried to a constant weight at 40–60 °C. The liquid wastes were collected for soluble by-product analyses.2.2. Characterization of BA and MA
To determine the morphology and structure of BA and MA, transmission electron microscope (TEM) images were recorded on a JEOL 2100 instrument (JEOL, Japan). The corresponding energy dispersive spectroscopy (EDS) data were obtained to identify the elemental composition of BA and MA. Fourier-transform infrared (FT-IR) spectra measurements were carried out on a NICOLET 6700 (Thermo Corporation, USA) in the range of 400 to 4000 cm−1 by the KBr method. The crystal type, phase and pattern of BA and MA were determined by the X-ray diffraction (XRD, D8 Advance, Bruker AXS, Germany) method over an angular range of 15–90° 2θ with Cu Kα radiation (40 kV, 40 mA).2.3. Soils
Lead contaminated soil was collected at a depth of 0–20 cm of cropland soil in a red sandstone area.17 All sample digging areas have been polluted for many years by surrounding chemical enterprises. The soil samples were collected with an equal amount at random excavation points and well-mixed. The original soil samples were air-dried at room temperature for 5 days, ground, and sieved with a 2 mm mesh. The soil samples were analyzed to obtain the pH value, organic matter (OM), cation exchange capacity (CEC), available phosphorus (P), potassium (K), and nitrogen (N), along with the total content of Pb (S1 in the ESI†). The ecological toxicity of lead in soil was evaluated by the toxicity characteristic leaching procedure (TCLP) before incubation experiments.2.4. Incubation procedure
100 g original soil sample was mixed with BA and MA with a weight rate of 2.5% and 5.0% in a 200 mL plastic beaker, respectively. A soil sample without BA or MA (0.0%) was used as the control. Each treatment had three replicates, with a total of 15 samples. All plastic beakers were incubated under greenhouse conditions for 60 days within the temperature range of 18–25 °C and with a moisture content of 15 ± 5% by the weighing method. Milli-Q water was used in order to maintain the water holding capacity of soil during the incubation period. The soil samples were collected at 7, 15, 30, and 60 days for pH analysis and lead toxicity measurements by the TCLP method. After 60 days, the classification of lead in soil was determined by the Tessier sequential extraction procedure.2.5. TCLP extraction of the soil sample
The TCLP method is widely used to evaluate the mobility, bioavailability and toxicity of heavy metals in soil and waste.5,18–20 Depending on the pH value of the soil sample, TCLP extraction was performed as follows. 5.7 mL glacial acetic acid was diluted to 1 liter with Milli-Q water. The pH of the solution was adjusted to 2.88 ± 0.05 by using NaOH and the final solution was used as an extraction solution of the TCLP method. Subsequently, 2 g soil sample was dispersed into 40 mL of the above extraction solution, shaken and mixed. After equilibration, the Pb concentration in the filtrate was measured by using inductively coupled plasma optical emission spectroscopy (ICP-OES).2.6. Sequential extraction of the soil sample
The Tessier sequential extraction method is widely used to determine the form change of metals during in situ remediation.21 In this work, the Tessier sequential extraction procedure was used to evaluate the change of lead speciation and solid-phase association of lead in soil to divide the lead speciation in the soil sample into five fractions with three replicates: (1) exchangeable (EXC), (2) carbonates (CA), (3) Fe–Mn oxides (Fe–Mn), (4) organic matter (OM), and (5) residue (RES). The Pb content in each speciation was determined by using ICP-OES. In order to ensure the accuracy of analysis results, a certified soil reference material (GBW07445, National Research Center for Certified Reference Materials, China) was used.2.7. Data analyses
For each incubation treatment, the mean pH value and lead content in each speciation were compared by using one-way analysis of variance (ANOVA). Statistically significant differences were determined by the Fisher least significant difference (LSD) test (P < 0.05).3. Results and discussion
3.1. Characterization of BA and MA
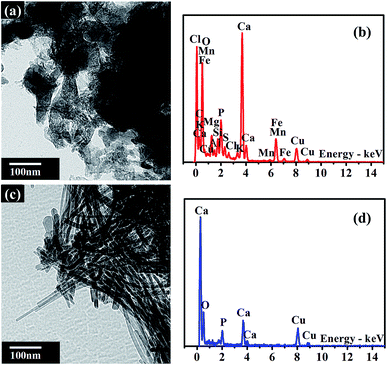
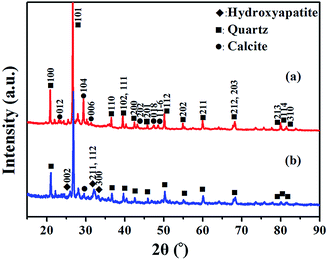
The morphology and composition of BA and MA were characterized by TEM and corresponding EDS analysis. As shown in Fig. 1a, the morphology of BA is irregular, with a significant amount of nanoparticles bonded together. The corresponding EDS spectrum shows that BA contains a lot of conventional elements, such as Ca, Si, O, Fe, K, Al, Mg, Cl, P, S, Na, Mn, and other trace elements. It is noticeable that the above elements are nutritional substances of soils and necessary minerals for plants and crops. The relative content of various elements in BA is given in the ESI (Table S2†). The EDS spectrum of BA shows that the relative content of Ca in BA is the highest. As a result, BA can provide enough Ca source for the hydrothermal reaction during the modification procedure. As reported by Steenari et al., the contents of Ca, K, Na, Mg and other salts in the ash of biofuel are so high with obvious alkaline characteristics.22 Similarly, BA used in this study is composed of numerous alkali metals and alkaline-earth metals, such as Ca, K, Na, and Mg with high alkalinity (Tables S1 and S2†), which can supply an alkaline environment for the hydrothermal reaction. In comparison with BA, MA exhibits a needle-like nanocrystalline structure, consisting of Ca, P, and O elements (Fig. 1b). The relative content of P increased significantly in MA compared with that in BA (Table S2†). These results unambiguously confirm the presence of insoluble phosphates in MA.The main compositions of crystal substances in BA and MA were examined by XRD analysis. As shown in Fig. 2a, BA mainly consists of quartz (SiO2) and calcite (CaCO3). However, the diffraction peaks of calcite in MA (Fig. 2b) weaken obviously and nearly disappear, and the diffraction peaks of hydroxyapatite (HA, Ca10(PO4)6(OH)2) appear in the XRD pattern of MA. These results indicate that needlelike HA nanoparticles are generated during the modification process of BA, in which Ca2+ and OH− are supplied by calcite and alkaline substances in BA. As shown in Fig. S1,† the soluble by-products during the modification process include sodium bicarbonate (NaHCO3) and sodium carbonate (Na2CO3). Therefore, all the above results indicate that the nanoscale hydroxyapatite should be formed according to the following reaction equation:
| 10CaCO3 + 6Na2HPO4 + 2H2O → Ca10(PO4)6(OH)2 + 8NaHCO3 + 2Na2CO3 |
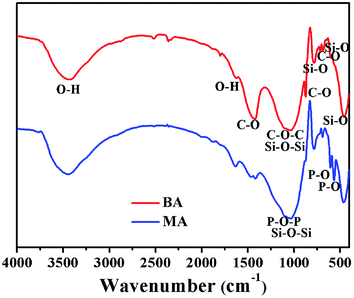
The FT-IR spectra of BA and MA are shown in Fig. 3. Accordingly, both BA and MA show strong absorption peaks at 1039–1034, 777–781, 686–690 and 457–463 cm−1, corresponding to the Si–O–Si asymmetric stretching vibration, Si–O–Si symmetric stretching vibration, Si–O in-plane bending vibration and Si–O out-of-plane bending vibration of SiO2, respectively. In addition, the presence of CO32− ions in BA can be deduced from the peaks at 1421, 875 and 715 cm−1, which is consistent with the presence of calcite in BA. In the FT-IR spectrum of MA, the peaks at 606 and 567 cm−1 indicate the presence of PO43− ions in MA. IR spectroscopic data of MA further prove that HA is generated during the modification process of BA.
3.2. Effect of the amendment on the pH value of soil
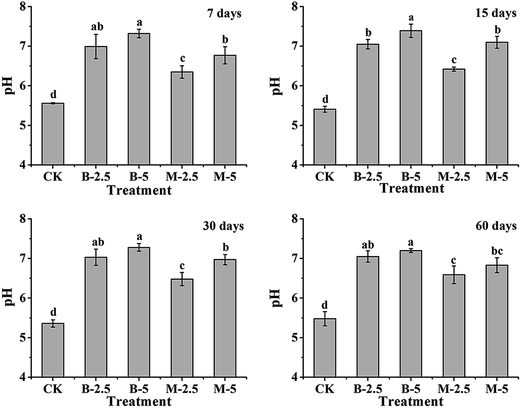
The pH value is an important parameter of soil properties, which can affect the immobilization and dissolution of heavy metals in soils.23 The pH variation of the soil samples was measured after incubation with BA and MA for 7, 15, 30 and 60 days, respectively. As shown in Fig. 4, both BA and MA increase (P < 0.05) the pH value of the soil significantly compared with the untreated soil (CK) during the incubation period. After incubation for 60 days, as for the CK sample, the pH values of the soil samples incubated with 2.5% BA and MA are increased to 7.05 and 6.59, and the pH values are increased to 7.20 and 6.83 with 5% BA and MA. For the same amendment, with the increase of the adding amount, there is a slight increase in the soil pH.For BA, the increase of soil pH can be explained by the dissolution of alkali salts in acid soil solution. For MA, the pH increase of soil can be due to (1) the effects of alkali salts that remained in MA and (2) the dissolution of HA and/or isomorphous substitution in the soil solution (OH− in HA is substituted by anions in soil solution, like F−, Cl−, and so on).24,25
Normally, the solubility and toxicity of heavy metals decrease with the increase of soil pH. However, large changes in soil pH should be avoided to make the soil a safe and green environment. During long-term incubation, MA shows a smaller influence and/or disturbance on/to the soil pH than BA with the same adding amount. The possible reasons are as follows: (1) the alkaline character of MA is “immobilized” in HA; (2) the solubility of HA is very low,26 and (3) the process of isomorphous substitution for OH− in soil solution is slow and incomplete, which mainly occurs on the surface of HA particles.
3.3. TCLP extraction of soil samples
The immobilization efficiency of the amendments for lead in the soil was evaluated by the TCLP extraction test. As shown in Table 1 and Fig. 5, the untreated soil samples are highly contaminated by toxic lead, in which the Pb2+ concentration is drastically higher than the toxicity level specified by the US EPA (Pb: 5 mg L−1).20,27 After incubation with BA and MA (2.5% and 5% by weight), the content of bioavailable lead reduces significantly and the degree of reduction increases with the increase of time and the dosage of amendments. After incubation with 2.5% and 5% BA for 60 days, toxic lead contents are reduced by about 33.9% and 52.6%, respectively; about 79.9% and 95.2% for MA-treated soil samples. It is obvious that MA can immobilize lead more effectively than BA even with low dosage. Moreover, it should be noted that the Pb2+ concentration by TCLP extraction is lower than the toxicity level specified by the US EPA when MA is used as a soil amendment.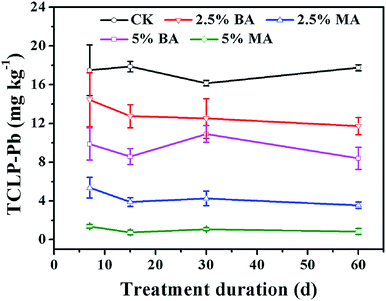 | ||
| Fig. 5 Concentrations of lead by TCLP extraction from the CK and soils treated with BA and MA after long-term incubation. | ||
The increase of soil pH is beneficial to the immobilization of available lead in soil due to precipitation. However, although the increase of the soil pH value by MA is lower than that by BA (Fig. 4), MA can immobilize the bioavailable lead more effectively than BA, which can be seen from Fig. 5. The excellent lead immobilization efficiency of MA can be owing to the existence of nanoscale HA, which is well-known for its favorable lead adsorption and immobilization ability based on cation exchange and surface metal complexation reactions.3,5,28,29,30
3.4. Fraction distribution of lead
To determine the speciation change of lead and immobility performance of BA and MA, Tessier sequential extraction was performed after incubation for 60 days. It can be seen from Table 2 that after incubation with 2.5% and 5% BA for 60 days, the exchangeable lead concentrations decrease obviously from 11.92% to 0.51% and 0.48%, respectively, which are primarily transformed to CA, Fe–Mn, and RES. With the increase of the adding amount of BA, more exchangeable lead is transformed to more stable speciation. The speciation change of lead after BA treatment can be explained by three reasons. First, Ca2+ in CaCO3 can be replaced by Pb2+ in soil solution due to the lower solubility product of PbCO3, which induces the increase of lead bound to the carbonate fraction. Second, the increase of soil pH by BA can lead to the formation of lead hydroxide, which induces the increase of lead bound to the Fe–Mn oxide fraction. Third, the increase of soil pH may cause the formation of some crystal minerals, which can transform lead into RES from other fractions.| Treatment | EXC | CA | Fe–Mn | OM | RES |
|---|---|---|---|---|---|
| a For each remediation treatment, mean values with different letters indicate statistically significant differences according to the LSD test (P < 0.05). EXC: exchangeable fraction, CA: carbonate fraction, Fe–Mn: Fe–Mn oxide fraction, OM: organic matter, and RES: residual fraction. | |||||
| CK | 11.92 ± 0.33a | 17.27 ± 0.14b | 23.52 ± 0.44b | 11.00 ± 0.11a | 36.28 ± 0.16d |
| 2.5% BA | 0.51 ± 0.03d | 22.44 ± 1.85a | 30.51 ± 1.72a | 8.94 ± 0.71b | 37.60 ± 0.90d |
| 5% BA | 0.48 ± 0.03d | 17.73 ± 0.32b | 23.42 ± 1.11b | 9.28 ± 0.64b | 49.09 ± 1.52b |
| 2.5% MA | 3.38 ± 0.20b | 11.63 ± 0.49c | 32.42 ± 1.04a | 7.11 ± 0.99c | 45.46 ± 1.50c |
| 5% MA | 1.52 ± 0.10c | 8.83 ± 0.36d | 23.05 ± 1.65b | 7.03 ± 0.55c | 59.58 ± 2.34a |
Compared with BA, MA reduces both the exchangeable lead concentration and the concentration of lead bound to the carbonate fraction effectively (Table 2). After incubation with 2.5% and 5% MA, the exchangeable lead concentrations decrease from 11.92% to 3.38% and 1.52%, respectively. The lead concentrations of CA are significantly decreased from 17.27% to 11.63% and 8.83%, respectively. In addition, the lead contents bound to EXC and CA are significantly reduced with the increase of the adding amount of MA. Moreover, it should be noted that the concentration of lead bound to RES in the soil treated with MA increases more than that in the soil treated with BA. It is known that the exchangeable heavy metal is considered as easily mobile and bioavailable one.21 Heavy metals bound to CA, Fe–Mn, and OM fractions are potentially bioavailable. The RES is the most stable among all five fractions and it is hard to be influenced by other conditions. The above results indicate that MA can promote the transformation of lead into a stable form more effectively than BA. Therefore, the durability of lead fixation by MA is stronger than that by BA due to the lower solubility of lead mineral species formed during the incubation than that of lead carbonate, lead hydroxide and so on.
4. Conclusions
In this study, a kind of true industrial waste (BA) was used as a low-cost raw material for the preparation of an amendment to reduce the environmental risk of lead in soil. According to the basic component and properties of BA, the ash was successfully modified through a hydrothermal reaction, in which Ca sources and the alkaline character of the ash play important roles in the formation of hydroxyapatite. After incubation with 5% BA and 5% MA for 60 days, the pH value of the soil samples increased from 5.46 to 7.20 and 6.83, and the lead concentration extracted from TCLP decreased by about 52.6% and 95.2%, respectively. And the concentration of lead bound to RES increased from 36.28% to 49.09% and 59.58%, respectively. The incubation results show that both BA and MA can reduce the content of available lead in soil. However, MA shows less disturbance to the soil pH, and it has higher immobilization efficiency and better immobilization stability to lead than BA. Therefore, MA is more significant than BA in reducing the environmental risk of lead and it has great potential as an amendment for soil contaminated by lead.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This project was financially supported by the National Key Research & Development Program (2018YFD0800300), the Project for Science and Technology Service of Chinese Academy of Sciences (CAS) (KFJ-SW-STS-172 & KFJ-EW-STS-016), the Hundred Talent Program of CAS (2010-735), the aided program for Science and Technology Innovative Research Team of Ningbo Municipality (Grant No. 2014B82010 and 2015B11002), the Natural Science Foundation of Ningbo (2016A610266) and the Zhejiang Province Financial Support (LGF19D060001 and R5110230).References
- CDC, Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention: Report of the Advisory Committee on Childhood Lead Poisoning Prevention of the Centers for Disease Control and Prevention, 2012, http://www.cdc.gov/nceh/lead/ACCLPP/Final_Document_030712.pdf Search PubMed.
- K. D. Bradham, C. M. Nelson, J. Kelly, A. Pomales, K. Scruton, T. Dignam, J. C. Misenheimer, K. Li, D. R. Obenour and D. J. Thomas, Environ. Sci. Technol., 2017, 51, 10005–10011 CrossRef CAS PubMed.
- H. Henry, M. F. Naujokas, C. Attanayake, N. T. Basta, Z. Q. Cheng, G. M. Hettiarachchi, M. Maddaloni, C. Schadt and K. G. Scheckel, Environ. Sci. Technol., 2015, 49, 8948–8958 CrossRef CAS PubMed.
- A. L. Juhasz, D. Gancarz, C. Herde, S. McClure, K. G. Scheckel and E. Smith, Environ. Sci. Technol., 2014, 48, 7002–7009 CrossRef CAS PubMed.
- X. D. Cao, L. N. Ma, Y. Liang, B. Gao and W. Harris, Environ. Sci. Technol., 2011, 45, 4884–4889 CrossRef CAS PubMed.
- H. B. Yin and J. C. Zhu, Chem. Eng. J., 2016, 285, 112–120 CrossRef CAS.
- L. Datko-Williams, A. Wilkie and J. Richmond-Bryant, Sci. Total Environ., 2014, 468, 854–863 CrossRef PubMed.
- G. M. Filippelli and M. A. Laidlaw, Perspect. Biol. Med., 2010, 53, 31–45 CrossRef CAS PubMed.
- D. B. Smith, W. F. Cannon, L. G. Woodruff, F. Solano, J. E. Kilburn and D. L. Fey, Geochemical and Mineralogical Data for Soils of the Conterminous United States, USGS, Reston, VA, 2013, https://pubs.usgs.gov/ds/801/pdf/ds801.pdf Search PubMed.
- R. Pode, Renewable Sustainable Energy Rev., 2016, 53, 1468–1485 CrossRef.
- S. Singh, L. C. Ram, R. E. Masto and S. K. Verma, Int. J. Coal Geol., 2011, 87, 112–120 CrossRef CAS.
- C. P. Liao, C. Z. Wu and Y. J. Yan, Fuel Process. Technol., 2007, 88, 149–156 CrossRef CAS.
- M. Narodoslawsky and I. Obernberger, J. Hazard. Mater., 1996, 50, 157–168 CrossRef CAS.
- M. Y. Zhao, Z. N. Han, C. D. Sheng and H. W. Wu, Energy Fuels, 2013, 27, 898–907 CrossRef CAS.
- L. Xu, H. B. Cui, X. B. Zheng, J. N. Liang, X. Y. Xing, L. G. Yao, Z. J. Chen and J. Zhou, Water Sci. Technol., 2018, 1, 115–125 CrossRef PubMed.
- Z. L. Liu, D. Tian, J. G. Hu, F. Shen, L. L. Long, Y. Z. Zhang, G. Yang, Y. M. Zeng, J. Zhang, J. S. He, S. H. Deng and Y. D. Hu, Sci. Total Environ., 2018, 634, 760–768 CrossRef CAS.
- H. B. Cui, J. Zhou, Q. G. Zhao, Y. B. Si, J. D. Mao, G. D. Fang and J. N. Liang, J. Soils Sediments, 2013, 13, 742–752 CrossRef CAS.
- X. X. Ye, S. H. Kang, H. M. Wang, H. Y. Li, Y. X. Zhang, G. Z. Wang and H. J. Zhao, J. Hazard. Mater., 2015, 289, 210–218 CrossRef CAS PubMed.
- M. L. F. M. Kede, F. V. Correia, P. F. Conceicao, S. F. Salles, M. Marques, J. C. Moreira and D. V. Perez, Int. J. Environ. Res. Public Health, 2014, 11, 11528–11540 CrossRef PubMed.
- U. S. EPA, Method 1311: Toxicity Characteristics Leaching Procedure, Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846, Office of Solid Waste, Washington D.C., 1996, https://www.epa.gov/sites/production/files/2015-12/documents/1311.pdf Search PubMed.
- A. Tessier, P. G. C. Campbell and M. Bisson, Anal. Chem., 1979, 51, 844–851 CrossRef CAS.
- B. M. Steenari and O. Lindqvist, Biomass Bioenergy, 1997, 13, 39–50 CrossRef CAS.
- X. D. Cao, L. Q. Ma, S. P. Singh and Q. X. Zhou, Environ. Pollut., 2008, 152, 184–192 CrossRef CAS PubMed.
- J. Boisson, A. Ruttens, M. Mench and J. Vangronsvel, Environ. Pollut., 1999, 104, 225–233 CrossRef CAS.
- Q. Y. Ma, T. J. Logan, S. J. Traina and J. A. Ryan, Environ. Sci. Technol., 1994, 28, 408–418 CrossRef CAS PubMed.
- A. Ito, K. Maekawa, S. Tsutsumi, F. Ikazaki and T. Tateishi, J. Biomed. Mater. Res., 1997, 36, 522–528 CrossRef CAS.
- R. Bade, S. Oh and W. S. Shin, Ecotoxicol. Environ. Saf., 2012, 80, 299–307 CrossRef CAS PubMed.
- Q. Y. Ma, S. J. Traina, T. J. Logan and J. A. Ryan, Environ. Sci. Technol., 1994, 28, 1219–1228 CrossRef CAS PubMed.
- E. Mavropoulos, A. M. Rossi and A. M. Costa, Environ. Sci. Technol., 2002, 36, 1625–1629 CrossRef CAS PubMed.
- Y. P. Xu, F. W. Schwartz and S. J. Traina, Environ. Sci. Technol., 1994, 28, 1472–1480 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9na00001a |
| This journal is © The Royal Society of Chemistry 2019 |