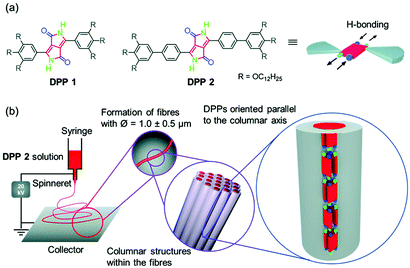
Anisotropic microfibres of a liquid-crystalline diketopyrrolopyrrole by self-assembly-assisted electrospinning†
Markus
Hecht
a,
Bartolome
Soberats
 b,
Jian
Zhu
c,
Vladimir
Stepanenko
a,
Seema
Agarwal
b,
Jian
Zhu
c,
Vladimir
Stepanenko
a,
Seema
Agarwal
 c,
Andreas
Greiner
c and
Frank
Würthner
c,
Andreas
Greiner
c and
Frank
Würthner
 *ab
*ab
aInstitut für Organische Chemie, Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: wuerthner@uni-wuerzburg.de
bCenter for Nanosystems Chemistry (CNC) & Bavarian Polymer Institute (BPI), Universität Würzburg, Theodor-Boveri-Weg, 97074 Würzburg, Germany
cMacromolecular Chemistry II and Bayreuth Center for Colloids and Interfaces, University of Bayreuth, Universitätsstrasse 30, Bayreuth, Germany
First published on 20th September 2018
Abstract
Electrospinning is a well-established technique for the preparation of nanofibres from polymer solution or melt, however it is rarely applied for small molecules. Here we report a unique example of a liquid-crystalline (LC) diketopyrrolopyrrole (DPP) dye that was successfully used for electrospinning. Micrometric fibres with anisotropic alignment of DPP dye were produced by this process as shown by polarized optical microscopy and selected area electron diffraction. This newly designed DPP dye self-assembles in solution by hydrogen bonding and π–π-interactions and forms columnar LC phases in the bulk. X-ray scattering and polarized FT-IR studies in the LC state revealed a hierarchical arrangement of DPP molecules into columnar structures. The successful preparation of anisotropic microfibers by electrospinning is attributed to the hydrogen bond-directed supramolecular polymerization of the new DPP dye in solution and its LC properties.
Conceptual insightsDiketopyrrolopyrrole (DPP) dyes are widely applied as red colour pigments (e.g. “Ferrari Red”) and organic electronic materials in devices. Their self-assembly into supramolecular materials with defined arrangement, however, is still challenging and their further hierarchical organization on macroscopic length scales has remained so far elusive. Toward this important goal for the implementation of self-assembled supramolecular materials in nanodevices, new processing methods are required to shape and orient the supramolecular materials in a specific direction. In this regard, we envisioned that the electrospinning technique might allow the preparation of fibres of small molecules with specific orientation within the fibres. Thus, we have designed a DPP dye that forms LC phases in bulk and self-assembles in solution. This design enables access to the benefits of both the self-assembly of small molecules in solution prior to the electrospinning process and the alignment of liquid crystals under external forces like electric fields. Indeed, electrospinning of the self-assembled DPP dye through hydrogen bonding and π–π-interactions produces anisotropic microfibres. Thus, here we present the first example of a small molecule DPP dye for the preparation of electrospun microfibres. |
Introduction
Self-assembly of organic molecules constitutes a most appealing bottom-up approach for the development of functional nanoscale materials.1–5 In this context, self-organizing materials based on supramolecular polymers,6–9 liquid crystals,10–13 and even covalent polymers14–16 are in the spotlight. Much effort has been devoted to the elucidation of self-assembly processes with particular attention on the self-assembly mechanisms,17 structure–functions relationship,18 and stimuli-responsive properties.19 These studies revealed the potential of supramolecular polymers, gels and liquid crystals in many applications such as organic electronics,20,21 photonics,22,23 or drug delivery.24,25 However, for the implementation of such molecular materials in devices new processing methods have to be developed. Albeit the advances in this direction are key to satisfy upcoming demands related to the miniaturization of components in modern nanoscale devices, so far comparatively little efforts have been devoted to the development of advanced processing technologies for supramolecular materials.In the case of liquid-crystalline (LC) materials, most approaches are focused on the alignment of the phases along 2D macroscopic arrays. Liquid crystals are usually susceptible to the molecular environment and due to their dynamic and adaptive properties they are easily aligned using chemically modified templating substrates or applying mechanical or electric forces.10,26,27 In this context, also electrospinning of LC polymers has been applied to prepare anisotropic fibres with well-defined internal structure.28–30 Electrospinning uses the deformation of droplets in an electric field by induced electrostatic charges leading to jets of material which, if the viscoelasticity is sufficient, form fibres.31 This technique is widely applied to prepare micro- or nanometric fibres from covalent polymers (solution and/or melts),32–36 but it has been rarely used for processing of small molecules37–42 and detailed information about the hierarchy and molecular organization of small molecules in such electrospun fibres is essentially absent. We envisioned that the electrospinning technique might be applicable for the fabrication of micrometric fibres based on hierarchical assemblies of mesogenic low-molecular weight dyes. For this purpose, we considered the use of organic dyes with the ability to self-assemble in solution into supramolecular polymers and to form LC phases in the bulk, i.e. supramolecular liquid crystals. We anticipated that dyes with such properties may be advantageous for this purpose due to: (1) their ability to entangle upon supramolecular polymerization in solution prior to the spinning process and (2) their tendency (as liquid crystals) to align into anisotropic domains under external forces (i.e. electric fields).
Herein, we report on the electrospinning of highly anisotropic microfibers of 1.0 ± 0.5 μm diameter from a diketopyrrolopyrrole (DPP) dye, namely DPP 2 (Fig. 1a). The anisotropic properties of DPP 2 microfibres are the result of a cooperative self-assembly through hydrogen bonds and π–π-interactions where the DPPs are oriented parallel to the fibre direction (Fig. 1b). Studies of the liquid crystallinity and supramolecular polymerization of DPP 1 and DPP 2 revealed the key factors for the successful preparation of anisotropic microfibres by electrospinning.
Results and discussion
Material design and electrospinning experiments
The molecular design of DPP 1 and DPP 2 consists of a DPP core, which is unsubstituted in lactam positions and functionalized with two 3,4,5-tris(dodecyloxy)phenyl groups in 3,6-positions. In the case of DPP 1 the wedge-shaped groups are directly connected to the core, while for DPP 2 a phenyl spacer connects core and dendrons (Fig. 1a). This design is expected to promote the hydrogen bond (H-bond) directed supramolecular polymerization in solution and to induce LC behaviour by nanosegregation in the bulk.43–45DPP 1 was prepared as previously described.46 The new DPP 2 was synthesized by Suzuki cross-coupling reaction of tert-butyloxycarbonyl (BOC)-protected 3,6-bis(4-bromophenyl)-substituted DPP and 3,4,5-tris(dodecyloxy)phenylboronic acid pinacol ester, followed by deprotection of BOC group by heat treatment (for detailed procedure and characterization see ESI†).In a previous work, we have reported the liquid crystallinity of DPP 1 which assembles via a π-stacked dimeric unit by quadruple H-bonds into a 1D supramolecular polymer structure that in turn organizes in a columnar LC phase.46 Our preliminary experiments confirmed that DPP 2 also forms LC phases (Fig. S1, ESI†). Furthermore, temperature-dependent UV-Vis experiments in toluene revealed that both DPP dyes self-assemble in solution into supramolecular polymers (Fig. S2, ESI†). Detailed studies of the LC behaviour and supramolecular polymerization are described later in this paper. The high propensity for supramolecular self-assembly makes DPP 1 and DPP 2 suitable candidates for electrospinning experiments.
Toluene was chosen as solvent for this process since both DPP 1 and DPP 2 self-assemble into aggregates in this solvent (Fig. S2, ESI† and vide infra). For this purpose, 20 mM solutions of both DPPs were prepared. In the case of DPP 1, the solution is of purple colour and low viscosity, while DPP 2 forms a dark red viscous solution that was shown to be a gel in the tube inversion test (Fig S3, ESI†). These solutions were electrospun on silicon and glass substrates by using a voltage of 20 kV and a flow rate of 0.1 ml h−1 (for details see ESI†). The substrates were then analysed by scanning electron microscopy (SEM) (Fig. 2a–c). While small droplets were obtained for DPP 1 (Fig. 2a), DPP 2 formed under the same conditions well-defined fibres with a length of several hundred micrometres and uniform thickness of 1 ± 0.5 μm exhibiting a certain rigidity (Fig. 2b and c).
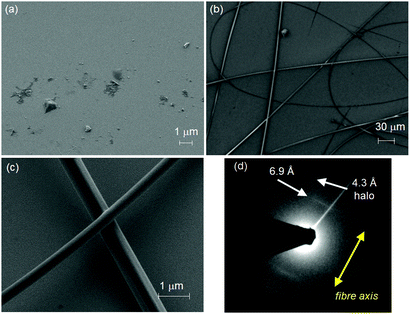 | ||
| Fig. 2 SEM images of electrospun (a) DPP 1 and (b and c) DPP 2 on silicon substrates. (d) Electron diffraction pattern of an electrospun fibre of DPP 2 (diameter ∅ = 500 nm) as obtained from SAED. | ||
To elucidate the anisotropic properties of the electrospun fibres of DPP 2, the material was investigated by polarizing optical microscopy (POM) (Fig. S4, ESI†). The fibres appear dark when they are oriented parallel to the analyzer or polarizer, but become bright (red colour) when the fibres are rotated by 45° to the analyzer due to their birefringence. This indicates that the fibres have a well-defined (macro-)molecular organization and the molecules as well as the supramolecular scaffolds forming the fibre are aligned in a specific direction. To get deeper insight into the exact arrangement of the DPPs, selected area electron diffraction (SAED) was measured on a spot of an electrospun fibre of DPP 2. The electron diffraction pattern showed a broad halo at a distance of 4.3 Å and a sharp signal at 6.9 Å (Fig. 2d). The latter appears (only) along the long axis of the fibre which is an indication of the anisotropic nature of the fibre. This signal matches well with the molecular length of a DPP molecule along its long molecular axis bearing two lactam units (6.0 Å) that connect two neighbour molecules by hydrogen bonds (ca. 6.0 + 0.8 Å) (Fig. S5, ESI†). Accordingly, POM and SAED experiments suggest that the DPP 2 electrospun fibre is composed of DPP molecules that are hierarchically organized with the long molecular axis of the DPP π-core parallel to the fibre axis. Thus, it is obvious that the electrospinning of DPP 2 in toluene produces anisotropic fibres with a well-defined packing arrangement of the DPP molecules. Such anisotropic properties have never been achieved by electrospinning of small molecules. In order to figure out their precise organization and the principles that govern the formation of these fibres, the LC properties and aggregation behaviour in solution of DPP 2 were studied in detail.
Liquid-crystalline properties
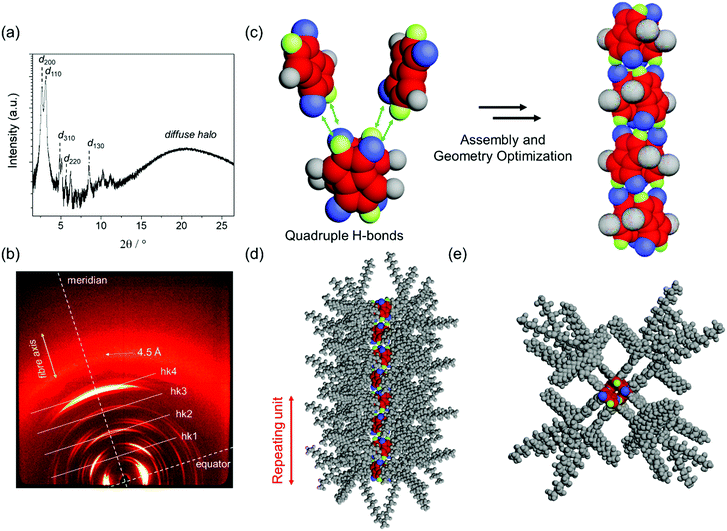
We have previously reported that DPP 1 self-assembles into DPP dimers that stack into a columnar structure via quadruple H-bonds, where each dimer is rotated by 90° along the columnar axis.46 These columns further arrange into a Colr phase between 0 °C and 45 °C and a Colh phase between 45 °C and 336 °C. In the present work, the LC properties of DPP 2 have been studied by the same techniques, i.e. POM, DSC, polarized FT-IR and X-ray experiments. DSC and POM experiments revealed that DPP 2 forms three phases: a soft-crystalline (SC) phase (from 0 °C to 177 °C) and two LC phases, the first one from 177 °C to 225 °C and the second one from 225 °C up to 270 °C, where the material melts (Fig. S6, ESI†). The respective phases of DPP 2 were identified by middle and wide angle X-ray scattering (MAXS and WAXS) studies of aligned fibres prepared with a home-made extruder. Both LC phases show sharp signals in the X-ray pattern, while a broad set of signals was observed for the SC phase. This latter phase shows a pattern pointing to a columnar phase but, due to the rather broad peaks, this phase could not be elucidated in detail (Fig. S7, ESI†). The MAXS pattern of the first LC phase at 210 °C clearly shows the 200, 110, 310, 220 and 130 reflections along the equator corresponding to a columnar rectangular (Colr) lattice (a = 65.4 Å, b = 31.5 Å) (Fig. 3a). By increasing the temperature to 265 °C, the X-ray pattern becomes simpler showing the 100, 110, 200, 210 and 300 reflections of a columnar hexagonal (Colh) lattice (a = 35.9 Å) on the equator (Fig. S8 and S9, ESI†).To gain insight into the molecular arrangement of DPP molecules in the LC phases, polarized FT-IR studies and further analyses of the MAXS and WAXS patterns of aligned DPP 2 samples were performed. From the polarization of the N–H stretching signals in homogenously aligned (by mechanical shearing) sample of DPP 2 onto KBr substrates, it was deduced that the lactam units and the H-bonds are oriented parallel to the columnar axis in all the SC and LC phases (Fig. S11, ESI†). This is also supported by the X-ray patterns of both LC phases that exhibit meridional and off-meridional reflections with a representative strong signal on the meridian at 6.8 Å. Taking into account the distance of hydrogen-bonding, this signal matches well with the distance between the two lactam units which is in agreement with the signal observed in the SAED pattern of the electrospun fibres (Fig. 2d). These observations support that the DPP cores are oriented with their long molecular axis parallel to the columnar axis in the LC phases. For the Colr phase, this signal at 6.8 Å was assigned to layer line hk4 while the other off-meridional reflections at 27.2 Å, 20.4 Å and 13.6 Å were assigned as hk1, hk2 and hk3, respectively (Fig. 3b). This implies a correlation between every fifth molecule along the columns with a rotation of 90° with each stratum. The number of molecules per columnar stratum (h = 6.8 Å) was estimated to be two, considering a density of 0.9 g cm−3 and a reasonable error of 5% of each parameter in the evaluation of the X-ray diffraction data (ESI†).47 Similar results were obtained from the WAXS and MAXS patterns of the DPP 2 Colh phase (Fig. S9, ESI†).
According to the X-ray data, the packing arrangement in the DPP 2 Colr and Colh phases were modelled by using Materials Studio software (Fig. 3c, d, e and Fig. S12, ESI†). The optimized structure of the Colr phase (Fig. 3c) supports the formation of dimers that are stacked via quadruple H-bonding by rotation of 90° (Fig. 3c–e). This columnar arrangement shows a correlation between every fifth molecule, giving a columnar unit cell composed of four dimers. This assembly mode is enabled by the alternate stack of two types of dimers (with mirror image relationship) as reported previously for DPP 1.46 The molecular arrangement in the Colh phase of DPP 1 shows a similar columnar organization, but in this case every third molecule correlates (Fig. S10, ESI†).46 The modelled molecular structures for all the LC phases were used as input for retrostructural analysis with the program CLEARER.48 The calculated diffraction patterns are in good agreement with the experimental data, supporting the proposed structural models (Fig. S8, S9, S13 and S14, ESI†).
It is noteworthy that both the X-ray patterns of the extruded fibres and the SAED pattern of the electrospun DPP 2 fibres show a signal at 6.8 Å, which corresponds to the centre–centre distance of hydrogen-bonded DPPs (Fig. S5, ESI†). Since this signal is observed in the SAED pattern in the parallel direction of the electrospun fibre, it can be concluded that the DPP cores are oriented parallel to the fibres (Fig. 1b) with a columnar arrangement identical or at least very similar to the one observed in the X-ray experiments of the LC phases.
Despite the relevant findings that unveil the hierarchical anisotropic self-assembly in the electrospun DPP 2 fibres (Fig. 1b), these experiments cannot explain why DPP 2 is able to form fibres by electrospinning but DPP 1 not, although respective intracolumnar arrangements are very similar. In order to elucidate the reason for this, we further explored the supramolecular polymerization of both DPPs in solution.
Supramolecular polymerization in solution
The supramolecular polymerization studies of DPP 1 and DPP 2 in solution were carried out by temperature- and concentration-dependent UV-Vis spectroscopy and atomic force microscopy (AFM) experiments. In chloroform (c = 3 × 10−5 M), both materials exhibit the optical features of monomerically dissolved DPPs49 with a strong absorption band at 519 nm and a vibronic progression at 483 nm and 452 nm for DPP 1 and at 531 nm with the vibronic progression at 495 nm and 462 nm for DPP 2. However, the spectra drastically change in less polar solvents like toluene that are known to reinforce hydrogen bonding,50 now revealing the characteristics of co-facially aggregated DPPs (Fig. S15, ESI†). Fig. 4b and d show the concentration-dependent spectra of DPP 1 and DPP 2 in toluene. Gradual increase of the concentration leads to a decrease of the absorptions bands corresponding to the monomer, while new bands originate at 510 nm, 540 nm and 590 nm for DPP 1 and at 478 nm, 508 nm and 542 nm for DPP 2 due to the formation of aggregated species of the double-stranded supramolecular polymers. The absorption spectrum of an electrospun fibre of DPP 2 shows similar optical features as the concentrated solution in toluene (Fig. S16, ESI†). In both experiments, the presence of isosbestic points at 423 nm and 530 nm for DPP 1 and at 426 nm and 548 nm for DPP 2 indicates the transformation between two distinct species. The calculated degree of aggregation was fitted with the established models showing the best results for the cooperative model of Goldstein and Stryer51–53 for both compounds. From this data analyses, nucleation and elongation constants of Knuc = 140 M−1 and Kelong = 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 were obtained for DPP 1, pointing at a strongly disfavoured dimer nucleus formation (cooperativity factor of σ = 3.7 × 10−3) (Fig. 4a). Very similar results were obtained for the supramolecular polymerization of DPP 2via its dimer nucleus with nucleation and elongation constants of Knuc < 10 M−1 and Kelong = 11
000 M−1 were obtained for DPP 1, pointing at a strongly disfavoured dimer nucleus formation (cooperativity factor of σ = 3.7 × 10−3) (Fig. 4a). Very similar results were obtained for the supramolecular polymerization of DPP 2via its dimer nucleus with nucleation and elongation constants of Knuc < 10 M−1 and Kelong = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 (cooperativity of σ = 3.9 × 10−4). Thus, both dyes show a rather similar self-assembly process in terms of mechanism (strongly cooperative) as well as binding constants. Temperature-dependent UV-Vis absorption studies of both DPPs confirmed the cooperativity of the self-assembly process (Fig. S17, ESI†).
000 M−1 (cooperativity of σ = 3.9 × 10−4). Thus, both dyes show a rather similar self-assembly process in terms of mechanism (strongly cooperative) as well as binding constants. Temperature-dependent UV-Vis absorption studies of both DPPs confirmed the cooperativity of the self-assembly process (Fig. S17, ESI†).
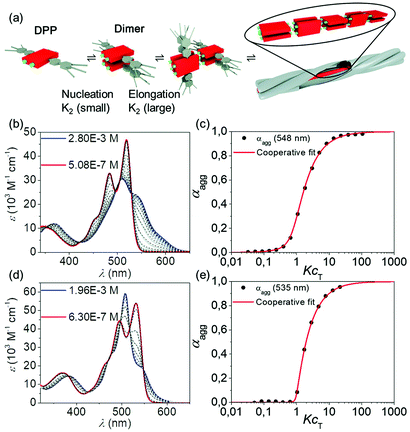 | ||
| Fig. 4 (a) Graphical illustration of the self-assembly process via the disfavoured dimeric nucleus. Concentration-dependent UV-Vis absorption spectra (coloured and grey lines) of (b) DPP 1 between 5.08 × 10−7 M and 2.80 × 10−3 M and (d) DPP 2 between 6.30 × 10−7 M and 1.96 × 10−3 M in toluene at 295 K and reconstructed spectra from the cooperative nucleation-elongation fit (black dashed lines). Plot of the experimental degree of aggregation αagg over the KcT (black dots) for (c) DPP 1 at 548 nm and (e) DPP 2 at 535 nm and the corresponding fit according to the cooperative nucleation-elongation model (red).51 | ||
The visualization of the aggregates in solution of both DPPs was achieved by AFM. For this purpose, solutions of DPP 1 (c = 1.0 × 10−4 M) and DPP 2 (c = 6.5 × 10−4 M) in toluene were spin-coated onto highly oriented pyrolytic graphite (HOPG) (Fig. 5a and b). These investigations showed that DPP 1 forms stiff fibres with a thickness of around 1.3 nm and a length of up to 800 nm. The thickness of the fibres is in good agreement with the width of the axis of the molecule along the wedge-shaped residues. This complies with the formation of 1D supramolecular polymers composed of dimeric DPP units as previously observed in the columnar LC phase of DPP 1.46DPP 2, on the other hand, forms more flexible fibres with a thickness of around 2.5 nm and a length of up to 800 nm. The flexibility of these 1D self-assemblies seems to promote the entanglement of the single 1D supramolecular polymers, which is not observed in DPP 1 assemblies (Fig. S3, ESI†).
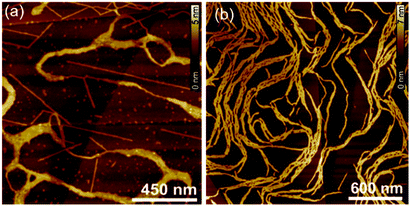 | ||
| Fig. 5 AFM height images of (a) DPP 1 (c = 1.0 × 10−4 M) and (b) DPP 2 (c = 6.5 × 10−4 M) spin-coated from solutions in toluene on HOPG. | ||
The concentration-dependent UV-Vis and AFM studies clearly show that DPP 1 and DPP 2 self-assemble into 1D fibres via a cooperative mechanism. Based on the AFM profiles, we assume that these structures consist of H-bonded DPP dimers, similar to those which compose a columnar slice in the LC phases (Fig. 3c–e). However, significant morphologic differences are found between the DPP 1 and DPP 2 fibres in AFM experiments (Fig. 5). While DPP 2 forms flexible fibres which lead to the gelation of toluene solutions at high concentrations, DPP 1 forms more rigid fibres that do not tend to entangle and hence do not lead to gelation of the solutions. Accordingly, it appears to be that the formation of entangled fibres and gels are a prerequisite for the electrospinning process of such small molecule based supramolecular polymers. As a control experiment, we also attempted to electrospin DPP 2 in a toluene/DMF mixture where no supramolecular polymers and gels are formed due to the disruption of the hydrogen bonds. This experiment failed to fabricate microfibres (Fig. S18, ESI†), proving the necessity of the formation of supramolecular polymer fibres in solution for the successful electrospinning process.
Conclusions
In this work, we have achieved the fabrication of anisotropic liquid-crystalline microfibres by electrospinning of hydrogen-bonded supramolecular polymers of DPP 2 in toluene. The presence of entangled supramolecular polymers in solution appears to be crucial for the success of the electrospinning process of small molecules. Furthermore, we have elucidated the molecular arrangement within the electrospun fibre by POM and SAED investigations showing a homogeneous arrangement of diketopyrrolopyrrole molecules within well-defined liquid-crystalline columnar domains. Accordingly, the electrospinning approach opens a new avenue for the creation of micrometre-sized fibres of organic molecules possessing unique electronic properties with well-ordered liquid-crystalline domains as desired for optoelectronic applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Bavarian State Ministry of Education, Science and the Arts for generous support for the newly established Key Laboratory for Supramolecular Polymers at the Center for Nanosystems Chemistry.Notes and references
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- T. Kato, M. Yoshio, T. Ichikawa, B. Soberats, H. Ohno and M. Funahashi, Nat. Rev. Mater., 2017, 2, 17001 CrossRef.
- F. Würthner, C. R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962–1052 CrossRef PubMed.
- B. M. Rosen, C. J. Wilson, D. A. Wilson, M. Peterca, M. R. Imam and V. Percec, Chem. Rev., 2009, 109, 6275–6540 CrossRef CAS PubMed.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef CAS PubMed.
- L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196–7239 CrossRef CAS PubMed.
- T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687–5754 CrossRef CAS PubMed.
- C. Rest, R. Kandanelli and G. Fernández, Chem. Soc. Rev., 2015, 44, 2543–2572 RSC.
- H. Kar, D. W. Gehrig, N. K. Allampally, G. Fernández, F. Laquai and S. Ghosh, Chem. Sci., 2016, 7, 1115–1120 RSC.
- J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. Gleeson and P. Raynes, Handbook of Liquid Crystals, Wiley-VCH, Weinheim, 2nd edn, 2014 Search PubMed.
- T. Kato, J. Uchida, T. Ichikawa and T. Sakamoto, Angew. Chem., Int. Ed., 2018, 57, 4355–4371 CrossRef CAS PubMed.
- D. J. Broer, C. M. W. Bastiaansen, M. G. Debije and A. P. H. J. Schenning, Angew. Chem., Int. Ed., 2012, 51, 7102–7109 CrossRef CAS PubMed.
- T. Wöhrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139–1241 CrossRef PubMed.
- C. J. Mueller, C. R. Singh, M. Fried, S. Huettner and M. Thelakkat, Adv. Funct. Mater., 2015, 25, 2725–2736 CrossRef CAS.
- F. Wieberger, C. Neuber, C. K. Ober and H. W. Schmidt, Adv. Mater., 2012, 24, 5939–5944 CrossRef CAS PubMed.
- G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176 CrossRef CAS PubMed.
- D. van der Zwaag, T. F. A. de Greef and E. W. Meijer, Angew. Chem., Int. Ed., 2015, 54, 8334–8336 CrossRef CAS PubMed.
- Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564–584 RSC.
- M. Wie, Y. Gao, X. Li and M. J. Serpe, Polym. Chem., 2017, 8, 127–143 RSC.
- B. R. Kaafarani, Chem. Mater., 2011, 23, 378–396 CrossRef CAS.
- A. P. H. J. Schenning and E. W. Meijer, Chem. Commun., 2005, 3245–3258 RSC.
- P. Lova, V. Grande, G. Manfredi, M. Patrini, S. Herbst, F. Würthner and D. Comoretto, Adv. Opt. Mater., 2017, 5, 1700523 CrossRef.
- H. Coles and S. Morris, Nat. Photonics, 2010, 4, 676–685 CrossRef CAS.
- A. Rösler, G. W. M. Vandermeulen and H.-A. Klok, Adv. Drug Delivery Rev., 2001, 53, 95–108 CrossRef.
- C. Guo, J. Wang, F. Cao, R. J. Lee and G. Zhai, Drug Discovery Today, 2010, 15, 1032–1040 CrossRef CAS PubMed.
- B. Soberats, E. Uchida, M. Yoshio, J. Kagimoto, H. Ohno and T. Kato, J. Am. Chem. Soc., 2014, 136, 9552–9555 CrossRef CAS PubMed.
- M. Yoshio, T. Mukai, H. Ohno and T. Kato, J. Am. Chem. Soc., 2004, 126, 994–995 CrossRef CAS PubMed.
- K. Nakashima, K. Tsuboi, H. Matsumoto, R. Ishige, M. Tokita, J. Watanabe and A. Tanioka, Macromol. Rapid Commun., 2010, 31, 1641–1645 CrossRef CAS PubMed.
- D. K. Kim, M. Hwang and J. P. F. Lagerwall, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 855–867 CrossRef CAS.
- Y. Wu, Q. An, J. Yin, T. Hua, H. Xie, G. Li and H. Tang, Colloid Polym. Sci., 2008, 286, 897–905 CrossRef CAS.
- D. H. Reneker, A. L. Yarin, H. Fong and S. Koombhongse, J. Appl. Phys., 2000, 87, 4531–4547 CrossRef CAS.
- S. Jiang, S. Agarwal and A. Greiner, Angew. Chem., Int. Ed., 2017, 56, 15520–15538 CrossRef CAS PubMed.
- T. D. Brown, P. D. Dalton and D. W. Hutmacher, Prog. Polym. Sci., 2016, 56, 116–166 CrossRef CAS.
- S. Agarwal, A. Greiner and J. H. Wendorff, Prog. Polym. Sci., 2013, 38, 963–991 CrossRef CAS.
- X. Shi, W. Zhou, D. Ma, Q. Ma, D. Bridges, Y. Ma and A. Hu, J. Nanomater., 2015, 2015, 140716 Search PubMed.
- J. D. Schiffman and C. L. Schauer, Polym. Rev., 2008, 48, 317–352 CrossRef CAS.
- A. Celebioglu and T. Uyar, Chem. Commun., 2010, 46, 6903–6905 RSC.
- X. Yan, M. Zhou, J. Chen, X. Chi, S. Dong, M. Zhang, X. Ding, Y. Yu, S. Shao and F. Huang, Chem. Commun., 2011, 47, 7086–7088 RSC.
- D. Kluge, J. C. Singer, B. R. Neugirg, J. W. Neubauer, H.-W. Schmidt and A. Fery, Polymer, 2012, 53, 5754–5759 CrossRef CAS.
- K. Wang, C.-Y. Wang, Y. Wang, H. Li, C.-Y. Bao, J.-Y. Liu, S. X.-A. Zhang and Y.-W. Yang, Chem. Commun., 2013, 49, 10528–10530 RSC.
- J. C. Singer, A. Ringk, R. Giesa and H.-W. Schmidt, Macromol. Mater. Eng., 2015, 300, 259–276 CrossRef CAS.
- X. Wang, Y. Han, Y. Liu, G. Zou, Z. Gao and F. Wang, Angew. Chem., Int. Ed., 2017, 56, 12466–12470 CrossRef CAS PubMed.
- T. E. Kaiser, H. Wang, V. Stepanenko and F. Würthner, Angew. Chem., Int. Ed., 2007, 46, 5541–5544 CrossRef CAS PubMed.
- S. Herbst, B. Soberats, P. Leowanawat, M. Lehmann and F. Würthner, Angew. Chem., Int. Ed., 2017, 56, 2162–2165 CrossRef CAS PubMed.
- S. Herbst, B. Soberats, P. Leowanawat, M. Stolte, M. Lehmann and F. Würthner, Nat. Commun., 2018, 9, 2646 CrossRef PubMed.
- B. Soberats, M. Hecht and F. Würthner, Angew. Chem., Int. Ed., 2017, 56, 10771–10774 CrossRef CAS PubMed.
- D. Sahoo, M. Peterca, E. Aqad, B. E. Partridge, P. A. Heiney, R. Graf, H. W. Spiess, X. Zeng and V. Percec, J. Am. Chem. Soc., 2016, 138, 14798–14807 CrossRef CAS PubMed.
- O. Sumner Makin, P. Sikorski and L. C. Serpell, J. Appl. Crystallogr., 2007, 40, 966–972 CrossRef CAS.
- M. Grzybowski and D. T. Gryko, Adv. Opt. Mater., 2015, 3, 280–320 CrossRef CAS.
- S. Ogi, V. Stepanenko, J. Thein and F. Würthner, J. Am. Chem. Soc., 2016, 138, 670–678 CrossRef CAS PubMed.
- R. F. Goldstein and L. Stryer, Biophys. J., 1986, 50, 583–599 CrossRef CAS PubMed.
- M. M. Smulders, M. M. Nieuwenhuizen, T. F. de Greef, P. van der Schoot, A. P. H. J. Schenning and E. W. Meijer, Chem. – Eur. J., 2010, 16, 362–367 CrossRef CAS PubMed.
- G. Fernández, M. Stolte, V. Stepanenko and F. Würthner, Chem. – Eur. J., 2013, 19, 206–217 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization, POM, UV-Vis, DSC, WAXS and MAXS data, molecular modelling and additional electrospinning experimental data. See DOI: 10.1039/c8nh00219c |
| This journal is © The Royal Society of Chemistry 2019 |