Hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets as efficient bifunctional electrocatalysts with superior stability for overall water splitting†
Tao
Zhang‡
ab,
Lifeng
Hang‡
bc,
Yiqiang
Sun
ab,
Dandan
Men
a,
Xinyang
Li
 a,
Lulu
Wen
ab,
Xianjun
Lyu
d and
Yue
Li
a,
Lulu
Wen
ab,
Xianjun
Lyu
d and
Yue
Li
 *a
*a
aKey Laboratory of Materials Physics and Anhui Key Laboratory of Nanomaterials and Nanotechnology, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei, 230031, P. R. China. E-mail: yueli@issp.ac.cn
bUniversity of Science and Technology of China, Hefei, 230026, P. R. China
cCollege of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, 518060, P. R. China
dCollege of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, P. R. China
First published on 12th April 2019
Abstract
Hierarchical hetero-Ni3Se4@NiFe LDH (layered double hydroxide) micro/nanosheets were prepared by coupling NiFe LDH nanosheets with Ni3Se4 micro-sized sheets, and could be used as effective bifunctional electrocatalysts for water splitting. Benefiting from the strong electronic interaction between the NiFe LDH nanosheets and Ni3Se4 micro-sized sheets, the hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets are endowed with enhanced charge transfer and reaction kinetics. As a result, the obtained hierarchical hetero-Ni3Se4@NiFe LDH/CFC (carbon fiber cloth) electrode shows optimized hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) properties in alkaline medium with a small overpotential of 85 mV and 223 mV at the current density of 10 mA cm−2, respectively. When the hierarchical hetero-Ni3Se4@NiFe LDH/CFCs are used as bifunctional electrodes for overall water splitting in a two-electrode electrolyzer, the current density of 10 mA cm−2 is derived at a cell voltage of 1.54 V. Moreover, due to the stability of the novel hierarchical hetero-micro/nanostructure, the as-prepared Ni3Se4@NiFe LDH/CFC exhibited high stability in the electrochemical processes over 100 h. This work highlights the importance of constructing efficient bifunctional electrocatalysts for overall water splitting by rational design of heterostructures.
New conceptsProgress toward electrochemical water splitting electrocatalysts with high activity and superior durability faces significant challenges due to the destruction of the crystal structure and inactivation of active sites, especially under alkaline conditions for industry application. If different functional electrocatalysts could be organized into hierarchical hetero-micro/nanomaterials, such novel structures would be endowed with abundant exposed edges and larger surface area, which offers more active sites for catalytic reactions. Moreover, advanced hierarchical hetero-catalysts could possess superior electrocatalytic stability due to such hierarchical micro/nanostructures. Based on this new concept, we assembled HER active Ni3Se4 micro-sized sheets and OER active NiFe LDH nanosheets into hierarchical hetero-Ni3Se4@NiFe LDH electrocatalysts with a simple and scalable thermal treatment method. Benefiting from the stability of the novel hierarchical hetero-micro/nanostructure and the strong electronic interaction between NiFe LDH and Ni3Se4, the hierarchical hetero-Ni3Se4@NiFe LDH catalysts were endowed with superior durability and high activity for water splitting. We expect that this work will inspire further promising synthetic methodologies for the rational design of efficient bifunctional electrocatalysts toward overall water splitting. |
Introduction
The critical shortage of fossil energy and the associated environmental crisis promote the urgent development of renewable energies.1–5 Water splitting driven by electricity or solar energy is one of the most effective strategies for renewable energy production by converting electrical energy into chemical energy stored by hydrogen fuels.6–10 The water electrolysis reaction consists of two half reactions: the oxygen evolution reaction (OER) at the anodic side and the hydrogen evolution reaction (HER) at the cathodic side, and it mainly relies on the use of noble-metal-based electrocatalysts.11–13 Currently, precious metal platinum (Pt) is known to exhibit the best catalytic performance for the HER, while the state-of-the-art catalysts for the OER are iridium dioxide (IrO2) and ruthenium dioxide (RuO2).14,15 Unfortunately, such noble metal-based materials with high cost and low earth-abundance are at high risk of corrosive dissolution onto the electrode or into the electrolyte solution, hampering their large-scale applications. Recently, a great deal of effort has been devoted to developing cost-effective and earth-abundant replacement catalysts with high activity. For instance, some transition metal oxides and hydroxides are beneficial for the OER in an alkaline medium,16 while transition metal sulfides, phosphides, nitrides, carbides, and selenides show potential applications for the HER in an acid electrolyte.17–20 During overall water splitting for industry applications, the electrocatalysts for the OER and HER simultaneously work in the same alkaline electrolyte.21 In order to address these challenges, developing earth-abundant, easily scalable, and stable electrocatalysts with excellent activity for overall water splitting is extremely urgent.Recent research has shown that rational design of heterogeneous electrocatalysts, especially with strong electronic interactions at the interface between the different parts, could enhance the charge transfer capability between the catalysts and electrode.22–24 Moreover, this unique design could further maximize the exposed active sites, facilitating the reaction kinetics of bifunctional electrocatalysts for overall water splitting. For example, Yu et al. prepared Co0.85Se/NiFe-LDH (layered double hydroxide) on exfoliated graphene as a bifunctional electrocatalyst for overall water splitting with a cell voltage of 1.67 V at 10 mA cm−2.25 Zhang et al. fabricated a bifunctional electrocatalyst (MoS2/Ni3S2 heterostructure) and the synergetic effect of the interface between MoS2 and Ni3S2 enhanced the overall water splitting performance.26 Moreover, Wu et al. integrated the active OER and HER components as heterostructures (Ni3N-NiMoN) for efficient overall water splitting.27 When Ni3N-NiMoN heterogeneous catalysts were used as the cathode and anode in a two-electrode electrolyzer, it only needs 1.54 V to drive a current density of 10 mA cm−2. Even though different kinds of bifunctional catalysts for overall water splitting have been synthesized, the performance, especially stability, is not satisfactory and their continuous operation is usually for less than 50 h. Therefore, it is highly imperative to develop better bifunctional catalysts with high activity and stability for practical water splitting. As reported in previous work, transition metal selenides are one kind of advanced HER electrocatalysts, but show relatively poor activity for the OER.28 NiFe-LDH, a representative two-dimensional (2D) material, is regarded as one of the most effective OER non-noble metal electrocatalysts.29,30 Compatibly integrating the transition metal selenides and NiFe-LDH in a designed manner, they may serve as a new bifunctional electrocatalyst for overall water splitting. In addition, if the different functional electrocatalysts could be organized into hierarchical hetero-micro/nanomaterials, such novel structures will be endowed with abundant exposed edges and larger surface area, which offers more active sites for catalytic reactions. Moreover, the advanced hierarchical hetero-catalysts might possess superior electrocatalytic stability due to such hierarchical micro/nanostructures. Also, a self-supported nanostructure fabricated on carbon cloth as a conductive and flexible substrate can facilitate the charge transfer and enhance the electrical conductivity.
Inspired by the above considerations, herein, we successfully prepared hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets on carbon fiber cloth (Ni3Se4@NiFe LDH/CFC) with a simple and scalable thermal treatment method. The as-prepared materials could be used as bifunctional electrocatalysts, which displayed highly efficient catalytic activity for the HER and OER with excellent stability in 1 M KOH. Benefiting from the strong electronic interaction between the NiFe LDH nanosheets and Ni3Se4 microsized sheets, the hierarchical hetero-Ni3Se4@NiFe LDH/CFCs were endowed with enhanced charge transfer and reaction kinetics. Thus, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrocatalysts used as bifunctional electrocatalysts displayed highly efficient HER and OER properties in alkaline medium with a small overpotential of 85 mV and 223 mV at a current density of 10 mA cm−2, respectively. Importantly, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrocatalysts used as a cathode and anode in a two-electrode electrolyzer could drive a current density of 10 mA cm−2 at a cell voltage of 1.54 V at room temperature. In addition, due to the high stability of the hierarchical hetero-structure, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC exhibited superior durability in electrochemical processes over 100 h. This work is indicative of a further promising potential route for constructing efficient bifunctional electrocatalysts for overall water splitting by rational design of heterostructures.
Results and discussion
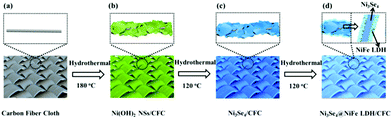
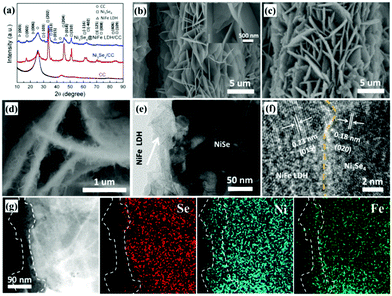
The hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrodes were constructed by a simple, safe, and low-cost three-step hydrothermal method, as illustrated in Fig. 1. This method could avoid the high-temperature annealing process in the presence of protective gas. Importantly, the synthesis steps are easy to scale up, which is beneficial for large-scale practical applications. Fig. S1 (ESI†) displays a digital photograph of the as-prepared samples, showing an apparent color change and uniformity during the synthesis process. The X-ray diffraction (XRD) spectrum of the as-prepared hierarchical hetero-Ni3Se4@NiFe LDH/CFC is shown in Fig. 2a, together with CFC and Ni3Se4 as references, for identifying the phase of the samples. From the pattern of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC, the broader peak located at 25.8° can be assigned to the (002) planes of C, and the four typical peaks at 16.9°, 33.7°, 45.3°, and 50.7° well matched with the (002), (202), (204), and (310) planes of Ni3Se4, respectively (PDF#18-0890). Obviously, the characteristic peaks at 11.7°, 23.2°, 38.7°, and 46.8° coincided very well with the (003), (006), (015), and (018) planes of the NiFe LDH, respectively.1,31 The hierarchical hetero-nanostructures of the samples were revealed by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). The Ni(OH)2 micro-sized sheets were firstly prepared on the surface of CFC by the hydrothermal reaction, as shown in Fig. S2 (ESI†) (the FESEM images, energy dispersive spectrum (EDS), and XRD result). Fig. 2b shows the morphology of the Ni3Se4 micro-sized sheets, which were preserved after the selenylation reaction of the Ni(OH)2 micro-sized sheets. The EDS elemental mapping images of the Ni3Se4 micro-sized sheets show that two elements (Se and Ni) were in a homogeneous distribution with atomic ratios of about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. S3 and S4, ESI†). Compared with the as-prepared Ni3Se4 micro-sized sheets, the hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets exhibited a representative hierarchical hetero-structure with ultrathin NiFe LDH nanosheets grown on the Ni3Se4 micro-sized sheets (Fig. 2c and d). From their EDS, one can find that the peaks from Se, Ni, and Fe were clearly discernible and the ratio of Se
3 (Fig. S3 and S4, ESI†). Compared with the as-prepared Ni3Se4 micro-sized sheets, the hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets exhibited a representative hierarchical hetero-structure with ultrathin NiFe LDH nanosheets grown on the Ni3Se4 micro-sized sheets (Fig. 2c and d). From their EDS, one can find that the peaks from Se, Ni, and Fe were clearly discernible and the ratio of Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe is 53.8
Fe is 53.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32.3
32.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.9, which is similar to the results from ICP-MS (Fig. S5, ESI†). The TEM images were performed to further explore the morphology as well as the interfaces between the NiFe LDH and Ni3Se4 micro-sized sheets. Fig. 2e and Fig. S6 (ESI†) indicate a typical TEM image of the hierarchical hetero-structure, which distinctly exhibits that NiFe LDH vertically grow on the Ni3Se4 micro-sized sheets. As shown in the high-resolution TEM image (HRTEM) of the hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets (Fig. 2f), the interface of the NiFe LDH and the Ni3Se4 exhibited lattice fringes with an interplanar spacing of 0.25 and 0.18 nm, respectively, which can be assigned to the (012) and (020) planes of the NiFe LDH and Ni3Se4, respectively. The EDS mapping images further evidenced the hierarchical hetero-structure, which clearly shows that the element Se is in the central part while both Ni and Fe are uniformly distributed throughout the whole composites (Fig. 2g). For comparison, NiFe LDH/CFC was also prepared by the same hydrothermal reaction procedure. The FESEM image, EDS element mapping image, EDS spectrum, and XRD spectrum show that the uniform NiFe LDH compactly grows on the CFC substrate (Fig. S7, ESI†).
13.9, which is similar to the results from ICP-MS (Fig. S5, ESI†). The TEM images were performed to further explore the morphology as well as the interfaces between the NiFe LDH and Ni3Se4 micro-sized sheets. Fig. 2e and Fig. S6 (ESI†) indicate a typical TEM image of the hierarchical hetero-structure, which distinctly exhibits that NiFe LDH vertically grow on the Ni3Se4 micro-sized sheets. As shown in the high-resolution TEM image (HRTEM) of the hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets (Fig. 2f), the interface of the NiFe LDH and the Ni3Se4 exhibited lattice fringes with an interplanar spacing of 0.25 and 0.18 nm, respectively, which can be assigned to the (012) and (020) planes of the NiFe LDH and Ni3Se4, respectively. The EDS mapping images further evidenced the hierarchical hetero-structure, which clearly shows that the element Se is in the central part while both Ni and Fe are uniformly distributed throughout the whole composites (Fig. 2g). For comparison, NiFe LDH/CFC was also prepared by the same hydrothermal reaction procedure. The FESEM image, EDS element mapping image, EDS spectrum, and XRD spectrum show that the uniform NiFe LDH compactly grows on the CFC substrate (Fig. S7, ESI†).
X-ray photoelectron spectroscopy (XPS) measurements were carried out to evaluate the elemental composition and chemical valence states of the as-prepared hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets. The survey spectra in Fig. S8 (ESI†) confirm the existence of Se, Ni, Fe, and O elements in the hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets. As indicated in Fig. 3a, two peaks centered at 856.5 and 873.2 eV are attributed to the main peaks of oxidized Ni 2p3/2 and Ni 2p1/2, respectively, and two other peaks located at 861.9 and 880.7 eV correspond to the shakeup satellite peaks (Sat.). This indicated the presence of Ni2+ and Ni3+ ions.1,32,33 For the XPS spectra of Fe 2p (Fig. 3b), two typical peaks located at 712.9 and 726.1 eV are assigned to Fe 2p3/2 and Fe 2p1/2, respectively, which can be assigned to Fe3+.34 All these features indicate that the Ni and Fe are present in the form of Ni and Fe oxidation states in NiFe LDH. In addition, the peak fitting of Se 3d shows that the peak at 54.9 eV corresponds to Se 3d from Ni3Se4 (Fig. 3c).35 The peak at 59.2 eV is attributed to the Se-O bonding structure which confirms the surface oxidation of Se species.36 We also compared the Ni peak in Ni3Se4 with that in the hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets. After coupling with the NiFe LDH ultrathin nanosheets, Ni 2p (Ni-Se) can also be observed for the hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets, which confirmed that the NiFe LDH nanosheets did not completely block the XPS signal of the Ni3Se4 micro-sized sheets (Fig. 3d). Moreover, the Ni 2p3/2 peaks shifted 0.22 eV to lower binding energy after coupling with the NiFe LDH ultrathin nanosheets. Compared with Ni 2p in NiFe LDH, the Ni 2p in the hierarchical hetero-Ni3Se4@NiFe LDH shifted 0.13 eV towards a lower binding energy. These results indicated the strong electronic interactions at the interface between the NiFe LDH and the Ni3Se4.31,37 The interaction between the NiFe LDH and Ni3Se4 will be beneficial for the reaction dynamics of both the HER and OER. Besides, the Brunauer–Emmett–Teller (BET) surface areas of the NiFe LDH/CFC, Ni3Se4/CFC, and hierarchical hetero-Ni3Se4@NiFe LDH/CFC were measured from the N2 adsorption and desorption isotherms (Fig. S9, ESI†). The NiFe LDH vertically grew on the Ni3Se4 nanosheets, maintaining the initial two-dimensional structures and forming special hierarchical structures. However, the NiFe LDH was fabricated on CFC, and the structure of LDH was collapsed, resulting in a smaller specific surface area. In this case, the surface area of the Ni3Se4@NiFe LDH/CFC (96.2 m2 g−1) is much larger than that of the NiFe LDH/CFC (10.7 m2 g−1) and Ni3Se4/CFC (11.5 m2 g−1). The higher surface area would provide more active sites and large contact areas for the electrocatalytic reactions.38,39
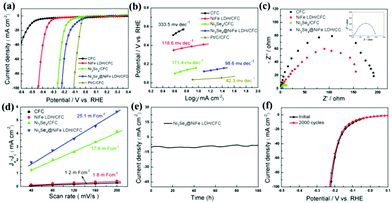
The electrocatalytic HER performance of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC was systematically investigated by using a three-electrode cell system in a 1.0 M KOH solution. In this work, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC with a geometric area of 1 × 1 cm2 directly served as the working electrode. For comparison, bare CFC, NiFe LDH/CFC, Ni3Se4/CFC, and Pt/C/CFC electrodes were also tested under the same conditions. Fig. 4a displays the representative polarization curves of five samples and the as-prepared hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrode exhibited excellent HER activity. As expected, the Pt/C/CFC exhibits the best HER activity and reaches a current density of 10 mA cm−2 at an overpotential of 36 mV. We can find that a very small overpotential of 85 mV can drive the current density of 10 mA cm−2 for the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrode, which is superior to that of CFC (490 mV), NiFe LDH/CFC (339 mV), and Ni3Se4/CFC (167 mV). The HER performance of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC established superior activity, compared with other recently reported HER catalysts in alkaline media (Table S1, ESI†). The remarkable enhancement of the HER activity derived from the strong interaction and efficient synergy between the outer NiFe LDH and inner Ni3Se4 micro-sized sheets. As shown in Fig. 4b, the Tafel slope of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC is 98.6 mV dec−1, smaller than that of CFC (333.5 mV dec−1), NiFe LDH/CFC (118.6 mV dec−1), and Ni3Se4/CFC (171.4 mV dec−1), respectively, which indicates the comparatively favorable catalytic kinetics of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC. Moreover, the Tafel slope value of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC also indicated that the Volmer–Heyrovesky pathway was the prominent rate-limiting step for the HER.40,41 Electrochemical impedance spectroscopy (EIS) measurements were utilized to study the electrode kinetics of the catalysts as shown in Fig. 4c. Herein, the Nyquist plots of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrode showed the diameter of the semicircle, which was much smaller than that of CFC, NiFe LDH/CFC, and Ni3Se4/CFC, indicating lower charge transfer resistance. The low charge transfer resistance reveals desirable electron transport and catalytic kinetics, which improved the activity of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC for water splitting. The double layer capacitances (Cdl) were obtained to investigate the electrochemical surface area (ECSA) of all the samples.13 The ECSA of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC is 25.1 mF cm−2, which is 20.9, 13.9, and 1.4 times that of CFC (1.2 mF cm−2), NiFe LDH/CFC (1.8 mF cm−2), and Ni3Se4/CFC (17.6 mF cm−2), respectively (Fig. 4d and Fig. S10, ESI†). This suggested that the hierarchical hetero-Ni3Se4@NiFe LDH/CFC contained many more catalytically active sites for electrolysis during the HER. Stability is another important criterion of electrocatalysts for practical applications. Fig. 4e indicates that the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrode exhibited excellent stability and showed a negligible decrease (85% current density maintained) even after 100 h of continuous operation (the stability tests in recently reported electrocatalysts were usually less than 20 h). Moreover, upon continuous cyclic voltammetry (CV) scanning for 2000 cycles, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrode shows negligible decays of the polarization curves, showing excellent stability, as illustrated in Fig. 4f.
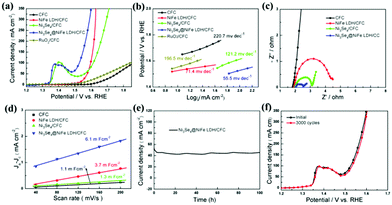
The Ni3Se4@NiFe LDH/CFC electrode was also further systematically explored with respect to the electrocatalytic OER in 1.0 M KOH. As shown in Fig. 5a, the LSV polarization curves of various electrocatalysts, namely, the CFC, NiFe LDH/CFC, Ni3Se4/CFC, hierarchical hetero-Ni3Se4@NiFe LDH/CFC and RuO2/CFC, displayed remarkable differences in the OER activities. Notably, the peaks that appeared around 1.4 V versus reversible hydrogen electrode (RHE) can be probably ascribed to the oxidation of Ni ions in the hierarchical hetero-Ni3Se4@NiFe LDH/CFC.35,42,43 The Ni3Se4@NiFe LDH/CFC electrode affords a current density of 10 mA cm−2 at a low overpotential of 223 mV (acquired from the chronopotentiometry measurement). Even though the current density achieved was 50 and 100 mA cm−2, the corresponding overpotential was only 250 and 290 mV, respectively. For comparison, the CFC (530 and 626 mV), NiFe LDH/CFC (347 and 370 mV), Ni3Se4/CFC (330 and 380 mV), and RuO2/CFC (470 and 610 mV) required much larger overpotential than the hierarchical hetero-Ni3Se4@NiFe LDH/CFC. The excellent OER performance of the Ni3Se4@NiFe LDH/CFC is also better than the recently reported OER catalysts (Table S2, ESI†). Fig. 5b shows the corresponding Tafel plots of different electrocatalysts. The Tafel slope of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC catalyst for the OER was measured to be 55.5 mV dec−1, which is smaller than that of the CFC, NiFe LDH/CFC, Ni3Se4/CFC, and RuO2/CFC catalysts, indicating more favorable reaction kinetics in the OER process. We also recorded Nyquist plots at a potential of 0.5 V, sweeping the frequency from 100 kHz to 0.1 Hz (Fig. 5c). The Nyquist plots obtained by EIS measurement indicate that the hierarchical hetero-Ni3Se4@NiFe LDH/CFC has a lower charge transfer resistance than the other reference catalysts. In addition, the Cdl values of the catalysts, directly proportional to ECSA, suggest that the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrode had more exposed catalytically active sites during the OER, as displayed in Fig. 5d and Fig. S11 (ESI†).44 The stability test of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC catalyst was further performed in O2-saturated 1 M KOH by long-term chronoamperometry and CV scanning for 3000 cycles (Fig. 3e and f). The electrocatalytic activity can maintain 90% of its initial value after 100 h of continuous operation at a current density of 50 mA cm−2 and the LSV curve shows negligible change after 3000 cycles. These results indicate the intrinsic superior OER activity of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC.
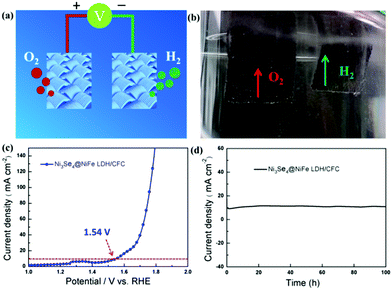
Finally, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC is further utilized as an efficient and robust bifunctional electrocatalyst for overall water splitting in a two-electrode system in 1 M KOH solution. The schematic diagram and digital photograph of the electrolytic cell during operation show the formation of H2 and O2 bubbles on the cathode and anode, respectively (Fig. 6a and b). Remarkably, the electrolyzer delivered a current density of 10 mA cm−2 in 1.0 M KOH at a cell voltage of 1.54 V at room temperature (Fig. 6c), which was superior to those of the recently reported overall water splitting catalysts (Table S3, ESI†). This also confirms that the synergy between NiFe LDH and Ni3Se4 is beneficial for overall water splitting. After 100 h of continuous electrolysis process, the electrolyzer still exhibited high activity for overall water splitting, demonstrating its high stability in the electrochemical processes over long time periods (Fig. 6d). These results further reveal that the as-obtained hierarchical hetero-Ni3Se4@NiFe LDH/CFC is a promising bifunctional electrocatalyst for highly efficient and stable water splitting electrocatalysts.
Importantly, the structure and composition of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC on the anode and cathode sides after the stability test for 100 h were systematically investigated. Displayed in Fig. S12 (ESI†) and the FESEM and EDX mapping images of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC after the OER stability test. We can find that it well retains the initial nanosheet morphology and the EDS mapping images further confirm a homogeneous distribution of the elements. Moreover, the XRD pattern of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrocatalyst after the stability test also matches well with the initial Ni3Se4 and NiFe LDH phase, indicating no obvious phase change in the hierarchical hetero-Ni3Se4@NiFe LDH/CFC after the OER (Fig. S13, ESI†). Fig. S14 (ESI†) shows the FSEM and EDS element mapping images of the catalysts for the cathode (HER) after the stability test. Compared with the initial one, there were also no obvious changes in morphology. In Fig. S15 (ESI†), the XPS spectrum of Ni 2p and Fe 2p of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC after the OER and HER stability test are almost identical to that of the initial sample, manifesting no obvious composition changes of Ni and Fe. These results sufficiently indicated the high structural stability of the hierarchical hetero-Ni3Se4@NiFe LDH.
Based on the abovementioned experimental results, the enhancement in the activity and durability of the hierarchical hetero-Ni3Se4@NiFe LDH/CFC could be attributed to several factors. (1) Strong electronic interactions at the interface between the NiFe LDH nanosheets and the Ni3Se4 micro-sized sheets verified by XPS measurement. (2) The few-layer NiFe LDH nanosheets vertically grow on the Ni3Se4 micro-sized sheets, leaving abundant exposed edges and larger surface area, which offers more active sites for catalytic reactions. (3) The hierarchical hetero-structure of the NiFe LDH and Ni3Se4 are favorable for diffusion of water molecules, ensuring gaseous product release. (4) The high stability of the hierarchical hetero-structure endows Ni3Se4@NiFe LDH with superior durability in electrochemical processes.
Conclusions
In summary, we have successfully engineered a new kind of hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets with a simple and scalable thermal treatment method, which show outstanding features as bifunctional electrocatalysts for overall water splitting. Benefiting from the strong electronic interaction between the NiFe LDH nanosheets and Ni3Se4 micro-sized sheets, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC was endowed with enhanced charge transfer and reaction kinetics. Thus, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC electrocatalyst exhibited excellent HER and OER properties in alkaline medium with a small overpotential of 85 and 223 mV at a current density of 10 mA cm−2, respectively. The two-electrode electrolyzer assembled using the material as a cathode and anode could afford a current density of 10 mA cm−2 at a cell voltage of 1.54 V at room temperature in 1 M KOH. Moreover, the hierarchical hetero-Ni3Se4@NiFe LDH/CFC exhibited high durability in the electrochemical processes over long time periods, due to the good structural stability of the hierarchical hetero-structure. This study will inspire further promising potential routes for the rational design of nanostructures as efficient bifunctional electrocatalysts for overall water splitting.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Science Fund for Distinguished Young Scholars (Grant No. 51825103), the Natural Science Foundation of China (Grant No. 51771188, 51571189), the Major Program of Development Foundation of Hefei Center for Physical Science and Technology (Grant No. 2017FXZY002), and the Anhui Provincial Natural Science Foundation (Grant No. 1708085QE92).References
- L. Yu, H. Zhou, J. Sun, F. Qin, F. Yu, J. Bao, Y. Yu, S. Chen and Z. Ren, Energy Environ. Sci., 2017, 10, 1820–1827 RSC.
- L. Hang, T. Zhang, Y. Sun, D. Men, X. Lyu, Q. Zhang, W. Cai and Y. Li, J. Mater. Chem. A, 2018, 6, 19555–19562 RSC.
- L. Meng, D. Rao, W. Tian, F. Cao, X. Yan and L. Li, Angew. Chem., Int. Ed., 2018, 57, 16882–16887 CrossRef CAS PubMed.
- P. Guo, Q. Ye, X. Y. Yang, J. Zhang, F. Xu, D. Shchukin, B. Wei and H. Wang, J. Mater. Chem. A, 2019, 7, 2497–2506 RSC.
- J. Jian, G. Jiang, R. D. Krol, B. Wei and H. Wang, Nano Energy, 2018, 51, 457–480 CrossRef CAS.
- C. Tang, N. Cheng, Z. Pu, W. Xing and X. Sun, Angew. Chem., Int. Ed., 2015, 54, 9351–9355 CrossRef CAS PubMed.
- X. Liu, W. Liu, M. Ko, M. Park, M. G. Kim, P. Oh, S. Chae, S. Park, A. Casimir, G. Wu and J. Cho, Adv. Funct. Mater., 2015, 25, 5799–5808 CrossRef CAS.
- L. Han, S. Dong and E. Wang, Adv. Mater., 2016, 28, 9266–9291 CrossRef CAS.
- H. Wang, X. Liu, S. Wang and L. Li, Appl. Catal., B, 2018, 222, 209–218 CrossRef CAS.
- R. Wei, Z. Huang, G. Gu, Z. Wang, L. Zeng, Y. Chen and Q. Liu, Appl. Catal., B, 2018, 231, 101–107 CrossRef CAS.
- Y. Sun, K. Xu, Z. Wei, H. Li, T. Zhang, X. Li, W. Cai, J. Ma, H. J. Fan and Y. Li, Adv. Mater., 2018, 30, 1802121 CrossRef PubMed.
- C. Guan, X. Liu, A. M. Elshahawy, H. Zhang, H. Wu, S. J. Pennycook and J. Wang, Nanoscale Horiz., 2017, 2, 342–348 RSC.
- L. Yan, L. Cao, P. Dai, X. Gu, D. Liu, L. Li, Y. Wang and X. Zhao, Adv. Funct. Mater., 2017, 1703455 CrossRef.
- J. Zheng, W. Zhou, T. Liu, S. Liu, C. Wang and L. Guo, Nanoscale, 2017, 9, 4409–4418 RSC.
- C. C. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed.
- T. Maiyalagan, K. A. Jarvis, S. Therese, P. J. Ferreira and A. Manthiram, Nat. Commun., 2014, 5, 3949–3956 CrossRef CAS PubMed.
- J. Tian, Q. Liu, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 9577–9581 CrossRef CAS PubMed.
- W. F. Chen, J. T. Muckerman and E. Fujita, Chem. Commun., 2013, 49, 8896–8909 RSC.
- C. Tan and H. Zhang, Chem. Soc. Rev., 2015, 44, 2713–2731 RSC.
- Y. Yan, L. Thia, B. Y. Xia, X. Ge, Z. Liu, A. Fisher and X. Wang, Adv. Sci., 2015, 2, 1500120 CrossRef PubMed.
- D. Merki, S. Fierro, H. Vrubel and X. Hu, Chem. Sci., 2011, 2, 1262–1267 RSC.
- Y. Yang, K. Zhang, H. Lin, X. Li, H. C. Chan, L. Yang and Q. Gao, ACS Catal., 2017, 7, 2357–2366 CrossRef CAS.
- X. D. Wang, Y. F. Xu, H. S. Rao, W. J. Xu, H. Y. Chen, W. X. Zhang, D. B. Kuang and C. Y. Su, Energy Environ. Sci., 2016, 9, 1468–1475 RSC.
- X. D. Yan, K. X. Li, L. Lyu, F. Song, J. He, D. M. Niu, L. Liu, X. L. Hu and X. B. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 3208–3214 CrossRef CAS PubMed.
- Y. Hou, M. R. Lohe, J. Zhang, S. Liu, X. Zhuang and X. Feng, Energy Environ. Sci., 2016, 9, 478–483 RSC.
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong, S. Liu, X. Zhuang and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 6702–6707 CrossRef CAS PubMed.
- J. Jia, M. Zhai, J. Lv, B. Zhao, H. Du and J. Zhu, ACS Appl. Mater. Interfaces, 2018, 10, 30400–30408 CrossRef CAS PubMed.
- Y. R. Zheng, M. R. Gao, Z. Y. Yu, Q. Gao, H. L. Gao and S. H. Yu, Chem. Sci., 2015, 6, 4594–4598 RSC.
- H. Liang, A. N. Gandi, C. Xia, M. N. Hedhili, D. H. Anjum, U. Schwingenschlögl and H. N. Alshareef, ACS Energy Lett., 2017, 2, 1035–1042 CrossRef.
- L. Chen, Y. Guo, H. Wang, Z. Gu, Y. Zhang, X. Li, H. Wang and C. Duan, J. Mater. Chem. A, 2018, 6, 4636–4641 RSC.
- H. Zhang, X. Li, A. Hähnel, V. Naumann, C. Lin, S. Azimi, S. L. Schweizer, A. W. Maijenburg and R. B. Wehrspohn, Adv. Funct. Mater., 2018, 28, 1706847 CrossRef.
- J. Liu, J. Wang, B. Zhang, Y. Ruan, L. Lv, X. Ji, K. Xu, L. Miao and J. Jiang, ACS Appl. Mater. Interfaces, 2017, 9, 15364–15372 CrossRef CAS.
- J. Wang, F. Chen, Y. Jin, Y. Lei and R. L. Johnston, Adv. Funct. Mater., 2017, 1700260 CrossRef.
- X. Han, C. Yu, J. Yang, C. Zhao, H. Huang, Z. Liu, P. M. Ajayan and J. Qiu, Adv. Mater. Interfaces, 2016, 3, 1500782 CrossRef.
- S. Anantharaj, J. Kennedy and S. Kundu, ACS Appl. Mater. Interfaces, 2017, 9, 8714–8728 CrossRef CAS PubMed.
- X. Xu, F. Song and X. Hu, Nat. Commun., 2016, 7, 12324–12330 CrossRef CAS PubMed.
- Z. Wang, S. Zeng, W. Liu, X. Wang, Q. Li, Z. Zhao and F. Geng, ACS Appl. Mater. Interfaces, 2017, 9, 1488–1495 CrossRef CAS PubMed.
- T. Zhang, Y. Sun, L. Hang, Y. Bai, X. Li, L. Wen, X. Zhang, X. Lyu, W. Cai and Y. Li, ACS Appl. Energy Mater., 2018, 1, 6250–6259 CrossRef.
- Y. Hou, M. R. Lohe, J. Zhang, S. Liu, X. Zhuang and X. Feng, Energy Environ. Sci., 2016, 9, 478–483 RSC.
- H. Jin, J. Wang, D. Su, Z. Wei, Z. Pang and Y. Wang, J. Am. Chem. Soc., 2015, 137, 2688–2694 CrossRef CAS PubMed.
- Y. Pan, K. Sun, S. Liu, X. Cao, K. Wu, W. Cheong, Z. Chen, Y. Wang, Y. Li, Y. Liu, D. Wang, Q. Peng, C. Chen and Y. Li, J. Am. Chem. Soc., 2018, 140, 2610–2618 CrossRef CAS PubMed.
- X. Xu, H. Liang, F. Ming, Z. Qi, Y. Xie and Z. Wang, ACS Catal., 2017, 7, 6394–6399 CrossRef CAS.
- J. Du, Z. Zou, C. Liu and C. Xu, Nanoscale, 2018, 10, 5163–5170 RSC.
- H. Liang, A. N. Gandi, D. H. Anjum, X. Wang, U. Schwingenschlogl and H. N. Alshareef, Nano Lett., 2016, 16, 7718 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00177h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |