Advances in portable electrospinning devices for in situ delivery of personalized wound care
Xu
Yan
 *abc,
Miao
Yu
cd,
Seeram
Ramakrishna
*abc,
Miao
Yu
cd,
Seeram
Ramakrishna
 de,
Stephen J.
Russell
*f and
Yun-Ze
Long
de,
Stephen J.
Russell
*f and
Yun-Ze
Long
 *abcd
*abcd
aIndustrial Research Institute of Nonwovens & Technical Textiles, College of Textiles & Clothing, Qingdao University, Qingdao 266071, China. E-mail: yanxu-925@163.com; yunze.long@163.com
bCollaborative Innovation Center for Eco-Textiles of Shandong Province, Qingdao University, Qingdao 266071, China
cCollaborative Innovation Center for Nanomaterials & Devices, College of Physics, Qingdao University, Qingdao 266071, China
dQingdao Junada Technology Co. Ltd, Qingdao International Academician Park, Qingdao 266199, China
eCenter for Nanofibers & Nanotechnology, Faculty of Engineering, National University of Singapore, Singapore
fSchool of Design, University of Leeds, UK. E-mail: S.J.Russell@leeds.ac.uk
First published on 9th May 2019
Abstract
Electrospinning and electrospun fibrous assemblies have attracted interest in a variety of biomedical fields including woundcare, tissue engineering and drug delivery, due to the large surface-area-to-volume ratio and high porosity of nanofibrous webs. Normally, wound dressings are manufactured well before the point of care, and then packaged and distributed for use at a later stage. More recently, in situ electrospinning of fibers directly onto wound sites has been proposed as a route to personalized wound dressing manufacture, tailored to the needs of individual patients. Practically, in situ deposition of nanofibers on to a wound could be envisaged using a portable or hand-held electrospinning device that is safe and easy to operate. This review focuses on recent advances in portable electrospinning technology and potential applications in woundcare and regenerative medicine. The main research challenges and future trends are also considered.
Introduction
Electrospinning and electrospun fibre assemblies are the subject of intensive research in multiple fields such as energy and the environment,1–4 healthcare5–12 and nanodevice development13–18 due in part to the large surface-area-to-volume ratio and high porosity of the as-spun fiber webs. In biomedical applications such as wound care, the nanoscale dimensions of the fibres contribute not only to the physical protection of wound sites from sources of infection, but also provide an environment for soft tissue regeneration by modulating gaseous exchange and promoting hemostasis whilst avoiding scar induction.8,11 The potential to mimic collagen fibrils in native extracellular matrix and human organs has also been widely reported.8–12Electrospun fibers can be produced from a multitude of natural and synthetic materials using a variety of electrospinning methods.5–12 Classical applications in tissue engineering have involved the production of an electrospun web, followed by culture in vitro prior to implantation.9,10 To aid in the control and reproducibility of dressings applied directly to wounds, portable devices have been developed and applied to understand the dynamics of fibre deposition.9,10In situ deposition of nanofibers directly on to wounds has been proposed to improve conformance to the wound bed, reduce pain and enable customization of the dressing according to individual patient needs.8,19 As the technology continues to develop, potential for highly portable, hand-held electrospinning devices suitable for advanced, personalized wound care has emerged.
Fundamentals of electrospinning and setups
Electrospinning is a well-known fiber-producing technique with origins dating back to 1934.20 Intensive global research interest in electrospinning for biomedical applications can be traced back to the rapid development of nanotechnology from the 1990s onwards.21–38 In brief, the process of electrospinning involves stretching a polymer solution (or melt) in an electrostatic field, and the formation of fibres as a volatile solvent is removed (or as the melt cools). Fibers are then deposited on various types of collector to form a nonwoven web, or can be assembled directly into other formats such as yarns.21–26 A typical electrospinning setup comprises three basic elements: a high voltage (HV) power supply, a spinneret and a grounded metal collector, as shown in Fig. 1a.27–38 In a typical electrospinning process, the polymer solution is first fed through a spinneret and a HV is applied to form the well-known Taylor cone39 (Fig. 1b) due to the interactions of the electrical charges in the polymer solution. Once the repulsive force within the charged solution is larger than its surface tension, a jet erupts from the tip of the Taylor cone. Generally, the jet is straight near the tip of the spinneret, but soon converts in to a whipping motion due to electrically-driven bending instability, as described in Fig. 1b.27–38 As the solvent evaporates, electrospun fibres are most commonly deposited as a web on the collector.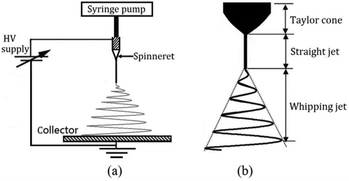 | ||
| Fig. 1 (a) Schematic of a typical electrospinning setup. (b) Illustration of instability and jet formation in the electrospinning process. Reproduced with permission from ref. 36, copyright from Taylor & Francis 2016. | ||
The randomly oriented arrangement of fibres in the collected web typically produced by a simple electrospinning setup such as that shown in Fig. 1 can limit potential applications.38 Consequently, it is necessary to control various aspects of the process, to achieve the required orientation and morphology of fibres in the web.16,27–38,40–56 Practically, this involves modifications to the spinneret and design of collectors, as summarised in various references.27–38 For example, to produce aligned networks of electrospun fibres a range of strategies can be employed, such as the use of rotating drums40,41 exterior frames,42 parallel electrodes43,44 and ring collectors.45 Patterned collectors can also be used to produce complex fibrous geometries46–48 and fixed tip as well as centrifugal spinnerets are capable of forming producing oriented nanofiber assemblies.52–54 Such modifications on simple electrospinning setups are amongst the methods that can be employed to customize the structure and properties of electrospun webs, extending the potential applications of the technology across various branches of regenerative medicine.38 However, in the case of portable, hand-held electrospinning equipment, modifications that rely on changes to the collector are not practicable, because the collector is the biological wound site.
Fundamentals of portable electrospinning devices
In addition to a biological collector, the versatility of portable electrospinning devices arises from a number of modified components including the spinneret and HV supply system. The degree of portability of each device varies depending on the nature of the modifications. This encompasses hand-held spinnerets connected to a conventional power supply system,57–64 use of a miniature high voltage power supply with a converter64–74 and use of alternative power supplies such as batteries or generators.65–80 The advantages and disadvantages of the various devices are listed in Table 1.| Type of portable device | Advantages | Disadvantages |
|---|---|---|
| Hand-held spinnerets | Flexibility in use; in situ spinning; higher voltage; accuracy of voltage and flow rate; safety in use; precise deposition. | Mains electricity dependent; heavy to transport; expensive. |
| Battery powered | Portability (small, light, handheld); flexibility in use; in situ spinning; safety in use; precise deposition; affordable. | Limited high voltage; limited by capacity of batteries |
| Generator powered | Sufficient power supply; portability (small, light); flexibility in use; in situ spinning; safety in use; affordable. | Limited, fixed and unstable high voltage; limited flow rate; uncontrollable deposition. |
The portability of these electrospinning systems, combined with their compatibility with the polymer systems found in existing dressings is advantageous in terms of the development of personalized wound care. Portable electrospinning devices can be potentially used for various wound types, including incised skin of variable depth,57 irregular grazed skin wounds,57 burned skin wounds,67,68 liver excised wounds59,61 and dural repairs.62 Moreover, in situ spinning and precise deposition provides opportunities to manage wound sites quickly, promoting wound healing.57–76 The safety in use partly depends on lower electrical current during processing (several milliampere) and easily adjustable operating distances between the spinneret and the wound site (∼2–12 cm). Furthermore, the small size, light-weight and the ability to avoid dependency on a mains power supply, make it possible for these devices to work both indoors and outdoors, which may be useful in emergency situations or remote locations.70,71,75,78 Recent advances in portable electrospinning device technology and their in situ applications in wound care will now be discussed.
Hand-held spinneret electrospinning devices and their in situ applications
To achieve in situ electrospinning, Edirisinghe et al. proposed a portable electrospinning device incorporating a portable handheld spinneret as shown in Fig. 2a.57 The device comprised a spray gun (Fig. 2b1), fitted with a single needle or two coaxial needles (Fig. 2b2). Poly(lactic-co-glycolic) acid (PLGA) particles (Fig. 2c1) and fibers (Fig. 2c2) as well as PLGA/Polymethysilsesquioxane (PMSQ) core–shell fibers (Fig. 2c3) were fabricated using this device at different angles and deposited on to a confined target area.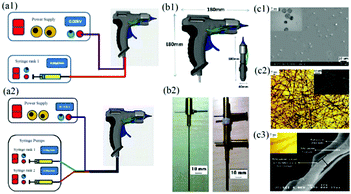 | ||
| Fig. 2 Schematic of a portable electrospinning setup with (a1) one or (a2) two syringes. (b1) The handheld spinneret was designed in the form of a spray gun shape, in which (b2) a single and multi-coaxial needle was assembled to produce (c1) particles, (c2) fibrous mats and (c3) coaxial fibers. Reproduced with permission from ref. 57, copyright from Elsevier B.V. 2012. | ||
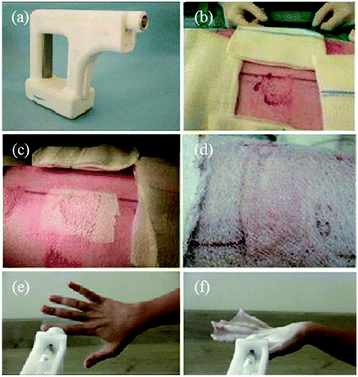
Subsequently, the portable electrospinning device was employed for in situ deposition of bio-products during the wound healing process.58 As shown in Fig. 3a, the portable apparatus provided a convenient means of depositing PLGA fibers onto a target area, such as incised wound, or a grazed wound surface (Fig. 3b–d). Moreover, fibers could be sprayed onto a burn wound site (Fig. 3e) within 300 s to form a thin protective film on the surface due to the electrostatic attraction forces.58 When the hand was flexed, the film could be easily removed (Fig. 3f).
 | ||
| Fig. 3 (a) Schematic of a portable electrospinning setup for in situ application of a wound dressing, including (b, c) the incised wound with edges and (d) a graze wound, (e) in situ electrospinning of a fiber-based film on to a burned hand and (f) removal of the film.58 Copyright from ICE Publishing 2013. | ||
Similarly, Long et al. designed an airflow-directed in situ electrospinning setup for rapid hemostasis.59 As can be seen in Fig. 4a, the in situ electrospinning device mainly consisted of four elements: a high voltage power supply, a syringe pump, an air pump, and a home-made coaxial spinneret. The air pump was connected to the coaxial spinneret to assist electrospinning and control the fiber deposition range, as illustrated in Fig. 4b. Recently, a conical aluminum auxiliary electrode has also been used to aid rapid and precise fiber deposition.60 Moreover, an airflow can also be applied to clean wound sites before in situ electrospinning commences. Using this apparatus, medical adhesive, 2-octyl cyanoacrylate (OCA) was electrospun into fibers and deposited into the wound,59,61 as shown in Fig. 4c.
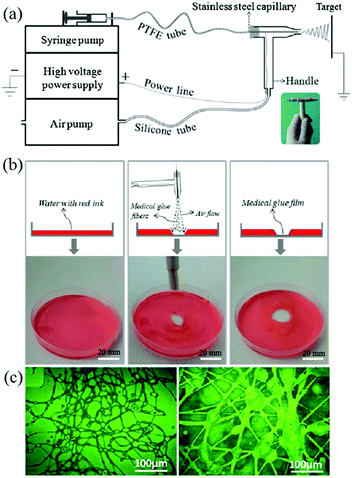 | ||
| Fig. 4 (a) Schematic illustration of the airflow-directed in situ electrospinning device with the home-made portable spinneret (inset). (b) Using this device, precise deposition of fibers consisting of medical adhesive (OCA) was achieved. (c) Optical images of OCA fibers fabricated by this device.59 Copyright from the Royal Society of Chemistry 2014. | ||
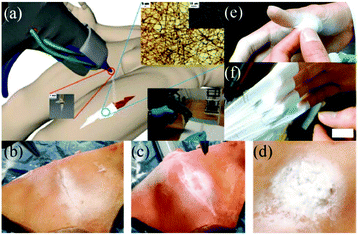
In vitro experiments using this device were carried out by electrospinning OCA fibers directly onto fresh pig liver and lung tissues, with a view to healing applications in small wounds and cuts.59 Moreover, an in vivo hemostasis experiment with this portable device in a pig model was also performed, (Fig. 5). As illustrated in Fig. 5a, the experimental procedure involves a series of steps. Firstly, the “diseased” liver tissue was removed, and then the wound surface was cleaned. OCA fibers were then immediately electrospun directly onto the wound for rapid hemostasis. The results of in vivo hemostasis experiments on pig liver are illustrated in Fig. 5b–h. It was noted that during this liver resection procedure, no other hemostats or sealants were used, and the process only took about 20 s. This in vivo hemostasis procedure has been successfully carried out in pigs more than twenty times, suggesting that a reliable approach could be developed. Furthermore, in comparing in situ precision electrospinning and traditional spraying of OCA medical adhesive for in vivo hemostasis, the former has advantages in terms of precise fibre deposition, high efficiency and low dosage.61 Subsequently, the same team explored in situ electrospinning of OCA to dural repair, as shown in Fig. 5i and j,62 further extending potential applications for airflow-directed in situ electrospinning devices.
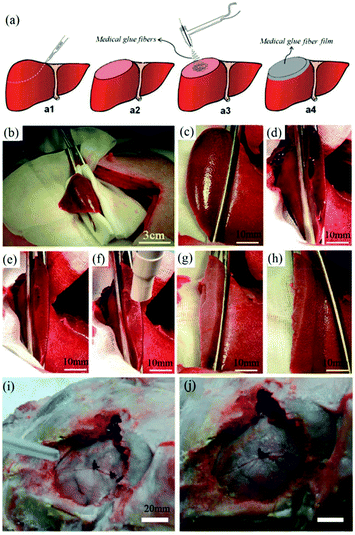 | ||
| Fig. 5 (a) Schematic illustration of the in vivo rapid hemostasis experimental procedure. The following are time-lapse images for pig liver wound hemostasis: (b) Firstly, a section of liver was exposed; (c) then the diseased tissue was fixed by hemostatic forceps; (d) the fixed liver was resected, (e) the site was cleaned, (f) the wound was further cleaned by airflow from the device, (g) the OCA adhesive fibers were electrospun in situ for 20 s onto the wound, (h) no blood or bile seeping was observed after removing the hemostatic forceps.59 Copyright from the Royal Society of Chemistry 2014. (i) The in situ electrospinning device was also used for dural repair, in which OCA adhesive fibers were precisely electrospun onto the dural defect. (j) The dural defect was apparently repaired by the OCA fibrous membrane.62 Copyright from Dove Medical Press 2016. | ||
More recently, home-made electrospinning apparatuses with portable spinnerets have been replaced by commercially produced devices.63 These commercially produced handheld electrospinning guns comprise both a gun-shaped spinneret (Fig. 6a) and an airflow duct. The electrospinning jets are therefore bound by the airflow as shown in Fig. 6b, such that electrospun fibers are precisely deposited onto the target area. The portability and controllable deposition of fibres from this device have further increased the potential of in situ electrospinning for applications in wound healing (Fig. 6c and d).
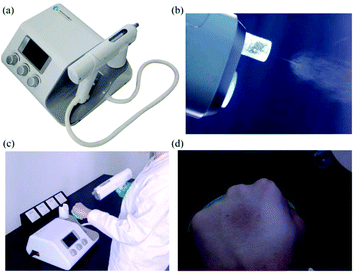 | ||
| Fig. 6 (a) Image of the electrospinning gun from Qingdao Junada Technology Co., Ltd. (b) Fibres being directed towards the target site. (c) Use of the handheld gun. (d) Direct deposition of fibres onto a human hand.63 | ||
While the electrospinning devices discussed so far are portable, only the spinneret component is handheld, with the rest of unit being too large and heavy to be conveniently held in the hand. To address this limitation, as well as the associated high cost of the equipment, Brako et al. proposed an inexpensive, portable electrospinning device,64 as shown in Fig. 7(a). This comprises both a miniaturised high precision microsyringe pump (Micrel mph+) and a miniature HV power supply, capable of generating up to 33 kV at 10 W (EMCO 4330+). By fully integrating the spinneret and the power supply unit in to a handheld unit, the whole apparatus becomes lighter in weight and smaller, such that it can be easily deployed in anywhere with mains power, Fig. 7b. Using this device, cellulose acetate (CA), and CA doped with silver nanoparticles have been successfully electrospun into fibers directly onto simulated wound sites, as shown in Fig. 7c–e. Such highly portable devices may suggest a route for the greater practical use of functional nanofibers in personalized advanced wound care.64
 | ||
| Fig. 7 Schematic diagram of the portable EHD device (a), and a photograph showing the assembly of the mini device handheld unit (b). (c–e) Snapshots of real-time recording of the device during deposition of fibres on to a simulated wound site.64 Copyright from the Authors. | ||
Battery operated portable electrospinning devices and their applications
Early in situ electrospinning devices of the type discussed so far have a degree of portability in that they consist of handheld spinnerets, but this is compromised by a heavy power supply and the need for a mains electrical connection. Several attempts have been made to dispense the limitation of the mains electrical connection, which has given rise to battery powered devices.Battery-operated handheld electrospinning apparatus have been reported for the spinning of biodegradable polymers directly onto soft tissue injuries to form a fibrous dressing in situ, as shown in Fig. 8a.27 Smith and Reneker also proposed a battery-powered device with a reservoir to provide a spinning solution, and described a process in which a fiber web is directly electrospun onto the affected skin areas.65 Greiner and collaborators from the University of Marburg disclosed a handheld electrospinning equipment which could be used for the direct deposition of nanofibers onto wounds Fig. 8b.32 It was reported that in such a device, a high voltage could be supplied by standard batteries and with a modular construction it was possible to spin from different polymer solutions, such that a variety of polymer carriers and drugs could be applied, depending on the type of wound.32 A device based on a similar principle was also proposed by University of Singapore, as shown in Fig. 8c.66 However, few technical details have been revealed about these battery-operated devices, or follow on research dealing with practical applications.
 | ||
| Fig. 8 Examples of battery-operated handheld electrospinning apparatus. (a) Reprint from Fig. 20 in ref. 27. Copyright from Elsevier 2003. (b) Handheld electrospinning device for wound dressing application. Inset: PEO fibers electrospun from aqueous solution onto a human hand.32 Copyright from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2007 (c) Portable electrospinning apparatus reported by Singaporean. | ||
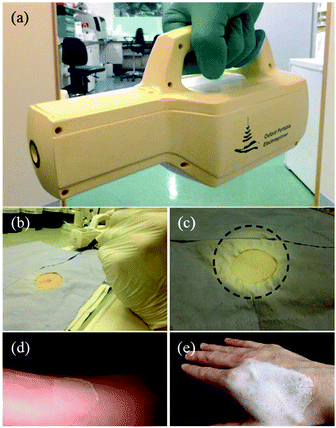
Recently, Josef Haik et al. studied the feasibility of a portable electrospinning device for production of directly applied wound dressings.67 A new portable handheld device manufactured by Nicast Ltd, Lod, Israel (Fig. 9a) was employed to electrospin biocompatible medical grade polyester, polycarbonate, and polyurethane polymers directly onto a wound site for in vivo studies, (Fig. 9b–d), and macroscopic evaluations on the donor site wound were performed at days 2, 7, and 14 in respect of adherence, wound exudate, presence of eschar, adverse skin reaction, wound closure, ease of dressing removal and time to complete healing.67 Only one minute was required for the creation of a dressing of a desirable and workable thickness, (http://www.liebertpub.com/wound and Fig. 9e–f). It was claimed the handheld device was convenient to use and effective in producing in situ nanofibrous dressings, applied at a distance from the wound, with the potential to reduce infection and cross-contamination rates. Moreover, this device is now sold and renamed as SpinCare™ System which including a hand-held device and sterile, pre-filled SpinKit™ solution syringe.68
 | ||
| Fig. 9 (a) Image of a handheld electrospinning device manufactured by Nicast Ltd., Lod, Israel. (b) Wound site on a pig model, and (c) electrospun fiber dressing applied to the wound. (d) After 14 days, the dressing was removed. (e) Fibers directly electrospun onto a human hand using the same device, and (f) the resulting wound dressing.67 Copyright from Mary Ann Liebert, Inc. 2017. | ||
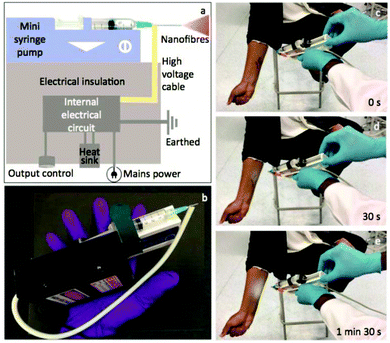
Long et al. designed a simple handheld battery-operated electrospinning apparatus (BOEA) involving a finger-pressed syringe as shown in Fig. 10.69,70 As described in Fig. 10a, the high-voltage supply in this device relied on batteries and a high-voltage converter. The positive electrode of the converter was connected to the needle on the syringe, while the negative electrode connected to a conductive metal foil which could be used to operate the device. It was reported that the charge could be transferred through the body (hand) by touching the metal foil to avoid charge accumulation. For practical application of the BOEA, two AAA batteries (3 V) provided a high voltage up to 10 kV, and the device was capable of continuous operation for more than 15 h with a negligible current and with an effective working distance in the range of 2 to 10 cm. Moreover, the combination of a battery and a high-voltage converter ensured the device was light-weight (about 120 g) and of small overall dimensions (5 cm in length, 3 cm in thickness and 10.5 cm in height), as illustrated in Fig. 10b, enabling a high degree of portability.70 The performance of the BOEA for solution electrospinning was also examined (Fig. 10c), and various polymers including polyvinyl pyrrolidone (PVP), polyvinylidene fluoride (PVDF) and polycaprolactone (PCL) (Fig. 10d–f) were successfully electrospun into fibers with diameters of the region of hundreds of nanometers.
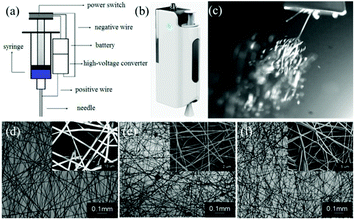 | ||
| Fig. 10 (a) Schematic diagram of a battery-operated portable handheld electrospinning apparatus.69 (b) Image of the device commercially available in China.63 (c) Electrospinning jets from the device captured by a high-speed camera, and resulting electrospun fiber morphologies: (d) PVP, (e) PVDF, and (f) PCL.70 Copyright from the Royal Society of Chemistry 2015. | ||
The application of this device for in situ electrospinning and the effects on wound healing have been examined.71 Owing to the portability of the BOEA, direct deposition of personalized nanofibrous dressings for wound healing has been demonstrated in Fig. 11a and b, where conformation to a three-dimensional target size can be readily accomplished. The BOEA device can be conveniently operated using one hand, and within a few minutes, a sufficiently robust web can be laid down to occlude the wound site from potential vectors of infection. The electrospun fibrous membrane showed good flexibility and compactness, and could be modulated by adjusting the deposition time, for example. An in vivo animal study was conducted using PCL fibers loaded with Ag-decorated mesoporous silica nanoparticles (Ag-MSNs) electrospun using the BOEA device onto wistar rats (Fig. 11c) to evaluate antimicrobial activity and biological efficacy. The in vitro and in vivo results confirmed the antimicrobial activity and bioavailability of the 5% of Ag-MSNs/PCL electrospun fibers, and antibacterial function was demonstrated against two predominant pathogenic bacteria responsible for wound infections, Staphylococcus aureusandEscherichia coli. Furthermore, in vivo studies demonstrated improved in situ deposition of nanofibers and wound healing compared to the control groups. After four weeks of post-treatment, it was possible to observe significant wound closure and complete re-epithelialization (Fig. 11c). Using the same device, PVP/iodine and PVP/PVP-Iodine complex (PVPI) were also successfully electrospun into fibers and deposited in situ to produce a personalized antibacterial wound dressing.81 Moreover, the possibility of using this apparatus for other applications such as filtration was proposed.82–84
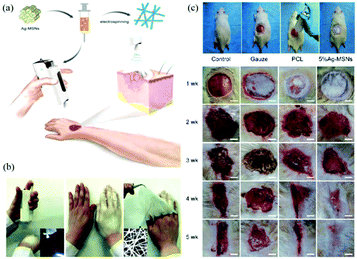 | ||
| Fig. 11 (a) Schematic illustration of in situ production of personalised wound dressings using the BOEA device, (b) directly electrospinning of a dressing using the BOEA in 2 minutes, (c) in situ electrospinning of PCL/Ag-MSNs fibrous dressings for in vivo wound healing on wistar rats.71 Reproduced from ref. 71 with permission from The Royal Society of Chemistry 2016. | ||
More recently, Long et al. considered the use of the BOEA for direct electrospinning of the medical adhesive, N-octyl-2-cyanoacrylate (NOCA) to make fibrous media suitable for soft tissue hemostasis, as illustrated in Fig. 12,72 which relates to liver hemostasis. In this particular application, it is important to control tissue adhesion after surgery, which can require precise deposition of the hemostatic medical adhesive.72 To achieve such precise deposition, a metal cone was attached to the spinning nozzle, as presented in Fig. 12a. It was found that the deposition range of the electrospun fibers could be adjusted by changing the size of the metal cone (see Fig. 12b). This modified BOEA was further used in the deposition of NOCA fibers onto the resection site of rat liver to realize rapid hemostasis within 10 s, as shown in Fig. 12c. Postoperative pathological results indicated reduced inflammatory response and tissue adhesion in this method compared with that of the airflow-assisted group. This suggested potential for the modified BOEA to be developed for emergency medical procedures, or for community patient care or home care situations, where the portability and ease of operation would be particularly advantageous. However, the voltage in this device was fixed, limiting the degree to which the physical properties dressings and dimensions of dressings produced in situ could be modified, and the spinning solution delivery depended on the pressure applied by the fingers to the trigger, which could vary during use, leading to inconsistent fibre deposition.
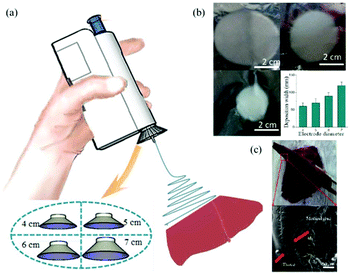 | ||
| Fig. 12 (a) Schematic diagram of the electric field-modified electrospinning NOCA fibers produced for liver resection hemostasis, (b) the size of deposition area as a function of the metallic cone diameter, (c) NOCA medical adhesive-based fibers were deposited on to the surface of the liver tissue using this electric-field assisted electrospinning device for hemostasis.72 Reproduced from ref. 72 with permission from The Authors. | ||
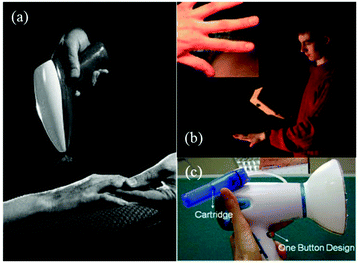
Ye et al. proposed a novel portable electrospinning apparatus, in which both the voltage and flow rate can be adjusted via a microcontroller over a range of values suitable for most electrospinning and electrospraying applications.73 This portable electrospinning device contained the three major components as shown in Fig. 13: (1) a high voltage converter; (2) a linear actuator and (3) a battery integrated in to the device to minimize its overall size (Fig. 13a). The total weight of the device was 1.11 kg. To generate the high voltage, a converter (model F101, EMCO High Voltage Corporation, US) was selected and combined with a pack of ten rechargeable NiMH AA batteries, which provided a voltage ranging from 0 to 14 kV. In addition, a T-NA linear actuator (model TNA08A25-S) in combination with the MAX-232 was used to form a syringe pump, which could provide a wide choice of flow rates between 0 and 10 mL h−1. As shown in Fig. 13b, cartridges were located in a separate compartment to allow safe and easy replacement, and the modified syringes are shown in Fig. 13c. Electrospinning can be performed directly from the cartridge (Fig. 13d) or with a pen extension to aid precision of fibre deposition on to the wound site (Fig. 13e). The pen extension was made of a 40 cm long Teflon tube ending with a pen-shaped casing. The ability of this device to produce submicron fibers and particles from several polymers was also examined.73 Using this device, PCL was electrospun into fiber webs direct from both the cartridge (Fig. 13f) and pen extension (Fig. 13g). Moreover, PLGA micro particles could be sprayed by this device (Fig. 13h). Several other polymers such as poly(p-dioxanone) (PDO), poly(3-hydroxybutyrate) (PHB), poly(vinyl acohol) (PVA), poly(ethylene oxide) (PEO) and poly(vinyl butyral) (PVB) could also be electrospun into fibers using this apparatus.73,74 Collectively, these results suggested that this portable electrospinning apparatus offered advantages in terms of cost, transportability and flexibility of use.
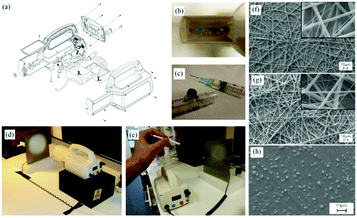 | ||
| Fig. 13 A battery-operated portable electrospinning device. (a) Exploded CAD view of the device assembly (b) separated compartment for removable cartridges, accessible via a removable lid and (c) cartridges. Fibers are electrospun directly from the device (d) or via a pen extension (e) connected to the device. Morphologies of PCL fibre webs electrospun using the device (f) the cartridge and (g) the pen extension. (h) Morphologies of PLGA micro particles produced using the device.73 Copyright from the Author(s) 2014. | ||
The possibility of in situ electrospinning directly onto skin was also demonstrated to highlight the potential of the apparatus for wound healing applications.73,74 Firstly, one can easily lift this device which underlines its portability (Fig. 14a). The results of in situ electrospinning experiments on both pig and human skin tissues have also been presented (Fig. 14b–e). Particularly by using the pen extension device, fibers could be precisely deposited onto a remarked area by the use of a mask (Fig. 14b and c). Moreover, with this apparatus, fibers can also be deposited in situ onto a human arm and hand for wound healing.
 | ||
| Fig. 14 (a) The portable electrospinning apparatus easily lifted by one hand. (b) In situ electrospinning of PDO fibers on to a pig skin defect using the portable apparatus and the pen extension, and (c) the skin defect fully covered by an electrospun fibrous dressing. Using this apparatus, PEO fibrous dressings could be directly electrospun onto a human (d) arm and (e) hand.73 | ||
Generator operated portable electrospinning devices and their applications
Despite good portability, battery life is one of the limitations of handheld devices. To achieve uninterrupted operation, Han et al. proposed a self-powered electrospinning setup based on a hand-operated Wimshurst generator, as shown in Fig. 15a and b.75 In contrast to battery-operated electrospinning devices, the high voltage supply is replaced by a Wimshurst generator capable of voltages of nearly 15 kV due to the electrostatic induction. As illustrated in Fig. 15a, during electrospinning, by turning the handle in the clockwise direction, positive and negative charges can be generated on different regions of the discs and stored in the two Leyden jars separately. High voltage can be generated between the needle and collector, and thus stable electrospinning jets can be detected using a high speed camera, (Fig. 15c). Several polymer solutions such as polystyrene (PS), PVDF, PCL and polylactic acid (PLA) have been electrospun into fiber webs using this method (Fig. 15d) as well as 3D structures containing a stack of fibres (Fig. 15e). Moreover, such self-powered electrospinning apparatus have been proposed for in situ electrospinning of wound dressings, especially for situations where there is no electricity supply, (Fig. 15f).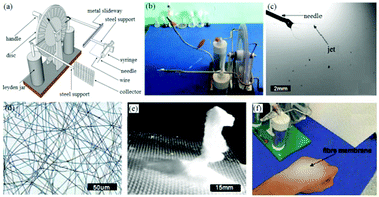 | ||
| Fig. 15 (a) Schematic diagram of a self-powered electrospinning apparatus based on a Wimshurst generator, (b) photograph of the device, (c) electrospinning using this device recorded by a high-speed camera, (d) optical microscope image of an electrospun PS fiber web and (e) 3D stacked structure of PS fibers. (f) In situ electrospinning of fibers directly onto the skin.75 Reproduced from ref. 75 with permission from The Royal Society of Chemistry 2015. | ||
Moreover, by replacing the needle and adding a heating gun, this self-powered apparatus could be used as a portable melt electrospinning setup, as shown in Fig. 16a and b.76 It was noted that this new apparatus could avoid the electrical interference that can be present in conventional melt electrospinning setups, and was more portable. Using this device, polymers such as PLA (Fig. 16c) and PCL (Fig. 16d) melts could be electrospun into fibers with diameters of about 20 μm. The absence of residual solvent in melt-electrospun fibers makes these fibers more suitable for direct, in situ application as wound dressings because of the reduced potential for toxicity (Fig. 16e–f). It was determined that the temperature of the melt-electrospun fibers on contact with the porcine liver tissue ranged from 33.6 to 24.2 °C (PCL fibers) as the spinning distance was increased from 8 to 10 cm, which is close to human body temperature. In addition, the adhesion of the PCL fibers to the liver tissue was measured at about 1.0–1.2 N.
 | ||
| Fig. 16 (a) Schematic diagram and (b) modified self-powered melt electrospinning apparatus, and the use of this device for the electrospinning of (c) PCL and (d) PLA fibers. (e) The in situ electrospinning of fibers directly onto porcine tissue (liver), characterized by (f) a long incision wound and (g) melt electrospun PCL fibers occluding the wound to provide a dressing.76 Reproduced from ref. 76 with permission from The Royal Society of Chemistry 2015. | ||
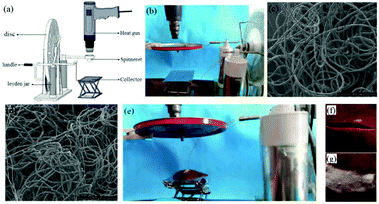
More recently, another self-powered electrospinning setup has been developed consisting of a rotating-disk triboelectric nanogenerator (R-TENG), a voltage doubling rectifying circuit (VDRC) and a simple spinneret, as shown in Fig. 17a.77 The energy for this device comes from the R-TENG, which forms the central component in the whole device. The R-TENG's design is shown in Fig. 17b–d. The working mechanism is based on triboelectrification and electrostatic induction85–88 and is capable of generating an alternating voltage up to 1400 V, and through the VDRC, a maximum constant direct voltage of 8.0 kV can be obtained, suitable for electrospinning. The performance of this self-powered electrospinning apparatus has been studied under different conditions, as indicated in Fig. 17e–g. Various polymers such as polyethylene terephthalate (PET), polyamide-6 (PA6), polyacrylonitrile (PAN), PVDF and thermoplastic polyurethanes (TPU) have been successfully electrospun into fibers as shown Fig. 17h–m, respectively.77 Given that this is a self-powered electrospinning system, potential applications can be contemplated where woundcare may need to take place in remote locations without any access to an electrical power supply.
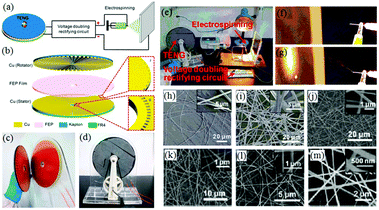 | ||
| Fig. 17 Structural design and performance of a self-powered electrospinning setup. (a) Schematic illustration of the self-powered electrospinning system. (b) Structural design of the R-TENG. (c) Photograph of the rotator and stator of the R-TENG. (d) Photograph of the handcranked R-TENG. (e) Photograph of the self-powered electrospinning apparatus. (f and g) High-speed photographs of the electrospinning process. SEM images of (h and i) the PET nanofibers at a magnification of 6× and 22×, and (j–m) PA6, PAN, PVDF, TPU nanofibers, respectively. Insets: Enlarged views of electrospun nanofibers.77 Copyright from American Chemical Society 2017. | ||
Although such self-powered electrospinning devices require no direct electrical supply or battery, the high voltage provided by the Wimshurst generator or R-TENG may not be very stable. To address this challenge, a solar cell hand operated portable electrospinning (SHPE) apparatus was introduced by Yan et al., as shown in Fig. 18a.78 The power supply of the SHPE apparatus included a solar cell, a hand generator, a set of rechargeable batteries and a high-voltage converter as displayed in Fig. 18b. This power supply relies on photoelectric and electromagnetic induction, as indicated in Fig. 18c. Moreover, the generated electricity can be stored in the rechargeable batteries and then translated into a high voltage (∼3.8 kV) through the high-voltage converter, which ensures a stable high voltage for solution or melt electrospinning at a fixed distance. An image of the SHPE apparatus and its performance for solution electrospinning is given in Fig. 18d and e, where the electrospinning jets can be clearly observed. Various polymers, including PLA (Fig. 18f), PS (Fig. 18g), PVP (Fig. 18h), PLGA (Fig. 18i) have been electrospun into fibers using this device.
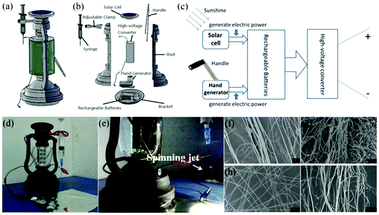 | ||
| Fig. 18 (a–c) Design of the SHPE device (a) 3D schematic representation, (b) exploded image of the SHPE device assembly and (c) schematic diagram of the power supply. (d) Image of the device, and (e) electrospinning performance using this device for solution electrospinning. (f–i) SEM images of as-spun fibers consisting of PLA, PS, PVP and PLGA, respectively.78 Reproduced from ref. 78 with permission from The Royal Society of Chemistry 2016. | ||
The SHPE apparatus may have potential applications for wound dressings, similar to previously reported portable devices.78 As suggested in Fig. 19a and b, the SHPE device allows in situ electrospinning of PLGA fibers directly onto the skin, even when outdoors, and the as-spun fibers form a thin fibrous membrane over the surface, like a second layer of skin, due to the electrostatic attraction forces. As illustrated in Fig. 19c, the as-spun PLGA fibrous membrane was flexible, compact and could be easily peeled away from the surface when required. These results demonstrate the potential for direct electrospinning of membranes directly on to injured tissues, outdoors, using a highly portable device with a stable power supply provided by a solar cell and a hand generator.
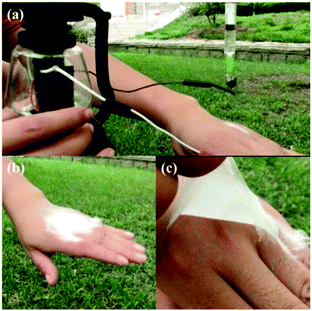 | ||
| Fig. 19 Optical images of the electrospinning PLGA fibers directly onto a hand using the SHPE device, outdoors. (a) The SHPE device can be operated with one hand. (b) A PLGA fibrous membrane was directly deposited onto a human hand. (c) The electrospun PLGA fibrous membrane exhibited high flexibility and compactness.78 Reproduced from ref. 78 with permission from The Royal Society of Chemistry 2016. | ||
In addition to solution electrospinning, the SHPE device can also be used for melt electrospinning by changing the spinneret into a conical metal nozzle, in which polymer particles could be heated into a melt by a heat gun or an alcohol lamp, as shown in Fig. 20a–c.78,79 Melt electrospinning jets can then be formed during the electrospinning process (Fig. 20c). Materials such as PLA, PCL and polyurethane (PU) have been melt-electrospun into microfibers using the SHPE device, (Fig. 20d–f). For outdoor use in remote locations, the heating system could take the form of an alcohol lamp, candle or a lighter.79
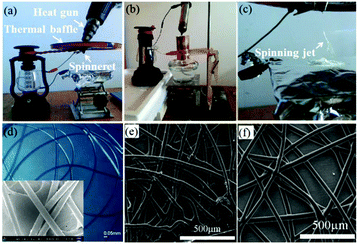 | ||
| Fig. 20 Optical images of the SHPE device for melt electrospinning with (a) heating gun and (b) alcohol lamp as the heating system,78,79 (c) melt electrospinning using the device, and SEM images of the as-spun (d) PLA, (e) PCL and (f) PU fibers. Reproduced from ref. 78 and 79 with permission from The Royal Society of Chemistry 2016, 2017. | ||
Generator-operated portable electrospinning apparatus are self-powered and can achieve stable performance during electrospinning, however, they do have the drawback of being larger than battery-operated devices. To achieve a smaller volume and weight, Duan et al. proposed a simple electrospinning setup using a piezoelectric (PZT) ceramic generator (Fig. 21a) to replace the high voltage supply system, facilitating the development of significantly smaller device (volume 5 × 1 × 1 cm3 and weight <10 g).80 A schematic image and photograph of this device are included in Fig. 21b and c. Using the PZT generator, a high pulsed voltage of about 56 kV was generated, sufficient to enable an electrospinning process. Using this apparatus, several kinds of polymer solutions such as PVP (Fig. 21d), PVDF (Fig. 21e) and PS were successfully electrospun into ultrathin fibers, confirming the feasibility of this apparatus. However, since a pulsed voltage is generated by the PZT, the electrospinning process cannot run continuously, and this device can only be used for demonstration purposes. Even so, this approach has established a fundamentally new approach to the design of ultra-portable electrospinning apparatus.
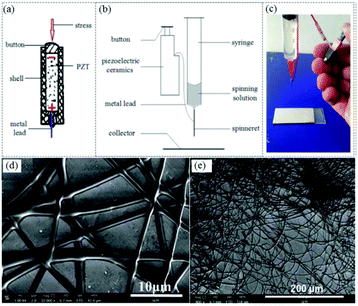 | ||
| Fig. 21 (a) Schematic of a PZT, (b) schematic diagram of the PZT-operated electrospinning apparatus, and (c) photograph of the prototype portable electrospinning apparatus. SEM images of electrospun (d) PVP and (e) PVDF fibers prepared by this device.80 Reproduced from ref. 80 with permission from The Royal Society of Chemistry 2016. | ||
Conclusion and future trends
Portable electrospinning devices are attracting interest from the scientific and medical communities as a potential means to generate a variety of personalized wound dressings at the point-of-care. In the past few years, significant advances have been made in terms of portable electrospinning devices, powered by either batteries or on-board electrical generators, and performance and portability have been systematically improved. Amongst the most important developments are the ability to control the deposition area of the electrospun fibres onto lesion sites and the ability to generate dressings in situ within minutes.Recent advances in portable electrospinning apparatus have mainly focused on the technical design rather than the materials utilized. Although different polymers have been electrospun into fibers using such portable devices, not all have been suitable for clinical translation except for medical adhesive. The in situ deposition processes have in many cases been suitable for demonstration rather than practical application. Moreover, most of these devices have relied upon solution electrospinning, which poses the risk of residual solvent being present in the fibres, potentially comprising wound healing. For improved in situ electrospinning, the use of toxic solvents in the formation of the fibres needs to be avoided, they should be based on appropriate polymer systems and there should be excellent conformation to the wound bed. Several advances in the evolving field of portable electrospinning apparatus can be foreseen in relation to wound healing, namely:
(i) Evaluation of a variety of functional materials suitable for portable electrospinning devices and direct deposition in to wound sites, including antimicrobials and growth factors. This may be combined with modified fibre structures resulting from the use of special spinnerets such as coaxial needles or solid needles to deliver core/shell and aligned structures.
(ii) Development of portable melt electrospinning apparatus to avoid solvent toxicity effects. Although several portable melt electrospinning devices have been reported, heating systems have been independent of the main device, compromising portability and convenience. Portable melt electrospinning devices with integrated heating and high-voltage supply systems, could provide a promising solution. In this way, electrostatic interference between the heating and high-voltage systems could be avoided.
(iii) Portable electrospinning devices present considerable opportunities for in situ electrospinning and direct deposition of nanofibers on to wound sites, independent of the wound size or depth. To enable further development towards personalized wound dressing devices, produced in situ at the bedside, further pre-clinical evaluation and in vivo experimentation is required.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51703102, 51373082 and 51673103), the Shandong Provincial Natural Science Foundation, China (ZR2016EMB09), and the Shandong Provincial Key Research and Development Plan (2016GGX102011).References
- X. F. Lu, C. Wang, F. Favier and N. Pinna, Adv. Energy Mater., 2017, 7, 1601301 CrossRef.
- S. Dharani, H. K. Mulmudi, N. Yantara, P. T. Trang, N. G. Park, M. Graetzel, S. Mhaisalkar, N. Mathews and P. P. Boix, Nanoscale, 2014, 6, 1675–1679 RSC.
- N. Wang, Y. Yang, S. S. Al-Deyab, M. El-Newehy, J. Y. Yu and B. Ding, J. Mater. Chem. A, 2015, 3, 23946–23954 RSC.
- S. Chen, G. S. Liu, H. W. He, C. F. Zhou, X. Yan and J. C. Zhang, Adv. Condens. Matter Phys., 2019, 2019, 6179456 Search PubMed.
- X. M. Sun, Q. Lang, H. B. Zhang, L. Y. Cheng, Y. Zhang, G. Q. Pan, X. Zhao, H. L. Yang, Y. G. Zhang, H. A. Santos and W. G. Cui, Adv. Funct. Mater., 2017, 27, 1604617 CrossRef.
- Y. Ding, W. Li, F. Zhang, Z. Liu, N. Z. Ezazi, D. Liu and H. A. Santos, Adv. Funct. Mater., 2019, 29, 1802852 CrossRef.
- J. Zhang, S. Li, D. D. Ju, X. Li, J. C. Zhang, X. Yan, Y. Z. Long and F. Song, Chem. Eng. J., 2018, 349, 554–561 CrossRef CAS.
- P. Zahedi, I. Rezaeian, S. O. Ranaei-Siadat, S. H. Jafari and P. Supaphol, Polym. Adv. Technol., 2010, 21, 77–95 CAS.
- R. F. Pereira, C. C. Barrias, P. L. Granja and P. J. Bartolo, Nanomedicine, 2013, 8, 603–621 CrossRef CAS PubMed.
- J. R. Dias, P. L. Granja and P. J. Bártolo, Prog. Mater. Sci., 2016, 84, 314–334 CrossRef.
- E. Mele, J. Mater. Chem. B, 2016, 4, 4801–4812 RSC.
- S. X. Chen, B. Liu, M. A. Carlson, A. F. Gombart, D. A. Reilly and J. W. Xie, Nanomedicine, 2017, 12, 1335–1352 CrossRef CAS PubMed.
- M. H. You, X. Yan, J. Zhang, X. X. Wang, X. X. He, M. Yu, X. Ning and Y. Z. Long, Nanoscale Res. Lett., 2017, 12, 360 CrossRef PubMed.
- B. Ding, M. Wang, X. F. Wang, J. Y. Yu and G. Sun, Mater. Today, 2010, 13, 16–27 CrossRef CAS.
- H. D. Zhang, X. Yan, Z. H. Zhang, G. F. Yu, W. P. Han, J. C. Zhang and Y. Z. Long, Int. J. Polym. Sci., 2016, 3021353 Search PubMed.
- B. Sun, Y. Z. Long, Z. J. Chen, S. L. Liu, H. D. Zhang, J. C. Zhang and W. P. Han, J. Mater. Chem. C, 2014, 2, 1209–1219 RSC.
- Y. Z. Long, M. Yu, B. Sun, C. Z. Gu and Z. Y. Fan, Chem. Soc. Rev., 2012, 41, 4560–4580 RSC.
- M. N. Liu, X. Yan, M. H. You, J. Fu, G. D. Nie, M. Yu, X. Ning, Y. Wan and Y. Z. Long, J. Appl. Polym. Sci., 2018, 135, 46342 CrossRef.
- B. Balakrishnan, M. Mohanty, P. R. Umashankar and A. Jayakrishnan, Biomaterials, 2005, 26, 6335–6342 CrossRef CAS PubMed.
- A. Formhals, Process and apparatus for preparing artificial threads, US Patent No. 1975504, 1934.
- J. Doshi and D. H. Reneker, J. Electrost., 1995, 35, 151–160 CrossRef CAS.
- D. H. Reneker and I. Chun, Nanotechnology, 1996, 7, 216 CrossRef CAS.
- X. Fang and D. H. Reneker, J. Macromol. Sci., Part B: Phys., 1997, 36, 169–173 CrossRef.
- H. Fong, I. Chun and D. H. Reneker, Polymer, 1999, 40, 4585–4592 CrossRef CAS.
- D. H. Reneker, A. L. Yarin, H. Fong and S. Koombhongse, J. Appl. Phys., 2000, 87, 4531–4547 CrossRef CAS.
- H. Fong, W. Liu, C. S. Wang and R. A. Vaia, Polymer, 2002, 43, 775–780 CrossRef CAS.
- Z. M. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, Compos. Sci. Technol., 2003, 63, 2223–2253 CrossRef CAS.
- D. Li and Y. Xia, Adv. Mater., 2004, 16, 1151–1170 CrossRef CAS.
- I. S. Chronakis, J. Mater. Process. Technol., 2005, 167, 283–293 CrossRef CAS.
- W. E. Teo and S. Ramakrishna, Nanotechnology, 2006, 17, R89 CrossRef CAS PubMed.
- S. Park, K. Park, H. Yoon, J. Son, T. Min and G. Kim, Polym. Int., 2007, 56, 1361–1366 CrossRef CAS.
- A. Greiner and J. H. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670–5703 CrossRef CAS PubMed.
- N. Bhardwaj and S. C. Kundu, Biotechnol. Adv., 2010, 28, 325–347 CrossRef CAS PubMed.
- B. Sun, Y. Z. Long, H. D. Zhang, M. M. Li, J. L. Duvaile, X. Y. Jiang and H. L. Yin, Prog. Polym. Sci., 2014, 39, 862–890 CrossRef CAS.
- M. Yu, R. H. Dong, X. Yan, G. F. Yu, M. H. You, X. Ning and Y. Z. Long, Macromol. Mater. Eng., 2017, 302, 1700002 CrossRef.
- C. J. Angammana and S. H. Jayaram, Part. Sci. Technol., 2016, 34, 72–82 CrossRef CAS.
- N. E. Zander, Polymers, 2013, 5, 19–44 CrossRef.
- G. Yang, X. Li, Y. He, J. K. Ma, G. L. Ni and S. B. Zhou, Prog. Polym. Sci., 2018, 81, 80–113 CrossRef CAS.
- G. I. Taylor, Proc. R. Soc. London, Ser. A, 1964, 280, 383 Search PubMed; G. I. Taylor, Proc. R. Soc. London, Ser. A, 1966, 291, 159 Search PubMed; G. I. Taylor, Proc. R. Soc. London, Ser. A, 1969, 313, 453 CrossRef.
- P. Katta, M. Alessandro, R. D. Ramsier and G. G. Chase, Nano Lett., 2004, 4, 2215 CrossRef CAS.
- J. A. Matthews, G. E. Wnek, D. G. Simpson and G. L. Bowlin, Biomacromolecules, 2002, 3, 232 CrossRef CAS PubMed.
- J. Zheng, X. Yan, M. M. Li, G. F. Yu, H. D. Zhang, W. Pisula, X. X. He, J. L. Duvail and Y. Z. Long, Nanoscale Res. Lett., 2015, 10, 475 CrossRef PubMed.
- D. Li, Y. Wang and Y. Xia, Nano Lett., 2003, 3, 1167 CrossRef CAS.
- D. Li, Y. Wang and Y. Xia, Adv. Mater., 2004, 16, 361 CrossRef CAS.
- P. D. Dalton, D. Klee and M. Moller, Polym. Commun., 2005, 46, 611 CrossRef CAS.
- D. Zhang and J. Chang, Adv. Mater., 2007, 19, 3664–3667 CrossRef CAS.
- H. Xu, H. Li and J. Chang, J. Mater. Chem. B, 2013, 1, 4182–4188 RSC.
- G. F. Yu, X. Yan, M. Yu, M. Y. Jia, W. Pan, X. X. He, W. P. Han, Z. M. Zhang, L. M. Yu and Y. Z. Long, Nanoscale, 2016, 8, 2944 RSC.
- Z. Sun, E. Zussman, A. L. Yarin, J. H. Wendorff and A. Greiner, Adv. Mater., 2003, 15, 1929 CrossRef CAS.
- D. Li and Y. Xia, Nano Lett., 2004, 4, 933 CrossRef CAS.
- G. Larsen, R. Spretz and R. Velarde-Ortiz, Adv. Mater., 2004, 16, 166 CrossRef CAS.
- J. Kameoka, R. Orth, Y. Yang, D. Czaplewski, R. Mathers, G. W. Coates and H. G. Craighead, Nanotechnology, 2003, 14, 1124 CrossRef CAS.
- D. Sun, C. Chang, S. Li and L. Lin, Nano Lett., 2006, 6, 839 CrossRef CAS PubMed.
- S. L. Liu, Y. Z. Long, Z. H. Zhang, H. D. Zhang, B. Sun, J. C. Zhang and W. P. Han, J. Nanomater., 2013, 8, 2514103 Search PubMed.
- M. Cai, H. He, X. Zhang, X. Yan, J. Li, F. Chen, D. Yuan and X. Ning, Nanomaterials, 2019, 9, 39 CrossRef PubMed.
- B. Sun, Y. Z. Long, S. L. Liu, Y. Y. Huang, J. Ma, H. D. Zhang, G. Shen and S. Xu, Nanoscale, 2013, 5, 7041–7045 RSC.
- P. Sofokleous, E. Stride, W. Bonfield and M. Edirisinghe, Mater. Sci. Eng., C, 2013, 33, 213–223 CrossRef CAS PubMed.
- W. K. Lau, P. Sofokleous, R. Day, E. Stride and M. Edirisinghe, Bioinspired, Biomimetic Nanobiomater., 2014, 3, 94–105 CrossRef.
- K. Jiang, Y. Z. Long, Z. J. Chen, S. L. Liu, Y. Y. Huang, X. Jiang and Z. Q. Huang, Nanoscale, 2014, 6, 7792 RSC.
- C. Song, X. X. Wang, J. Zhang, G. D. Nie, W. L. Luo, J. Fu, S. Ramakrishna and Y. Z. Long, Nanoscale Res. Lett., 2018, 13, 273 CrossRef PubMed.
- R. H. Dong, C. C. Qin, X. Qiu, X. Yan, M. Yu, L. Cui, Y. Zhou, H. D. Zhang, X. Y. Jiang and Y. Z. Long, Nanoscale, 2015, 7, 19468 RSC.
- F. Y. Lv, R. H. Dong, Z. J. Li, C. C. Qin, X. Yan, X. X. He, Y. Zhou, S. Y. Yan and Y. Z. Long, Int. J. Nanomed., 2016, 11, 4213 CrossRef CAS PubMed.
- http://www.qdjunada.com/Product.aspx?funId=24 .
- F. Brako, C. Luo, D. Q. M. Craig and M. Edirisinghe, Macromol. Mater. Eng., 2018, 303, 1700586 CrossRef.
- D. J. Smith, D. H. Reneker, A. T. McManus, H. L. Schreuder-Gibson, C. Mello and M. S. Sennett, Electrospun fibers and an apparatus therefor, US Patent, 6753454, 2004 Search PubMed; D. Smith and D. H. Reneker, PCTUS0027737, 2001.
- Y. J. Liu, R. Ramaseshan, Y. X. Dong, A. Kumar and S. Ramakrishna, A portable electrospinning apparatus, WO Patent AppPCTSG2008000444, 2010 Search PubMed.
- J. Haik, R. Kornhaber, B. Blal and M. Harats, Adv. Wound Care, 2017, 6, 166–174 CrossRef PubMed.
- https: //nanomedic.com/spincare/product-description/ .
- Y. Z. Long, S. Liu, K. Liu, L. Li, S. C. Xu, B. Sun and H. D. Zhang, Portable handheld electrostatic spinning device, China Patent CN201210229010, 2012 Search PubMed.
- S. C. Xu, C. C. Qin, M. Yu, R. H. Dong, X. Yan, H. Zhao, W. P. Han, H. D. Zhang and Y. Z. Long, Nanoscale, 2015, 7, 12351 RSC.
- R. H. Dong, Y. X. Jia, C. C. Qin, L. Zhan, X. Yan, L. Cui, Y. Zhou, X. Y. Jiang and Y. Z. Long, Nanoscale, 2016, 8, 3482 RSC.
- W. L. Luo, J. Zhang, X. Qiu, L. J. Chen, J. Fu, P. Y. Hu, X. Li, R. J. Hu and Y. Z. Long, Nanoscale Res. Lett., 2018, 13, 278 CrossRef PubMed.
- P. A. Mouthuy, L. Groszkowski and H. Ye, Biotechnol. Lett., 2015, 37, 1107–1116 CrossRef CAS PubMed.
- C. Y. Chui, P. A. Mouthuy and H. Ye, Biotechnol. Lett., 2018, 40, 737–744 CrossRef CAS PubMed.
- W. P. Han, Y. Y. Huang, M. Yu, J. C. Zhang, X. Yan, G. F. Yu, H. D. Zhang, S. Y. Yan and Y. Z. Long, Nanoscale, 2015, 7, 5603 RSC.
- C. C. Qin, X. P. Duan, L. Wang, L. H. Zhang, M. Yu, R. H. Dong, X. Yan, H. W. He and Y. Z. Long, Nanoscale, 2015, 7, 16611 RSC.
- C. J. Li, Y. Y. Yin, B. Wang, T. Zhou, J. Wang, J. Luo, W. Tang, R. Cao, Z. Yuan, N. Li, X. Du, C. Wang, S. Zhao, Y. Liu and Z. L. Wang, ACS Nano, 2017, 11, 10439–10445 CrossRef CAS PubMed.
- X. Yan, M. Yu, L. H. Zhang, X. S. Jia, J. T. Li, X. P. Duan, C. C. Qin, R. H. Dong and Y. Z. Long, Nanoscale, 2016, 8, 209 RSC.
- X. Yan, X. P. Duan, S. X. Yu, Y. M. Li, X. Lv, J. T. Li, H. Y. Chen, X. Ning and Y. Z. Long, RSC Adv., 2017, 7, 33132 RSC.
- X. P. Duan, X. Yan, B. Zhang, Z. G. Zhang, M. Yu, H. D. Zhang, D. P. Yang and Y. Z. Long, RSC Adv., 2016, 6, 66252 RSC.
- G. S. Liu, X. Yan, F. F. Yan, F. X. Chen, L. Y. Hao, S. J. Chen, T. Lou, X. Ning and Y. Z. Long, Nanoscale Res. Lett., 2018, 13, 309 CrossRef PubMed.
- X. Yan, M. H. You, T. Lou, M. Yu, J. C. Zhang, M. G. Gong, F. Y. Lv, Y. Y. Huang and Y. Z. Long, Nanoscale Res. Lett., 2016, 11, 540 CrossRef PubMed.
- B. Zhang, Z. G. Zhang, X. Yan, X. X. Wang, H. Zhao, J. Guo, J. Y. Feng and Y. Z. Long, Nanoscale, 2017, 9, 4154 RSC.
- X. Wang, W. Z. Song, M. H. You, J. Zhang, M. Yu, Z. Fan, S. Ramakrishna and Y. Z. Long, ACS Nano, 2018, 12, 8588–8596 CrossRef CAS PubMed.
- Z. L. Wang, ACS Nano, 2013, 7, 9533–9557 CrossRef CAS PubMed.
- Y. Xie, S. Wang, S. Niu, L. Lin, Q. Jing, J. Yang, Z. Wu and Z. L. Wang, Adv. Mater., 2014, 26, 6599–6607 CrossRef CAS PubMed.
- T. Jiang, L. M. Zhang, X. Chen, C. B. Han, W. Tang, C. Zhang, L. Xu and Z. L. Wang, ACS Nano, 2015, 9, 12562–12572 CrossRef CAS PubMed.
- H. J. Qiu, W. Z. Song, X. X. Wang, J. Zhang, Z. Fan, M. Yu, S. Ramakrishnaa and Y. Z. Long, Nano Energy, 2019, 58, 536–542 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |



