Plasmonic catalysis for the Suzuki–Miyaura cross-coupling reaction using palladium nanoflowers
Iyad
Sarhid†
a,
Ibrahim
Abdellah†
 *b,
Cyril
Martini
b,
Vincent
Huc
*b,
Cyril
Martini
b,
Vincent
Huc
 b,
Diana
Dragoe
b,
Patricia
Beaunier
c,
Isabelle
Lampre
b,
Diana
Dragoe
b,
Patricia
Beaunier
c,
Isabelle
Lampre
 a and
Hynd
Remita
a and
Hynd
Remita
 *a
*a
aLaboratoire de Chimie Physique, Univ Paris-Sud UMR 8000 CNRS, Université Paris-Saclay, 91405 Orsay cedex, France. E-mail: hynd.remita@u-psud.fr
bInstitut de Chimie Moléculaire et des Matériaux d’Orsay, Univ Paris-Sud UMR 8182 CNRS, Université Paris-Saclay, 91405 Orsay cedex, France. E-mail: ibrahim_abdellah@hotmail.com
cSorbonne Université, CNRS, Laboratoire de Réactivité de Surface, UMR 7197, F-75005 Paris Cedex 05, France
First published on 18th January 2019
Abstract
Plasmonic catalysis enables the realization of reactions using solar light with less energy and time consumption. Pd nanoflowers synthesized by a radiolytic reduction of PdII(acac)2 in ethanol under a CO atmosphere exhibit a broad plasmon band in the visible–near infrared domain. These plasmonic nanostructures showed remarkably enhanced the photocatalytic activity for Suzuki–Miyaura reactions under visible light irradiation.
Introduction
Palladium nanostructures have attracted a lot of interest in the last few decades because of their applications in catalysis and electrocatalysis, in hydrogen storage, in the field of sensors as well as in nanomedicine.1–7 Pd is used in a large number of industrially important reactions such as hydrogenation of unsaturated organic compounds and a number of C–C coupling reactions. Moreover, Pd nanoparticles (NPs) are efficient catalysts for environmental pollution abatement and automotive emission control.8–10C–C coupling reactions are involved in various organic transformations and polymer syntheses.11–13 C–C bonds forming reactions (such as the Suzuki–Miyaura,14–16 Heck,17–19 and Sonogashira20–22 reactions) are catalyzed by transition metals such as palladium. One of the most important chemical reactions in the pharmaceutical and fine-chemical industries is the Suzuki–Miyaura reaction, a C–C cross-coupling reaction between aryl halides and organoboron compounds, which has evolved to be a very useful method for the production of highly complex molecules.23 In the early 1990s, Reetz et al. reported the development of palladium clusters stabilized by alkylammonium cations or polymers and their catalytic activity toward the Suzuki–Miyaura reaction, giving moderate to good yields of the products.24 A few years later, the group of El-Sayed demonstrated that colloidal Pd nanoparticles were efficient catalysts for the Suzuki–Miyaura reaction in aqueous media, confirming that the reaction took place at the surface of the nanoparticles and that it is easy to separate the nanoparticles from the reaction products due to their solubility in water.25 Palladium-catalyzed C−C coupling reactions involve a thermally activated oxidative addition between a transition metal catalyst and organic substrates. The more electron-rich is the Pd complex, the faster is this oxidative addition. Very recently, some studies have shown that this charge transfer can be activated by light via a photocatalytic process, allowing a number of different organic transformations.26–28 A study of light-activated Suzuki–Miyaura reactions mediated by Pd-nanodots stabilized on MoS2 semiconductor nanosheets led to the observation of very high turnover frequencies, demonstrating the efficiency of the photocatalytic process. Mechanistic studies of this reaction suggested that an efficient electron–hole pair generation occurs in MoS2.29 Pd nanoparticles on semiconducting (2H)WS2 nanosheets, under visible light, have also been shown to catalyze Suzuki–Miyaura reactions at room temperature in protic organic solvents, giving very high yields. Mechanistic studies have shown that the photoexcited electrons produced in the reaction are transferred from the (2H)WS2 