Optical manipulation of animal behavior using a ruthenium-based phototrigger†
Yeraldith
Rojas Pérez
and
Roberto
Etchenique
 *
*
Departamento de Química Inorgánica, Analítica y Química Física, INQUIMAE, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, CONICET, Intendente Güiraldes 2160, Ciudad Universitaria, Pabellón 2, Buenos Aires (C1428EGA), Argentina. E-mail: rober@qi.fcen.uba.ar
First published on 29th October 2018
Abstract
A visible-light activatable caged compound based on a ruthenium-polypyridine complex was used to elicit the feeding response of the freshwater cnidarian Hydra vulgaris. The phototrigger delivers L-arginine in a clean reaction under irradiation with blue or green light. The synthesis, characterization and application mode of this caged arginine are described. A combination of fiber-optics setup and a high absorbance medium allows the precise control of uncaging in the submillimetric range, needed to address the zone where activation takes place.
Introduction
Phototriggers, also called caged-compounds, are widely used in neurophysiology as superb tools to control neural circuits with subcell accuracy, low invasivity and exquisite timing. They are comprised of a photoremovable group that “cages” the biological activity of the “caged” molecule. After being irradiated, the bio-molecule is freed and its physiological action is elicited.1–3 First phototriggers were active solely in the UV range4,5 but newer ones can be excited at longer wavelengths6–9 in one and two-photon regimes.10,11 Among phototriggers, ruthenium polypyridines present some interesting characteristics which make them near ideal as caged compounds. They present an irradiation window in the blue-green portion of the spectrum, high solubility and stability in aqueous solutions, very fast photoreaction kinetics and the absence of side reactions. Moreover, they are able to cage almost any molecule presenting a basic nitrogen moiety, a group present in many molecules of biological interest.Hydra vulgaris is a small cnidarian, typical of freshwaters, with a very simple body plan: just a foot, a body and a mouth surrounded by several tentacles. It is virtually immortal, not showing senescence, and therefore constitutes a convenient model to study regeneration.12,13Hydra has been genetically engineered and recently used as a model for the study of neural circuits, given its simple nervous system and its transparency.14,15
In spite of its simplicity, it is capable of exhibiting many different behaviors depending on the environment cues. One of the most interesting features is its feeding mechanism: a prey that touches its tentacles can be quickly grabbed and directed to the Hydra mouth with a fast tentacle movement which is initiated by chemical signals. Reduced glutathione and L-arginine are among the molecules known to elicit full or partial feeding response.16–18 The capability of generating patterns through natural-like events would allow the study of the subsequent nervous pathways, a big deal for knowing the basic neural coding in simple nets. However, most studies of this kind are performed under very loose control, given the difficulty in eliciting the response by means of a chemical signal with 3D resolution and no simultaneous mechanical perturbation.
In this work we present a ruthenium-based caged arginine and a method to deliver the amino acid with submillimeter resolution even using single-photon excitation. We characterized its photo-chemistry and photophysics and showed that this strategy can be used to elicit the feeding behavior of Hydra vulgaris in a physiological manner.
Experimental
Syntheses
All chemicals were purchased from Sigma-Aldrich and used as received without further purification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v mixture of ethanol and water, and refluxed under N2. The solution was filtered and 1.2 mL of 1 M trimethylphosphine in THF was added using a syringe. The reaction was followed using UV-visible (UV-Vis) spectroscopy. In some cases, additional phosphine solution was added. Once the UV-Vis spectrum was stable, methanol and excess phosphine were removed by rotary evaporation. The resulting oily solid was dissolved in 20 mL of 96% EtOH and rotary evaporated to remove traces of remaining phosphine. This procedure was repeated five times. The dry product was then dissolved in 2 mL of absolute EtOH and precipitated by adding 30 mL of THF. The solid was immediately filtered, dried and used with no further purification.
2 v/v mixture of ethanol and water, and refluxed under N2. The solution was filtered and 1.2 mL of 1 M trimethylphosphine in THF was added using a syringe. The reaction was followed using UV-visible (UV-Vis) spectroscopy. In some cases, additional phosphine solution was added. Once the UV-Vis spectrum was stable, methanol and excess phosphine were removed by rotary evaporation. The resulting oily solid was dissolved in 20 mL of 96% EtOH and rotary evaporated to remove traces of remaining phosphine. This procedure was repeated five times. The dry product was then dissolved in 2 mL of absolute EtOH and precipitated by adding 30 mL of THF. The solid was immediately filtered, dried and used with no further purification.
Spectroscopic measurements and photolysis
The optical bench used for UV-Vis measurements consists of a 532 nm diode pumped solid state laser directed towards a four-faceted cuvette, kept at 25 °C and stirred. The absorbance is monitored perpendicularly to the laser path by using an OceanOptics PC2000 diode-array spectrophotometer run by using OOIChem software.Quantum yield measurements of photouncaging were performed by recording full absorption spectra while the photoreaction occurs. Then, the quantum yield of photolysis (ϕPD) was adjusted as a parameter in order to fit eqn (1) to the measured spectra.
NMR spectra were obtained with a 500 MHz Bruker AM-500. The compound was photolyzed inside the unopened NMR test tube with an array of high power LEDs centered at 525 ± 20 nm (full width at half maximum, FWHM).
In vivo uncaging onto Hydra vulgaris
Hydra were maintained in artificial freshwater (T = 18 °C) in the dark, and fed freshly hatched Artemia nauplii once a week or more frequently when necessary. The animals were kept on a reverse daytime, 12 hours out of phase from local time. The animals were never fed the day before the experiments.15 minutes before an experiment, a Hydra or a freshly cut tentacle was immersed in a 35 mm diameter Petri dish filled with freshwater containing 100μM to 1 mM of [RuBi-Arg]Cl. The video images of the tentacles were recorded from above in a dark field configuration. The video images of the whole Hydra were recorded by using two microscope cameras: one above and one at one side, in order to register all movements. Illumination for imaging was performed with a NIR (800 nm) LED in order to prevent any uncaging or undesired effects of light on Hydra. A 445 nm laser was focused and directed through a 62 μm diameter fiber optics to the uncaging position. The fiber was kept in position by means of an XYZ manual micromanipulator. The uncaging light pulse was fixed to 3 seconds. Image manipulation was done with ImageJ software.
Results and discussion
Arginine can be coordinated to Ru(II) to form the complex [Ru(bpy)2PMe3Arg]2+ (RuBi-Arg, bpy = 2,2′-bipyridine, PMe3 = trimethylphosphine) in two main ways: through its amino group or through its guanidinium group. Coordination of basic nitrogens to Ru occurs readily when the coordinating group is not protonated. As the pKa of amine and guanidinium residues in Arg are 9.04 and 12.48, respectively, it is quite simple to obtain a pure amino-coordinated complex by keeping the pH around 9. Under these conditions, a deprotonated amino, protonated guanidinium is the main species of Arg. On the other hand, a synthesis at higher pH could yield a mixture of both amino and guanidine-coordinated complexes, implying the need for further cumbersome purification of the complex (Fig. 1a). We have chosen the first scheme to simplify the synthesis.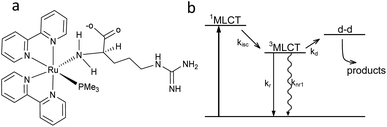 | ||
| Fig. 1 (a) Structure of [Ru(bpy)2PMe3Arg]2+ (RuBi-Arg). (b) Scheme of the Ru-bipyridine uncaging mechanism. | ||
The basic scheme of the photoreaction pathway is depicted in Fig. 1b. Briefly, irradiation at around 450 nm promotes the complex to a 1MLCT excited state which later decays to the triplet state, from which a dissociative d–d state can be populated. The overall process lasts for tens of nanoseconds, the time after which a monodentate ligand is expelled and replaced by a solvent (water) molecule.19,20
RuBi-Arg presents a deep orange colour as PF6 or a chloride salt. This colour arises from its strong MLCT absorption with a maximum at 443 nm. Irradiation on this band provokes the delivery of Arg to form [Ru(bpy)2PMe3(H2O)]2+. Fig. 2a shows a part of the NMR spectrum during photolysis with a 525 nm LED set, where it can be seen that arginine is released (signals A and D). The remaining aquo complex also appears, showing some degree of cis–trans isomerization (see the ESI†). Fig. 2b shows the quantitative photolysis of RuBi-Arg by means of a 532 nm laser while UV-Vis spectra were obtained. The quantum yield of the photoreaction can be obtained by fitting of the numerical integration.
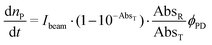 | (1) |
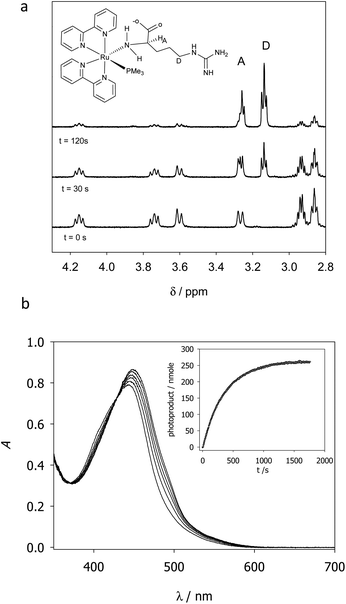 | ||
| Fig. 2 (a) NMR spectra of RuBi-Arg during photolysis in D2O at 525 nm. (b) UV-vis spectra of a solution 130 μM RuBi-Arg during photolysis in H2O (T = 25 °C, pH = 7, λ = 532 nm, p = 7.35 mW). Inset: Moles of products vs. time. Solid line: Fitting of eqn (1) for ϕPD = 0.21. | ||
Evidence about the coordination site between Arg and Ru can also be obtained from the 1H-NMR spectra. Signals at 4.16, 3.74, 3.61, and 3.27 ppm (t = 0 s) correspond to the hydrogens of the coordinated aminos in the two diastereomeric forms of the complex (ΔRu/L-Arg and ΛRu/L-Arg respectively). The fact that these signals appear even in D2O as the solvent indicates that no isotopic exchange is possible. This is a typical characteristic of amine-coordinated Ru-bpy complexes.6 No signals but the ones corresponding to the amino-coordinated cis-RuBi-Arg are present in the spectrum.
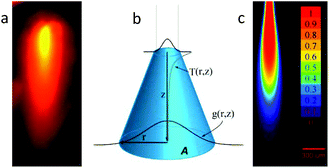
Multiphoton excitation exhibits real z-axis sectioning, and the complete photochemical action can be reduced to the focal volume. The price to obtain this exquisite precision is the use of very expensive femtosecond lasers. Conversely, linear optics cannot be used to achieve this goal. This fact is particularly troublesome when a precise 3D localization is required in thick environments. In order to be able to elicit a physiological response in a small 3D volume using a simple laser diode, we devised a fiber-optics probe that, under specific conditions, can be used to achieve submillimetric focal activation. Usually, linear excitation is done in a low absorption regime, and thus a full cone of light is directed far from the focal plane. In our case, we have chosen to use high absorption conditions and fiber optics irradiation. This configuration prevents the zones far from the fiber tip to be irradiated, given that most of the light was already absorbed in the vicinity of the tip. One of the keys to achieve this high absorption is the use of blue (445 nm) irradiation, in order to take advantage of the high molar absorptivity of RuBi-Arg at this wavelength. The quantum yield at 445 nm was measured in the same way, yielding ϕPD(445) = 0.17 (see ESI Fig. 3†). Fig. 3a shows the basic definitions of the coordinates used to model the uncaging setup. On the other hand, an experimental measurement of the emission of the complex excited with 445 nm light from the optical fiber tip can be seen in Fig. 3b. It can be noted that the emission diminishes abruptly due to the inner filter effect of the surrounding solution.
The analysis of the irradiation system was performed using cylindrical coordinates. The origin of the vectors (r, z) is located at the center of the fiber tip. The beam is considered to have a radial Gaussian dependence g(r, z) with minimum waist w0 at z = 0. The area A covered by the conical beam increases with z as A = NA2z2 + w02, with NA being the numerical aperture of the fiber, which can be measured in the experimental system. Supposing similar absorptivities of RuBi-Arg and the aquo product, the differential amount of Arg that appears at any point (r, z) is:
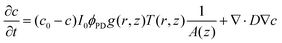 | (2) |
Hydra vulgaris feeds on small aquatic invertebrates. When a prey touches one of its tentacles, a nematocyst21–23 stings the animal, paralyzing it. Simultaneously, chemical cues from the prey indicate Hydra that there is a presence of a suitable piece of food, and initiates a neural response that ends on the sudden shrinkage of the tentacle, opening of the mouth and help from other tentacles to facilitate engulfing the prey. Among the molecules that can elicit one or more of these mechanisms are some small peptides like reduced glutathione and some derivatives, and a few amino acids.17 Maximum knowledge about this behaviour is gained from “bulk” experiments, in which a given amount of the chemical is added to a medium where many Hydra are placed, and the statistical response of the animals is recorded.23 On the other hand, the small size and high sensitivity of Hydra to even tiny mechanical perturbations make single tentacle experiments in whole animals under near physiological conditions almost impossible to perform, and to our knowledge no such experiment has been done to test chemical signaling and their correlates.
Fiber-optics irradiation of RuBi-Arg solutions constitutes a virtually ideal tool to activate the feeding response with no side effects. We have decoupled direct mechanical perturbations (like fast injection) from the chemical perturbation (Arg concentration increase). Both the capsule and the fiber optics are kept stationary while light is directed from the far end of the fiber through a remote electronic shutter to avoid any mechanical movement from being conducted to the tip end. The short range of Arg photodelivery allows a precise enough localization of the chemical action.
A first test of the setup used to uncage arginine with a fiber was done by directing light to a detached tentacle of a Hydra. Fig. 4 shows four characteristic frames of a video taken during the procedure.
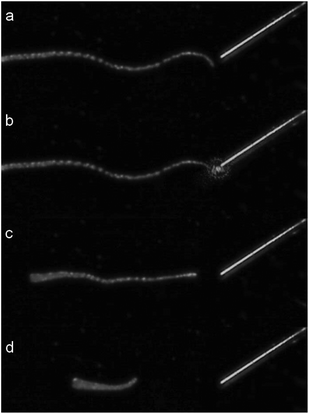 | ||
| Fig. 4 Sequence of a single tentacle shrinkage of Hydra vulgaris elicited by uncaging of arginine from a solution of 400 μM RuBi-Arg using the fiber optics illumination depicted in Fig. 3. (a) 1 second before irradiation. (b) 0 s. (c) 4.9 s. (d) 8.3 s laser irradiation (445 nm) from 0 to 3.5 s. | ||
A couple of seconds after Arg is photodelivered near the tentacle end, contraction begins until its total length shortens to less than one third of its original extension. As the tentacle is not attached to any massive body, the contraction keeps its mass center roughly unchanged. Although some similar experiments can be done by direct application of a free drug with a picospritzer injection, the uncaging procedure is the only one that guarantees a mechanical perturbation free environment.
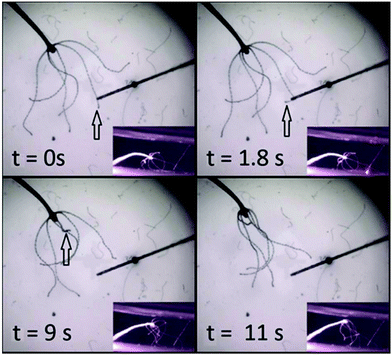
RuBi-Arg phototriggers at concentrations ranging from 80 μM to 1 mM activated through a fiber optics setup were also used to initiate stereotypical behaviours in whole animals. Fig. 5 shows four frames of a video in which a complete Hydra was placed in a bath containing 1 mM RuBi-Arg (Cl− salt). After a few minutes, the photodelivery of Arg was induced through irradiation using a 445 nm laser on a 62 μm diameter fiber optics for 3 seconds. The complete sequence of the response both in images and video are given in the ESI.† After 1.8 seconds, one of the mechanisms of the feeding response is initiated, leading to tentacle shrinkage at 9 s (see the arrow). Later, other tentacles are directed to the mouth axis, resembling the final stage of the feeding response. However, the movement of the excited tentacle toward the mouth and the subsequent aperture was not observed, in concordance with previous findings.17
The subtle but precise movements of the tentacle tip immediately after chemical detection, typical in shrimp-induced feeding response were also observed, as can be seen in the video given in the ESI.†
Similar responses were obtained using concentrations as low as 77 mM, as can be seen in ESI Fig. 5.†
In order to confirm that Arg release is the unique promoter of the feeding response, rather than light itself, a possible photoredox process due to MLCT irradiation or the aquo complex product, two additional tests were done. Firstly, irradiation was directed to Hydra tentacles in Hydra artificial freshwater, in the absence of any Ru complex (n = 4). Secondly, a solution containing 1 mM of the aquo product [Ru(bpy)2PMe3(H2O)]2+, a complex with a similar chemical structure and photophysics of RuBi-Arg was used (n = 3). In neither case was observed feeding response in Hydra as shown in ESI Fig. 6 (videos are available in the ESI†)
Conclusions
In conclusion, we have shown that visible-light excitable caged compounds can be used not only at the cellular or tissue level of preparations, or inner organs in entire animals, but also to elicit responses from the external medium. The use of a fiber optics and a high absorbance medium constitutes a suitable method to allow 3D precise stimulation in a bulk medium with negligible simultaneous mechanical perturbation. However, only volumes that are accessible to the fiber can be excited in this way. This precise method opens the way to a gamut of experiments where the neural correlate of the mechanisms elicited by chemical signals could be elucidated by means of imaging techniques. Further research in this direction is being conducted.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Agency for Science and Technology Promotion, CONICET, and the University of Buenos Aires. R. E. is a member of CONICET.Notes and references
- S. R. Adams and R. Y. Tsien, Annu. Rev. Physiol., 1993, 55, 755–784 CrossRef CAS PubMed.
- G. C. R. Ellis-Davies, Nat. Methods, 2007, 4(8), 619–628 CrossRef CAS PubMed.
- C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Angew. Chem., Int. Ed., 2012, 51, 8446–8476 CrossRef CAS PubMed.
- J. Kaplan, B. Forbush III and J. Hoffman, Biochemistry, 1978, 17(10), 1929–1935 CrossRef CAS PubMed.
- J. W. Walker, J. A. McCray and G. P. Hess, Biochemistry, 1986, 25, 1799–1805 CrossRef CAS PubMed.
- O. Filevich and R. Etchenique, Photochem. Photobiol. Sci., 2013, 12, 1565–1570 RSC.
- L. Lameijer, D. Ernst, S. Hopkins, M. Meijer, S. Askes, S. Le Dévédec and S. Bonnet, Angew. Chem., Int. Ed., 2017, 56, 11549–11553 CrossRef CAS PubMed.
- A. Bahreman, J. Cuello-Garibo and S. Bonnet, Dalton Trans., 2014, 43, 4494 RSC.
- J. Knoll, B. Albani and C. Turro, Acc. Chem. Res., 2015, 48, 2280–2287 CrossRef CAS PubMed.
- G. Borth, T. Gallavardin, D. Odgen and P. I. Dalko, Angew. Chem., Int. Ed., 2013, 52, 4526–4537 CrossRef PubMed.
- R. Araya, V. Andino-Pavlovsky, R. Yuste and R. Etchenique, ACS Chem. Neurosci., 2013, 4(8), 1163–1169 CrossRef CAS PubMed.
- K. Glauber, C. E. Dana and R. E. Steele, Curr. Biol., 2010, 20, 964–965 CrossRef PubMed.
- B. Galliot, Int. J. Dev. Biol., 2012, 56, 411–423 CrossRef CAS PubMed.
- C. Dupre and R. Yuste, Curr. Biol., 2017, 27, 1085–1097 CrossRef CAS PubMed.
- N. Ji and S. W. Flavell, Curr. Biol., 2017, 27, 297–317 CrossRef PubMed.
- W. R. Loomis, Annals of New York Academic of Sciences, 1955, 62(9), 211–227 CrossRef.
- K. Hanai, J. Comp. Physiol., 1981, 144, 503–508 CrossRef CAS.
- R. Kulkarni and S. Galandes, J. Visualized Exp., 2014,(93), e52178 Search PubMed.
- L. Zayat, O. Filevich, L. M. Baraldo and R. Etchenique, Philos. Trans. R. Soc., A, 2013, 371, 20120330 CrossRef PubMed.
- D. Pinnick and B. Durham, Inorg. Chem., 1984, 23, 1440–1445 CrossRef CAS.
- B. Quinn, F. Gagné and C. Blaise, Int. J. Dev. Biol., 2012, 56, 613–625 CrossRef CAS PubMed.
- J. A. Carter, C. Hyland, R. E. Steele and E. M. S. Collins, Biophys. J., 2016, 110(5), 1191–1201 CrossRef CAS PubMed.
- H. Lenhoff, J. Gen. Physiol., 1961, 45(2), 331–344 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR characterization of the compounds and a video of Hydra optical manipulation. See DOI: 10.1039/c8pp00467f |
| This journal is © The Royal Society of Chemistry and Owner Societies 2019 |