nanosheets to the palladium nanoparticles, while the photogenerated holes are transferred to the protic organic solvents, thereby increasing the electron density of the palladium nanoparticles and favoring the oxidative addition of the aryl halide.30 The plasmonic properties of metal nanostructures can also be used to activate or induce catalytic reactions.31 Small spherical Pd nanoparticles are known to exhibit a plasmon absorption band in the UV range. Moreover, it has been shown that Pd nanodiscs exhibit broad, localized surface plasmon resonance bands with a higher sensitivity to the disc ratio compared to Ag nanodiscs.32 Trinh et al. demonstrated direct plasmonic harvesting of visible to NIR light energy using Pd nanoplates (with a well-defined and tunable longitudinal surface plasmon resonance (LSPR) peak) in a Suzuki coupling reaction between iodobenzene and phenylboronic acid.33 The authors found that the catalytic activity of the Pd nanoplates was 2.5 and 2.7 times higher than that of Pd octahedrons and cubes, respectively, under illumination. By calculating the increase in the temperature of the hot electrons in the Pd nanoplates, it has been shown that the enhanced catalytic activity of the Pd nanoplates was mainly a result of the plasmonic photocatalytic effect by the plasmon induced hot electrons. A recent review summarizes the advances in heterogeneous photocatalyzed C–C cross-coupling reactions under visible and near-infrared light irradiation.34
We have synthesized Pd nanoflowers made up of connected nanosheets displaying a broad absorption plasmon band in the visible and near-IR ranges.35,36 We report here for the first time the application of the plasmonic properties of these palladium nanoflowers under visible light irradiation in Suzuki–Miyaura cross-coupling reactions.
Experimental
Materials
All reagents of high purity were obtained from commercial suppliers and used without purification. Potassium carbonate (K2CO3), phenylboronic acid, iodobenzene, 4-nitrobromobenzene, 4-nitroiodobenzene and iodotoluene were purchased from Acros. Ethanol, palladium acetylacetonate (Pd(acac)2), potassium phosphate (K3PO4), cesium carbonate (Cs2CO3), and potassium hydroxide (KOH) were obtained from Aldrich. Carbon monoxide (purity 99.99%) was purchased from Air Liquide.Synthesis of Pd nanoflowers
20 mL of ethanol solution containing Pd(acac)2 (10−3 M) were transferred in a small Pyrex bottle, which was then sealed with a rubber septum. The solution was flushed with flowing argon, and then CO (1 atm) was carefully passed through the sample at a very low flow rate for 15 min. As CO is very toxic, the bubbling was done with caution in a very well ventilated hood with external evacuation. Immediately after CO bubbling, the samples were exposed to gamma irradiation with a 60Co panoramic source for 1 hour at a dose rate of 3 kGy h−1. A dose of 3 kGy is necessary to complete the full reduction of Pd2+ into Pd0. The light yellow solution turned dark blue after irradiation. The radiolytic reduction of PdII(acac)2 at 10−3 mol L−1 in ethanol under a CO atmosphere led to Pd nanostructures.36The Pd nanostructures are stable in ethanol solution for few days in the dark and for few hours under light irradiation.
It has to be noted that PdII(acac)2 solutions are stable under CO for many hours: there is no evolution of the absorption spectrum and black large Pd particles (which precipitate) are formed only after a few days. Indeed, CO reduces Pd(II) very slowly and the direct reduction by CO is negligible at the scale of hours.
Characterization
UV-visible absorption spectra were recorded using a single beam Hewlett-Packard HP 8453 diode array spectrophotometer.Transmission electronic microscopy (TEM) observations were made using a JEOL JEM 100 CXII TEM instrument operated at an accelerating voltage of 100 kV and a JEOL JEM 2011 operated at 200 kV. Prior to the observations, a few droplets of the Pd suspension in ethanol were deposited and dried on carbon-coated copper TEM grids.
XPS analysis was performed using a K Alpha (Thermo Fisher) spectrometer at a base pressure in the low 10−9 mbar range, equipped with a monochromatic source (AlKα = 1486.7 eV). A spot size of 400 μm was used, corresponding to an irradiated area of approximately 1 mm2. A hemispherical analyser was operated at 0° take off angle in the constant analyser energy mode, at a pass energy of 200 eV and a step of 1 eV for the collection of survey scans and a pass energy of 50 eV and a step of 0.1 eV for the collection of narrow scans. Charge compensation was accomplished by means of a “dual beam” flood gun. Drops of a suspension in ethanol were deposited on silica plates and allowed to dry in a load lock prior to transfer into the analysis chamber in order to minimize the contact with air. The recorded XPS spectra were analyzed by means of Avantage software provided by the manufacturer.
Gas chromatography (GC) analyses were performed on a Varian 430-GC gas chromatograph and GC coupled with mass spectrometry (GC-MS) analyses was performed on a Thermo Scientific DSQ apparatus.
Catalytic reactions
A Schlenk tube equipped with a magnetic stirring bar was charged with a haloarene (0.54 mmol), boronic acid (0.6 mmol), a base (1 mmol) and 4 mL of ethanol solution containing 0.43 mg of palladium nanosheets. The flask was evacuated and backfilled with argon three times. The reaction mixture was irradiated under stirring with a 300 W Xe lamp equipped with a water cell filter to absorb the near-IR radiation and a 475 nm cut-off filter to avoid direct photoactivation of the aromatic compounds. The mixture was then filtered and analyzed by gas chromatography (GC) or gas chromatography coupled with mass spectrometry (GC-MS).Results and discussion
Pd nanoflowers as catalysts
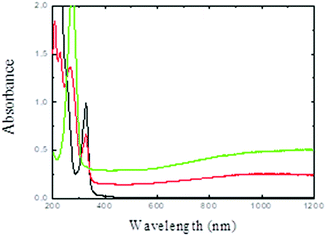
The ethanol solution containing PdII(acac)2 was irradiated under a CO atmosphere. PdII can be reduced by the solvated electrons and ethanol radicals induced by solvent radiolysis as well as by CO− formed by the reaction of CO with the solvated electrons.36,37The initial light-yellow solution turned dark blue after irradiation. Fig. 1 shows the UV-visible absorption spectra of the solution before and after gamma-irradiation. After irradiation, the absorption peak at 326 nm associated to the PdII(acac)2 complex vanishes and a new band appears at 275 nm (associated to free acetylacetone). While small spherical palladium nanoparticles exhibit a plasmon resonance band in the UV range, in our case, a broad plasmon band in the 400–1200 nm region is observed. Pd nanodisks and nanosheets are known to exhibit broad absorption bands in the visible and near infrared region.35,36
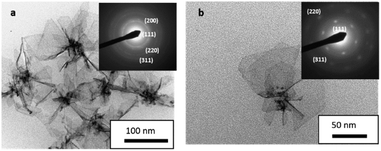
The TEM images show flower like nanostructures (150 nm). These nanostructures synthesized by radiolysis are made up of very thin Pd nanosheets (petals) with truncated hexagonal shapes (30–50 nm sheet dimensions) (Fig. 2). Most of the sheets originate from a seed. These sheets are very thin, so sometimes the borders are rolled. The selected area electron diffraction (SAED) pattern (Fig. 2a inset) can be fully indexed to the cubic system of Pd. An SAED pattern for only one sheet (Fig. 2b inset) clearly shows the good crystallinity. This result indicates the total reduction of the Pd(acac)2 complex. Complete characterization of these nanostructures has been reported previously.35
Previous studies show that CO plays an important role in defining the morphology of nanosheets and nanoflowers and intermediate unstable palladium carbonyl clusters are probably involved in the nucleation/growth process.35,36
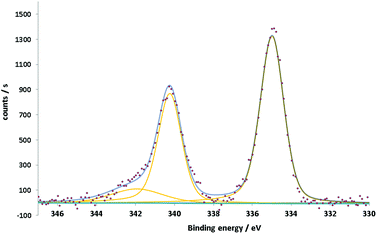
The XPS spectra of the Pd nanoflowers show two main asymmetric peaks at 335 and 340.2 eV (Fig. 3). This double feature, separated by 5.2 eV, is specific to the 3d5/2–3d3/2 spin–orbit splitting of the Pd3d core shell. The component Pd3d3/2 has a more pronounced asymmetry due to the interference coming from the Pd3d5/2 plasmon loss peak at around 342 eV.38,39 The peak positions, aligned following the aliphatic component of C1s set at 284.8 eV, together with their asymmetry and the presence of the plasmon loss peak confirm the metallic nature of Pd.
Catalytic studies
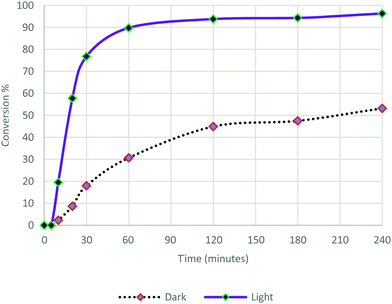
These Pd nanoflowers exhibiting absorption plasmon in the visible range in ethanol solutions were used as catalysts in the Suzuki–Miyaura cross coupling reaction between iodobenzene and phenylboronic acid using Cs2CO3 as the base (Scheme 1). The reaction was performed under an argon atmosphere, at room temperature, either in the dark or under visible light irradiation (λ > 475 nm). The formation of biphenyl was monitored by GC over a period of 240 min (Fig. 4). | ||
| Scheme 1 Reaction between iodobenzene (1 a) and phenyl boric acid (1 b) catalyzed by Pd nanoflowers. | ||
After 5 min latency, light irradiation induces an increase both in the reaction rate and in the conversion. The kinetics of the reaction was much faster and the conversion rate observed for this reaction was dramatically higher under irradiation compared to that carried out in the dark. After 20 min of reaction, 57% conversion was observed under irradiation, and only 9% in the dark. The reaction continued to proceed quickly under light illumination to reach 90% conversion at 60 min and 96% after 240 min, while the reaction in the dark proceeded slowly up to 53% conversion at 240 min. These results confirm that photocatalysis is indeed taking place. The Pd nanoflowers are absorbed in the visible region as shown in Fig. 1. Plasmon resonance excitation can induce heat and hot electrons can be induced on the surface of the nanostructures. These two effects may also be reinforced by the formation of “hot spots”, where the electric fields are concentrated.
This is likely to accelerate the initial oxidative addition reactions and the subsequent steps of the Suzuki–Miyaura reaction as well, (i.e. the transmetallation and the reductive elimination reactions).
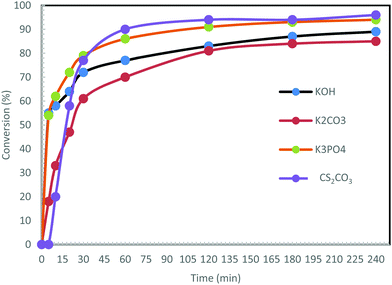
Under similar conditions to those described above, the reaction was carried out using other bases: K3PO4, KOH and K2CO3 (Fig. 5).
In contrast to Cs2CO3, no induction time at the beginning of the reaction is observed upon using the other bases. The use of KOH and K3PO4 initiated the reaction very quickly. Over 54% conversion was noted after 5 min. The reaction with K2CO3 was the slowest (only 47% conversion after 15 min), and the least efficient with a maximum conversion of 85%. The reactions led to maximum conversions of 89% with KOH and 94% with K3PO4 after 240 min. To conclude, the best bases for this reaction were found to be Cs2CO3 and K3PO4.
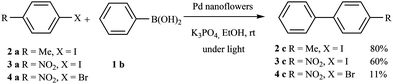
Finally, in order to confirm that the reaction is a true cross-coupling reaction and not a simple homocoupling reaction, other halogenated compounds were employed, such as 4-iodotoluene, 1-iodo-4-nitrobenzene and 1-bromo-4-nitrobenzene. The reactions were carried out under the same conditions, with light irradiation for 240 min using K3PO4 as the base (Scheme 2).
In these reactions, only cross-coupling products were observed. The conversion was very good (80%) in the case of 4-iodotoluene, while, surprisingly, the conversion was lower with the highly activated 1-iodo-4-nitrobenzene (60% conversion), and very low with 1-bromo-4-nitrobenzene (11% conversion). Irradiation of the plasmonic Pd nanostructures induces hot electrons and holes, therefore the Pd nanostructures are charged. So, the adsorption of more polar organic halides is favored, which facilitates the first step of the reaction (the oxidative addition of the aryl halide). This can explain the difference in the reactivity with 4-iodotoluene, 1-iodo-4-nitrobenzene and 1-bromo-4-nitrobenzene.
Wang et al. reported plasmonic Suzuki–Miyaura coupling reactions under light activation (using a laser and under solar irradiation) with Au–Pd nanostructures made up of PdNPs on the surface of Au nanorods.40 They demonstrated that light was absorbed by the Au cores and the PdNPs promoted the Suzuki coupling reactions. Au–Pd nanoalloys supported on ZrO2 tested for the Suzuki–Miyaura coupling reaction under visible-light illumination gave high yields of the target products, because the activation energy of Suzuki–Miyaura coupling was reduced.41 In this reported study, gold plasmon was used to activate the reaction as the Au–Pd spherical nanoparticles are absorbed in the visible range because of Au plasmon. Light absorption due to the LSPR of the gold-based nanoparticles played an important role in enhancing the catalyst performance. The enhancement of the photocatalytic activity was mainly attributed to the hot electrons rather than a thermal effect.41 Pd NP decorated Au nanorods (NRs) were also used for the Suzuki–Miyaura coupling reaction under light illumination, the excitation of the plasmon of the Au NRs being used to activate the catalytic reaction.42
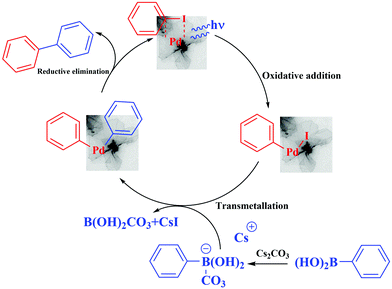
The activation effect of the Suzuki–Miyaura cross-coupling catalyzed by the palladium nanosheets can be explained according to the literature.29,41 The irradiation of the palladium nanosheets generate “hot” electrons, then the electron-rich palladium nanosheets undergo oxidative addition with the aryl halide, followed by transmetallation of in situ formed aryl borate (the boronic acid is transformed after the reaction with base into a borate anion, which facilitates the transmetallation). Also, it has been shown that the photogenerated holes in photocatalysts such as g-C3N4 or [Ru(pby)3]2+ can activate arylboronic acids by cleaving the C–B bonds or transforming the C–B bonds into C–O bonds in the presence of O2 and H2O.43,44 Finally, reductive elimination gave the biphenyl desired products, which can be facilitated by the “hot” holes. Plasmon excitation also induces heat. The local enhanced temperature on the Pd nanosheets can also be partly responsible for the increase in the reaction kinetics (Fig. 6).
Pd nanoflowers after catalysis
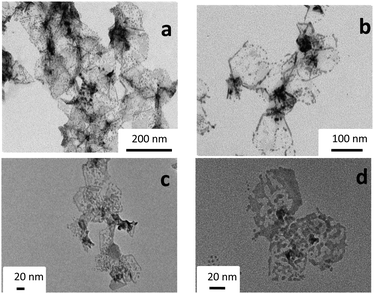
The TEM images of the Pd catalysts after 30 minutes of reaction under illumination show the evolution of the Pd nanostructures: the nanoflowers and nanosheets are damaged. The cores and the borders resist damage, but gradually the thinner parts are destroyed to form small Pd nanoparticles (around 4–5 nm) assembled in hexagonal shapes (Fig. 7a and b). The small spherical Pd nanoparticles exhibit a plasmon in the UV region and no absorption in the visible range. After 3 h of catalytic reaction under visible irradiation, no further major change in the shape was observed: the small Pd nanoparticles and the fragmented sheets are observed in the TEM images (Fig. 7c and d). Surprisingly, the hexagonal shapes are still observed after 3 h reaction. These Pd nanosheets are probably partly covered by the reaction components during the catalytic reaction, which can contribute in maintaining the initial shape (but in the same time decrease the contrast of part of the nanosheets in TEM observations). This coverage by the reaction components could also contribute to catalyst poisoning.Careful analysis of the conversion rates (see Fig. 4 and 5) shows that the reaction kinetics are fast until about 30 min, and then slow down. This behavior can be due to the transformation of the Pd nanosheets into small nanoparticles, which cannot be activated under visible light compared to the Pd nanosheets. Oxidation/reduction of Pd during catalytic cycles can lead to some dissolution or leaching of the Pd during the reaction.
This plasmonic catalysis leads to leaching and reshaping of the Pd nanosheets into more thermodynamically stable spherical nanoparticles (see Fig. 7). A recent review by Eremin and Ananikov discusses the transformations of molecular catalysts, leaching, aggregation and various interconversions of metal complexes, clusters and nanoparticles that occur during catalytic processes.45 In the Suzuki–Miyaura cross coupling reactions, a wide range of different Pd species was found in the reaction mixture containing complexes with small and bulky NHC-ligands. Small clusters were detected by MS techniques, and nanoparticles were observed in the precipitate using transmission electron microscopy. There is still a debate in the literature about the question of the true active species in these reactions. It is supposed that very small Pd clusters are more efficient for Suzuki reactions.46–49 The first step of the Suzuki–Miyaura catalytic cycle corresponds to the addition of a halogen, which induces the oxidation of Pd. This oxidation probably leads to partial dissolution (or leaching) of palladium, and this can explain the partial reshaping of the Pd nanoflowers.
Conclusions
Plasmonic catalysis enables the realization of reactions using solar light with less energy and time consumption. Palladium nanosheets exhibit a broad plasmon absorption band in the visible–near infrared domain. The catalytic activity of the Pd nanosheets for Suzuki–Miyaura reactions was remarkably enhanced under visible light irradiation. The reaction was carried out using iodobenzene, phenylboronic acid or Cs2CO3 as the base, in the dark and under visible light irradiation. Indeed, the results showed that the conversion under light irradiation was 96% versus 53% in the dark. Our findings can be explained by the plasmonic effect, which increases the reactivity of the palladium nanostructures. Plasmon resonance excitation induces heat and hot electrons are induced on the Pd surface. To optimize the reaction conditions, different bases were used under light irradiation, and the kinetics of the reactions showed that Cs2CO3 and K3PO4 were efficient bases for this reaction in the presence of palladium nanosheets. Finally, different haloarenes were subjected to the optimized conditions. The highest conversion was obtained with iodotoluene (80%) and surprisingly highly activated 1-iodo- or 1-bromo-4-nitrobenzene led to lower conversions. Calculations and molecular simulations will be performed to estimate the heat effect induced by plasmon excitation and its contribution to the reaction kinetics. These Pd nanostructures show partial dissolution of Pd and morphological changes during the Suzuki–Miyaura catalytic reaction, and light irradiation induces an enhancement of the kinetics, but could also accelerate Pd dissolution. To enhance the stability of the nanostructures for catalytic applications and increase their turnover frequencies, we will try in future work to support these nanosheets or to modify them with carbides for example. These Pd nanoflowers with a high surface area could potentially be employed as plasmonic catalysts in other C–C forming reactions, such as Sonogashira, Heck, and Stille reactions.Studies are still needed to better understand plasmonic palladium-photocatalyzed organic reactions from fundamental and mechanistic points of view in order to design more effective photocatalysts for a larger number of organic reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the C’Nano IdF and the DIM NanoK for financial support.References
- F. Ksar, G. K. Sharma, F. Audonnet, P. Beaunier and H. Remita, Nanotechnology, 2011, 22, 305609 CrossRef CAS PubMed
.
- T. Redjala, H. Remita, G. Apostolescu, M. Mostafavi, C. Thomazeau and D. Uzio, Gas and Oil: Science and Technology, 2006, 61, 789–797 CrossRef CAS
.
- F. Ksar, G. Surendran, L. Ramos, B. Keita, L. Nadjo, E. Prouzet, P. Beaunier, A. Hagège, F. Audonnet and H. Remita, Chem. Mater., 2009, 21, 1612–1617 CrossRef CAS
.
- G. Surendran, F. Ksar, L. Ramos, B. Keita, L. Nadjo, E. Prouzet, P. Beaunier, P. Dieudonné, F. Audonnet and H. Remita, J. Phys. Chem. C, 2008, 112, 10740–10744 CrossRef CAS
.
- S. Ghosh, H. Remita, P. Kar, S. Choudhury, S. Sardar, P. Beaunier, P. S. Roy, S. K. Bhattacharya and S. K. Pal, J. Mater. Chem. A, 2015, 3, 9517–9527 RSC
.
- S. Ghosh, A.-L. Teillout, D. Floresyona, P. De Oliveira, A. Hagège and H. Remita, Int. J. Hydrogen Energy, 2015, 40, 4951–4959 CrossRef CAS
.
- A. Dumas and P. Couvreur, Chem. Sci., 2015, 6, 2153–2157 RSC
.
- D. Astruc, Inorg. Chem., 2007, 46, 1884–1894 CrossRef CAS PubMed
.
- B. Karimi, S. Abedi, J. H. Clark and V. Budarin, Angew. Chem., Int. Ed., 2006, 45, 4776–4779 CrossRef CAS
.
- M. S. Kwon, N. Kim, C. M. Park, J. S. Lee, K. Y. Kang and J. Park, Org. Lett., 2005, 7, 1077–1079 CrossRef CAS
.
- N. G. Schmidt, E. Eger and W. Kroutil, ACS Catal., 2016, 6, 4286–4311 CrossRef CAS PubMed
.
- N. Corrigan, S. Shanmugam, J. T. Xu and C. Boyer, Chem. Soc. Rev., 2016, 45, 6165–6212 RSC
.
- I. Hussain, J. Capricho and M. A. Yawer, Adv. Synth. Catal., 2016, 358, 3907 CrossRef CAS
.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS
.
- A. Suzuki, J. Organomet. Chem., 1999, 576, 147–168 CrossRef CAS
.
- F. S. Han, Chem. Soc. Rev., 2013, 42, 5270–5298 RSC
.
- A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed., 1994, 33, 2379–2411 CrossRef
.
- C. Amatore and A. Jutand, Acc. Chem. Res., 2000, 33, 314–321 CrossRef CAS PubMed
.
- V. Farina, Adv. Synth. Catal., 2004, 346, 1553–1582 CrossRef CAS
.
- K. Sonogashira, J. Organomet. Chem., 2002, 653, 46–49 CrossRef CAS
.
- Y. Liang, Y. X. Xie and J. H. Li, J. Org. Chem., 2006, 71, 379–381 CrossRef CAS PubMed
.
- R. Chinchilla and C. Najera, Chem. Rev., 2007, 107, 874–922 CrossRef CAS PubMed
.
- I. Maluenda and O. Navarro, Molecules, 2015, 20, 7528–7551 CrossRef CAS PubMed
.
- M. T. Reetz, R. Breinbauer and K. Wanninger, Tetrahedron Lett., 1996, 37, 4499–4502 CrossRef CAS
.
- Y. Li, X. M. Hong, D. M. Collard and M. A. El-Sayed, Org. Lett., 2000, 2, 2385–2388 CrossRef CAS PubMed
.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed
.
- K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS PubMed
.
- M. D. Karkas, J. A. Porco and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683–9747 CrossRef CAS PubMed
.
- H. H. Shin, E. Kang, H. Park, T. Han, C. H. Lee and D. K. Lim, J. Mater. Chem. A, 2017, 5, 24965–24971 RSC
.
- F. Raza, D. B. Yim, J. H. Park, H.-I. Kim, S.-J. Jeon and J.-H. Kim, J. Am. Chem. Soc., 2017, 139, 14767–14774 CrossRef CAS PubMed
.
- S. Linic, U. Aslam, C. Boerigter and M. Morabito, Nat. Mater., 2015, 14, 567–576 CrossRef CAS PubMed
.
- C. Langhammer, Z. Yuan, I. Zoric and B. Kasemo, Nano Lett., 2006, 6, 833–838 CrossRef CAS PubMed
.
- T. T. Trinh, R. Sato, M. Sakamoto, Y. Fujiyoshi, M. Haruta, H. Kurata and T. Teranishi, Nanoscale, 2015, 7, 12435–12444 RSC
.
- Q. Gu, Q. Jia, J. Long and Z. Gao, ChemCatChem, 2019, 11, 669–683 CrossRef CAS
.
- P. F. Siril, L. Ramos, P. Beaunier, P. Archirel, A. Etcheberry and H. Remita, Chem. Mater., 2009, 21, 5170–5175 CrossRef CAS
.
- T. Redjala, G. Apostolescu, P. Beaunier, M. Mostafavi, A. Etcheberry, D. Uzio, T. Thomazeau and H. Remita, New J. Chem., 2008, 32, 1403–1408 RSC
.
- R. Derai, H. Remita and M. O. Delcourt, Radiat. Phys. Chem., 1991, 38, 483–486 CrossRef CAS
.
- M. C. Militello and S. J. Simko, Surf. Sci. Spectra, 1994, 3, 387–394 CrossRef CAS
.
- L. S. Kibis, A. I. Titkov, A. I. Stadnichenko, S. V. Koscheev and A. I. Boronin, Appl. Surf. Sci., 2009, 255, 9248–9254 CrossRef CAS
.
- F. Wang, C. H. Li, H. J. Chen, R. N. Jiang, L. D. Sun, Q. Li, J. F. Wang, J. C. Yu and C. H. Yan, J. Am. Chem. Soc., 2013, 135, 5588–5601 CrossRef CAS PubMed
.
- S. Sarina, H. Zhu, E. Jaatinen, Q. Xiao, H. Liu, J. Jia and J. Zhao, J. Am. Chem. Soc., 2013, 135, 5793–5801 CrossRef CAS
.
- X.-H. Li, M. Baar, S. Blechert and M. Antonietti, Sci. Rep., 2013, 3, 1743 CrossRef
.
- F. Wang, C. Li, H. Chen, R. Jiang, L.-D. Sun, Q. Li, J. Wang, J. C. Yu and C.-H. Yan, J. Am. Chem. Soc., 2013, 135, 5588–5601 CrossRef CAS
.
- Y.-Q. Zou, J.-R. Chen, X.-P. Liu, L.-Q. Lu, R. L. Davis, K. A. Jørgensen and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 784–788 CrossRef CAS PubMed
.
- D. B. Eremin and V. P. Ananikov, Coord. Chem. Rev., 2017, 346, 2–19 CrossRef CAS
.
- J. Lemo, K. Heuze and D. Astruc, Inorg. Chem. Acta., 2006, 359, 4909–4911 CrossRef CAS
.
- A. K. Diallo, C. Ornelas, L. Salmon, J. R. Aranzaes and D. Astruc, Angew. Chem., Int. Ed., 2007, 46, 8644–8648 CrossRef CAS PubMed
.
- C. Ornelas, A. K. Diallo, J. Ruiz and D. Astruc, Adv. Synth. Catal., 2009, 351, 2147–2154 CrossRef CAS
.
- C. Deraedt and D. Astruc, Acc. Chem. Res., 2014, 53, 8445–8449 CAS
.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |