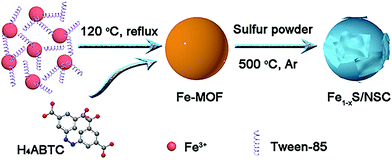
Fe1−xS/nitrogen and sulfur Co-doped carbon composite derived from a nanosized metal–organic framework for high-performance lithium-ion batteries†
Yingying
Liu
a,
Ming
Zhong
a,
Lingjun
Kong
a,
Ang
Li
a,
Xiaowen
Sun
a,
Danhong
Wang
a and
Xian-He
Bu
 *abc
*abc
aSchool of Materials Science and Engineering, National Institute for Advanced Materials, TKL of Metal and Molecule Based Material Chemistry, Nankai University, Tianjin 300350, P.R. China. E-mail: buxh@nankai.edu.cn
bKey Laboratory of Advanced Energy Materials Chemistry (Ministry of Education) College of Chemistry, Nankai University, Tianjin 300071, P. R. China
cCollaborative Innovation Center of Chemical Science and Engineering, Tianjin 300072, P.R. China
First published on 5th October 2018
Abstract
Iron sulfide (Fe1−xS), possessing unique superiorities, including high theoretical capacity, easy access to raw materials, and environment friendliness, has stood out from various anode materials of lithium-ion batteries (LIBs). However, there are still some critical obstacles that need to be overcome before its practical applications. For instance, the huge volume change that occurs during the lithium ion insertion/extraction process leads to a rapid decay in electrochemical performance. Moreover, it is generally difficult to achieve a perfect rate performance for Fe1−xS due to its poor intrinsic conductivity. Herein, we have reported a facile method to synthesize a Fe1−xS/N, S co-doped carbon composite (Fe1−xS/NSC) using a nanosized Fe-based metal–organic framework as the precursor. Compared with Fe1−xS, the as-synthesized Fe1−xS/NSC composite exhibited higher cycling stability and rate capability as an anode for LIBs. For example, a high specific capacity of 1135 mA h g−1 (0.1 A g−1) and 707 mA h g−1 (1 A g−1) could be maintained after 100 cycles and 200 cycles, respectively. Most impressively, a reversible capacity of 586 mA h g−1 was delivered at higher density of 5 A g−1 in the rate test.
Introduction
Owing to the superiorities of high energy density and operating voltage as well as low environmental pollution, lithium-ion batteries (LIBs) stand out from the diverse energy storage and conversion devices, and have dominated the commercial market of portable electronics and electric vehicles (EVs).1–3 However, the commercial graphite anode suppresses the development of emerging storage applications requiring high energy density on account of its limited theoretical capacity (372 mA h g−1). Thus, it is urgent to further improve the electrochemical performance of LIBs, which is required for higher capacity and long-term cycling stability. Notable results have been achieved in pursuing alternative anode materials for LIBs. Transition metal sulfides are considered as an ideal choice due to their unique physicochemical properties,4,5 high lithium storage capacities achieved via conversion reactions as well as higher conductivity than that of metal oxides.6,7 Among various transition metals, iron is one of the most abundant resources in the earth's crust and iron sulfides possess high theoretical capacities (FeS2: 894 mA h g−1, FeS: 609 mA h g−1). Therefore, iron sulfides are competitive candidates to replace the graphite anode. Nonetheless, the irreversible electrode pulverization problem that arises from the large volume change during the charge/discharge process is still unavoidable, leading to rapid capacity fading and poor rate capability.8,9Thus far, various strategies have been dedicated to avoid the volume change in electrode materials, including the construction of core–shell structures,10,11 hollow structures,12,13 and multi-shelled hollow structures.14–16 In addition to abovementioned methods, reducing the particle size into nanoscale may be a feasible option to weaken the mechanical stress during the lithium ion insertion/extraction process and solve the pulverization issue to some extent.17 Moreover, nanosized electrode materials can shorten the diffusion pathway of lithium ions, which is beneficial to the rate performance.18,19
Unfortunately, Fe1−xS shows poor intrinsic conductivity, which significantly limits its rate performance. Recently, compounding Fe1−xS with carbonaceous materials has been verified to be another attractive strategy to achieve enhanced electrochemical performance.8,9 The carbon component can significantly improve the conductivity of Fe1−xS and buffer the volume change, resulting in improved rate performance and cycling stability. For example, Fe1−xS-embedded carbon microsphere nanocomposites were prepared by a solvothermal method and exhibited excellent rate capability.8 Sun et al. reported an Fe1−xS@CNTs composite that achieved a superior rate performance.9 Thus, Fe1−xS-based materials are promising as anodes for LIBs.
Metal–organic frameworks (MOFs) that possess the advantages of large specific surface area, high porosity, and adjustable pore structure have been widely researched in many fields, such as gas adsorption, catalysis, drug delivery and energy storage. In addition, MOFs can be converted into porous materials or hollow materials by an in situ pyrolysis process.13,20–22 Particularly, MOF-derived metal sulfide/carbon materials usually exhibit multiple structural and compositional advantages, which may address the current problems of the large volume change of metal sulfides for high cyclic stability and capacity. For example, Zou et al. synthesized a carbon/CoS2 composite derived from ZIF-67 templates.17 Wu et al. reported a ZnxCo1−xS@C-CNTs nanocomposite using Zn-Co-ZIF@CNTs as the precursor.23
Herein, we developed a one-step approach to synthesize a Fe1−xS/N, S co-doped carbon composite (Fe1−xS/NSC) through the simultaneous decomposition and sulfidation of a nanosized Fe-MOF and have evaluated Fe1−xS/NSC as an anode for LIBs. During the discharge/charge process, the carbon component could not only buffer the volume change, but also improve the electrical conductivity of Fe1−xS/NSC and inhibit the dissolution of formed polysulfides effectively.24 In addition, N, S co-doped carbon could further improve the storage capacity of lithium ions. The as-obtained Fe1−xS/NSC nanocomposite thus presents excellent rate performance and superior long-term cycling stability.
Experimental section
Synthesis of Fe-MOF precursor
Fe-MOF was prepared according to a previous reference.25 Typically, 1.5 g iron(III) nitrate nonahydrate, 0.5 g 3,3′,5,5′-azobenzenetetracarboxylic acid (H4ABTC), 1 mL tert-butylamine (0.95 M in deionized water) and Tween-85 were dissolved in a mixed solution (50 mL DMSO, 10 mL CH3CN, and 10 mL deionized water). Then, the mixture was vigorously stirred and refluxed at 120 °C for 3 h. The precipitates were collected by centrifugation and dried at 60 °C overnight after washing with ethanol for three times. H4ABTC ligand was synthesized referring to the published literature.26Synthesis of Fe1−xS/NSC
In a typical synthesis process, 0.2 g Fe-MOF and 0.2 g sulfur powders were loaded into a combustion boat and then calcined at 500 °C for 2 h at a rate of 2 °C min−1 in Ar atmosphere.Synthesis of Fe1−xS
A combustion boat loaded with 50 mg commercial Fe2O3 precursor and 0.15 g thioacetamide (TAA) was transferred to a tube furnace under Ar atmosphere. The sulfurization temperature was set at 500 °C with a heating rate of 1 °C min−1 and maintained for 5 h.Characterization
Powder X-ray diffraction (XRD) patterns were recorded on a Rigaku MiniFlex600 X-ray diffraction meter with Cu Kα radiation (λ = 1.5406 Å) in the 2θ range of 3–80°. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Scientific ESCALAB 250Xi to characterize the surface composition of Fe1−xS/NSC. Nitrogen adsorption isotherms were recorded on ASAP 2020 (Micromeritis) at 77 K, and the surface area was calculated by Brunauer–Emmett–Teller (BET) measurements. Panoramic field-emission scanning electron microscopy (FESEM) images were obtained on a JSM-7800. Transmission electron microscopy (TEM) was conducted on a JEM-2800. Elemental analysis was performed on a Vario EL-CUBE.Electrochemical measurements
The working electrodes were fabricated by mixing active materials, Ketjenblack (KB) and polyvinylidenefluoride (PVDF) with a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using N-methyl-2-pyrrolidinone (NMP) as the solvent. The loading amount of the active materials per electrode was maintained at 0.8–1.1 mg. CR 2025 coin cells were assembled in a glovebox using a metallic lithium foil as both the counter and the reference electrode, Celgard 2400 microporous membrane as the separator and 1 M LiPF6 dissolved in ethylene carbonate/dimethyl carbonate/diethyl carbonate (EC
1 using N-methyl-2-pyrrolidinone (NMP) as the solvent. The loading amount of the active materials per electrode was maintained at 0.8–1.1 mg. CR 2025 coin cells were assembled in a glovebox using a metallic lithium foil as both the counter and the reference electrode, Celgard 2400 microporous membrane as the separator and 1 M LiPF6 dissolved in ethylene carbonate/dimethyl carbonate/diethyl carbonate (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC
DEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 1
DMC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v%) as the electrolyte. Galvanostatic charge and discharge tests were conducted between the potentials ranging from 0.01–3 V (vs. Li/Li+) on LAND-CT2001A battery testers. Cyclic voltammetry (CV) was conducted in the range of 0.01–3 V versus Li/Li+ at a scan rate of 0.1 mV s−1 on a VersaSTAT 4 electrochemical workstation. Electrochemical impedance spectroscopy (EIS) was performed on a VersaSTAT 4 electrochemical workstation in the frequency range of 100 mHz to 100 kHz.
1 v%) as the electrolyte. Galvanostatic charge and discharge tests were conducted between the potentials ranging from 0.01–3 V (vs. Li/Li+) on LAND-CT2001A battery testers. Cyclic voltammetry (CV) was conducted in the range of 0.01–3 V versus Li/Li+ at a scan rate of 0.1 mV s−1 on a VersaSTAT 4 electrochemical workstation. Electrochemical impedance spectroscopy (EIS) was performed on a VersaSTAT 4 electrochemical workstation in the frequency range of 100 mHz to 100 kHz.
Results and discussion
The synthesis process of the Fe1−xS/NSC composite is described in Scheme 1. First, uniform Fe-MOF nanoparticles were synthesized by refluxing a mixed solution of DMSO/CH3CN/H2O containing Fe3+ and H4ABTC at 120 °C for 3 h. Then, Tween-85 as a surfactant was asses to adjust the size of the Fe-MOF. FESEM images show that the obtained Fe-MOF exhibits spherical morphology with size distribution ranging from 200 nm to 1 μm (Fig. S1†). When the added amount of Tween-85 is 3 mL, the Fe-MOF has the smallest size and uniform morphology. Hence, Fe-MOF (3 mL) was chosen as the precursor for the preparation of Fe1−xS/NSC (please note that Fe-MOF (3 mL) is referred to as Fe-MOF in the following sections). The diffraction peaks of the Fe-MOF precursor matches well with simulated and previously reported results,25 implying that Fe-MOF was successfully synthesized (Fig. S2a†). The crystal structure of Fe-MOF is shown in Fig. S2b.† Then, the Fe-MOF precursor was uniformly mixed with S powder and the mixture was annealed at 500 °C in argon atmosphere. During this process, Fe-MOF reacts with sulfur powder to form iron sulfides. Moreover, the N element from ligands and the S element from the additive sulfur source were doped to form the N, S co-doped carbon matrix.XRD pattern was firstly recorded to identify the phase of the as-obtained sample. The result is shown in Fig. 1a. The broad diffraction peak located at about 25° could be attributed to the (002) plane of graphitic carbon, and the diffraction peaks at 29.9°, 33.7°, 43.7° and 53.1° were assigned to (200), (2 0 11), (2 0 22) and (220) planes of pyrrhotite Fe1−xS (JCPDs No. 29-726), respectively. As clearly shown in the crystal structure of Fe1−xS (Fig. 1b), iron ion occupies an octahedral site and exhibits a vacancy, while the sulfur ion resides in the trigonal prismatic site. XPS spectra were collected to further evaluate the elemental composition and the valence states of Fe1−xS/NSC. As shown in Fig. S3,† the characteristic peaks of Fe, S, N, and C elements can be clearly found in the full survey spectrum. The C, S and N contents analyzed by the elemental analysis were 30.0, 30.3 and 3.5 wt%, respectively. The high-resolution Fe 2p spectrum (Fig. 1c) clearly certifies the presence of the three chemical states of iron. The peak located at 707.0 eV can be assigned to metallic Fe (1.98 atom%), which is ascribed to the minute amount of Fe2+ and Fe3+ reduced by carbon during high-temperature calcination.27 Moreover, the peaks located at 714.8 (11.42 atom%) and 727.9 eV (13.55 atom%) indicate the existence of Fe3+. The other peaks at 710.8 (33.41 atom%) and 724.4 eV (39.64 atom%) were associated with Fe2+.9 As shown in Fig. 1d, the high-resolution spectrum of S 2p can be deconvoluted into five sub-peaks. The peaks at 161.0, 162.3, 163.5 and 164.7 eV correspond to S 2p and the peak at 168.0 eV can be ascribed to C-SOx-C.28,29 The high-resolution spectrum of C 1s exhibits four peaks located at 284.6, 285.0, 285.7 and 288.4 eV, corresponding to C–C, C–S–C, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively (Fig. 1e).30 The existence of C–N and C–S–C bonds indicate N, S-doping in the carbon matrix, which can significantly improve the electrochemical performance of Fe1−xS. It is widely acknowledged that N-doped carbon materials exhibit higher electrical conductivity originating from the conjugated effect between the graphene π-system and the lone pair of nitrogen, which could facilitate the rate performance of the Fe1−xS anode.31,32 In addition, carbon doped with N and S atoms can possess more active sites for lithium storage.33 Furthermore, the high-resolution N 1s spectrum can be deconvoluted into four peaks located at 398.4, 399.5, 400.8, and 405.3 eV, which are assigned to pyridinic N, pyrrolic N, graphitic N and oxidized N, respectively (Fig. 1f).34 Raman spectra of Fe1−xS/NSC and Fe1−xS were recorded to further investigate the carbon composition of the material. Two distinct carbon peaks could be observed in Fe1−xS/NSC. D band located at 1322 cm−1 originates from the disorder in carbon atoms and structural defects, and the G band at 1573 cm−1 can be attributed to graphitized carbon (Fig. S4†). The ID/IG value (1.09) indicates a large number of defects on the carbon substrate, which is ascribed to the doping of nitrogen and sulfur.35,36 The BET specific surface area of the Fe1−xS/NSC was estimated to be 23.1 m2 g−1 and the pore-size distribution indicated typical microporous and mesoporous characteristics with the sizes in the range of 1 to 15 nm (Fig. S5†).
O bonds, respectively (Fig. 1e).30 The existence of C–N and C–S–C bonds indicate N, S-doping in the carbon matrix, which can significantly improve the electrochemical performance of Fe1−xS. It is widely acknowledged that N-doped carbon materials exhibit higher electrical conductivity originating from the conjugated effect between the graphene π-system and the lone pair of nitrogen, which could facilitate the rate performance of the Fe1−xS anode.31,32 In addition, carbon doped with N and S atoms can possess more active sites for lithium storage.33 Furthermore, the high-resolution N 1s spectrum can be deconvoluted into four peaks located at 398.4, 399.5, 400.8, and 405.3 eV, which are assigned to pyridinic N, pyrrolic N, graphitic N and oxidized N, respectively (Fig. 1f).34 Raman spectra of Fe1−xS/NSC and Fe1−xS were recorded to further investigate the carbon composition of the material. Two distinct carbon peaks could be observed in Fe1−xS/NSC. D band located at 1322 cm−1 originates from the disorder in carbon atoms and structural defects, and the G band at 1573 cm−1 can be attributed to graphitized carbon (Fig. S4†). The ID/IG value (1.09) indicates a large number of defects on the carbon substrate, which is ascribed to the doping of nitrogen and sulfur.35,36 The BET specific surface area of the Fe1−xS/NSC was estimated to be 23.1 m2 g−1 and the pore-size distribution indicated typical microporous and mesoporous characteristics with the sizes in the range of 1 to 15 nm (Fig. S5†).
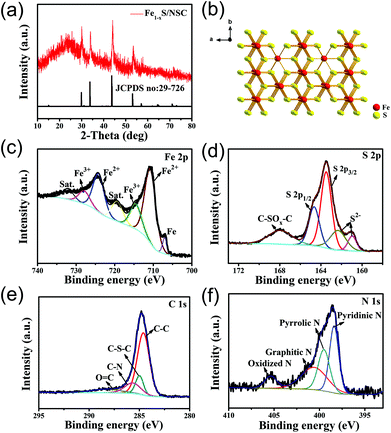 | ||
| Fig. 1 (a) XRD pattern of the Fe1−xS/NSC. (b) Crystal structure of Fe1−xS. (c) High-resolution Fe 2p, (d) S 2p, (e) C 1s, and (f) N 1s XPS spectra of the Fe1−xS/NSC sample. | ||
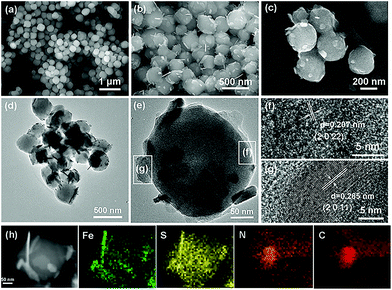
The FESEM image (Fig. 2a) reveals that the Fe-MOF precursor possesses uniform nanospherical morphology with a diameter of 200–300 nm. After the sulfuration process, the as-obtained Fe1−xS/NSC maintains a sphere-like morphology similar to that of the precursor, while Fe1−xS nanosheets are produced and decorated on the Fe1−xS/NSC nanosphere surface as a result of heterogeneous sulfuration (Fig. 2b and c).28 In agreement with the FESEM analysis, Fe1−xS/NSC nanospheres decorated with nanosheets are observed in low resolution TEM images (Fig. 2d and e). The lattice fringes of 0.207 nm and 0.265 nm can be detected in high resolution TEM images (Fig. 2f and g), corresponding to the (2 0 22) plane of Fe1−xS/NSC nanospheres and the (2 0 11) plane of Fe1−xS nanosheets, respectively. The elemental mapping images indicate the unique structure with the uniform distribution of Fe, S, N and C elements within the Fe1−xS/NSC hybrid, and further confirm the existence of Fe1−xS on the surface of the nanosheets (Fig. 2h).
For comparison, Fe1−xS was prepared using commercial Fe2O3 as the precursor. The preparation method of transforming Fe2O3 into Fe1−xS through the sulfuration process by using TAA as the sulfur resource referred to the study by Srinivasan et al.37 The XRD pattern of Fe1−xS was consistent with that of Fe1−xS/NSC, except for the disappearance of the carbon peak, indicating the successful preparation of the Fe1−xS phase (Fig. S6†). In addition, the FESEM images (Fig. S7†) reveal that the Fe1−xS material displays a very irregular morphology. Based on the advantages of the structure and composition, the as-prepared Fe1−xS/NSC composite was applied to the anode of LIBs, and the corresponding electrochemical results are presented in the following section.
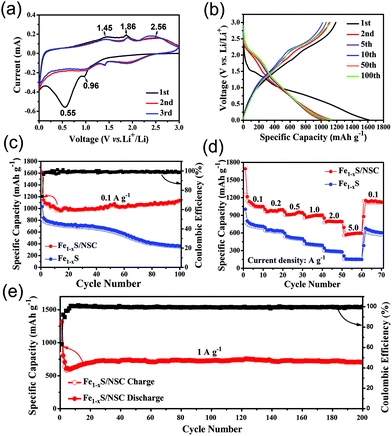
In order to study the lithium-ion storage mechanism of the Fe1−xS/NSC electrode for LIBs, CV curves were recorded at a scan rate of 0.1 mV s−1 (Fig. 3a). During the initial cathodic scan, a broad peak at 0.55 V can be attributed to the formation of the solid electrolyte interface (SEI) film, which is the key reason for the irreversible capacity. In addition, the CV curves of the 2nd and 3rd cycles overlap well, which reveals the good reversibility of the electrode materials. In the subsequent cathodic scan, the cathodic peak at 0.96 V is related to the conversion of Fe1−xS into Li2S and Fe, which can be described using the following equations:28,38
| Fe1−xS + 2Li+ + 2e− → Li2Fe1−xS2 | (1) |
| Li2Fe1−xS2 + 2Li+ + 2e− → 2Li2S + (1 − x) Fe0 | (2) |
In anodic scans, three oxidation peaks can be observed at 1.45 V, 1.86 V and 2.56 V, corresponding to the multi-step reaction. The peak at 1.45 V can be ascribed to the formation of Li2Fe1−xS2, and the peak at 1.86 V is attributed to the conversion of Li2Fe1−xS2 to Li2−yFe1−xS2. Another broad peak at around 2.56 V indicates the formation of iron sulfide.8,9,28,38,39Fig. 3b exhibits the typical charge/discharge voltage profiles of Fe1−xS/NSC for the 1st, 2nd, 5th, 10th, 50th and 100th cycle at 0.1 A g−1. The initial discharge and charge capacities were 1614 and 1188 mA h g−1, respectively, giving a high initial efficiency (CE) of 73.6%. The initial capacity loss may have resulted from the formation of the SEI film, which could consume a part of lithium ions.13 During the 2nd cycle, the discharge and charge capacities decreased to 1144 and 1104 mA h g−1, respectively, which indicates that the capacity loss is only ca. 5%. This high CE indicates the good reversibility of the electrode during the lithiation/delithiation process. In addition, the charge–discharge curves of the 5th, 10th, 50th, and 100th cycle were nearly coincident with that of the 2nd cycle, indicating the excellent cycling stability of the Fe1−xS/NSC electrode.
To further elucidate the cycling stability, Fe1−xS/NSC and Fe1−xS were discharged/charged at 0.1 A g−1 for 100 cycles. As shown in Fig. 3c, the decay of the specific capacity of the Fe1−xS/NSC electrode was probably caused by the formation of polysulfide anions and side reactions.24 Moreover, the capacities show an increasing trend after 15 cycles, which is normally attributed to the presence of a possible activation process in the electrode and the reversible growth and the dissolution of the polymeric gel-like film.40–42 Compared with the Fe1−xS/NSC electrode, the Fe1−xS displayed a remarkable decreasing trend for the specific capacity, suggesting the poorer cycling stability. The capacity of 1135 mA h g−1 for Fe1−xS/NSC could be achieved after 100 cycles at 0.1 A g−1, which was almost three times that for Fe1−xS (349 mA h g−1). The capacity of Fe1−xS/NSC was higher than the theoretical value due to the reversible formation and decomposition of the polymeric-like film from the electrolyte.43 In addition to the high reversible capacity and excellent cycling stability, the rate performance is also an important indicator for high performance LIBs. In rate capability tests, the average specific capacities of Fe1−xS/NSC were determined as 1089, 980, 929, 890, 794, and 586 mA h g−1 at 0.1, 0.2, 0.5, 1.0, 2.0, and 5.0 A g−1, respectively (Fig. 3d). With the increase in current density, the specific capacity of Fe1−xS/NSC decreased due to polarization and insufficient diffusion process at high current density.44 When the current density returns to 0.1 A g−1, the specific capacity of Fe1−xS/NSC can retain a capacity of 1135 mA h g−1, which further reveals the superior reversibility of the Fe1−xS/NSC electrode. In comparison, the bare Fe1−xS electrode exhibited worse rate performance. The specific discharge capacities of Fe1−xS/NSC were 686, 611, 488, 380 and 148 mA h g−1 with the increase in the current density from 0.1 A g−1 to 5.0 A g−1. In brief, Fe1−xS/NSC shows superior electrochemical performance than bare Fe1−xS, which is mainly attributed to the N, S co-doped carbon. The Fe1−xS/NSC electrode was further evaluated at a high current density of 1.0 A g−1. As exhibited in Fig. 3e, the electrode delivers a discharge specific capacity of 707 mA h g−1 after 200 cycles, indicating the superior cycling stability and rate performance of the Fe1−xS/NSC electrode.
Electrochemical impedance spectroscopy analysis provides an insight into the superior performance of the Fe1−xS/NSC electrode. As shown in Fig. S8,† the diameter of the semicircle is associated with charge transfer resistance (Rct), while the straight line is attributed to the diffusion of lithium ions. The EIS results indicate that the Fe1−xS/NSC electrode exhibits lower resistance and better diffusion properties compared with those of the Fe1−xS electrode owing to the existence of the conductive carbon matrix.45Fig. 4 shows the comparison of the rate performance between Fe1−xS/NSC prepared in this study and the previously reported iron sulfide/carbon composites.8,39,46–50 The Fe1−xS/NSC electrode displays higher capacities at the same current density compared with other iron sulfide/carbon composites. Furthermore, the cycling performance comparison between Fe1−xS/NSC and previously reported iron sulfide/carbon matrix composites is detailed in Table S1.† These excellent lithium storage properties make Fe1−xS/NSC very competitive among numerous iron sulfide/carbon composites. Furthermore, the Fe1−xS/NSC electrode could still retain the initial morphology to some extent after 100 cycles (Fig. S9†), which confirms the good structural stability of the Fe1−xS/NSC electrode.
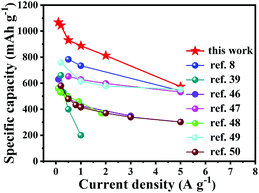 | ||
| Fig. 4 Comparison of rate capabilities of Fe1−xS/NSC composite prepared in this study with the previously reported iron sulfide-based electrodes. | ||
These excellent electrochemical performances of Fe1−xS/NSC are mainly ascribed to the small particle size of Fe1−xS and the introduction of N, S co-doped carbon. First, the ultra-small Fe1−xS nanoparticles shorten the diffusion distance of lithium ions, which significantly enhances the rate of their insertion/extraction, and thus induces excellent rate capacity. Second, the porous carbon matrix not only increases the conductivity of Fe1−xS/NSC and provides a good supporting matrix to increase the mechanical stability, but also effectively buffers the volume change during the lithium ion insertion/extraction process. Finally, doping carbon with N and S elements can also enhance the electronic conductivity of the carbon matrix as well as induce more topological defects on the surface of the Fe1−xS/NSC material, thus increasing active sites for lithium storage.
Conclusions
In summary, a unique Fe1−xS/N, S co-doped carbon composite has been successfully synthesized using a nanosized Fe-MOF as the precursor through a one-step sulfidation strategy. When evaluated as an anode for LIBs, the Fe1−xS/NSC electrode exhibited excellent rate performance and cycling stability. In particular, it achieved a reversible capacity as high as 1135 mA h g−1 after 100 cycles at 0.1 A g−1, a high capacity of 586 mA h g−1 at 5.0 A g−1, and even an impressively large special capacity of 707 mA h g−1 after 200 cycles at 1.0 A g−1. The improved performance could be attributed to the unique structure, nanocrystallization, and the integration of Fe1−xS with N, S co-doped carbon. This study provides a surfactant-assisted strategy to prepare nanoscale MOF materials and offers an insight into realizing the role of N and S co-doped carbon in developing high performance anode materials for LIBs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the NSFC (21421001 and 21531005), 111 Project (B18030) and the NSF of Tianjin (17JCYBJC20000).Notes and references
- J.-M. Tarascon and M. Armand, Nature, 2008, 451, 652–657 CrossRef.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243–3262 RSC.
- V. Aravindan, Y.-S. Lee and S. Madhavi, Adv. Energy Mater., 2015, 5, 1402225 CrossRef.
- K. Chang and W. Chen, Chem. Commun., 2011, 47, 4252–4254 RSC.
- J. Zhang, L. Yu and X. W. Lou, Nano Res., 2017, 10, 4298–4304 CrossRef CAS.
- Y. Liu, Z. Wang, Y. Zhong, M. Tade, W. Zhou and Z. Shao, Adv. Funct. Mater., 2017, 27, 1701229 CrossRef.
- R. Wu, D. P. Wang, X. Rui, B. Liu, K. Zhou, A. W. Law, Q. Yan, J. Wei and Z. Chen, Adv. Mater., 2015, 27, 3038–3044 CrossRef CAS PubMed.
- B. Wu, H. Song, J. Zhou and X. Chen, Chem. Commun., 2011, 47, 8653–8655 RSC.
- Y. Xiao, J.-Y. Hwang, L. Belharouak and Y.-K. Sun, ACS Energy Lett., 2017, 2, 364–372 CrossRef CAS.
- J. Zhang, K. Wang, Q. Xu, F. Cheng and S. Guo, ACS Nano, 2015, 9, 3369–3376 CrossRef CAS.
- X. Li, A. Dhanabalan, L. Gu and C. Wang, Adv. Energy Mater., 2012, 2, 238–244 CrossRef CAS.
- L. Zhang, H. B. Wu, S. Madhavi, H. H. Hng and X. W. Lou, J. Am. Chem. Soc., 2012, 134, 17388–17391 CrossRef CAS.
- F. Zou, X. Hu, Z. Li, L. Qie, C. Hu, R. Zeng, Y. Jiang and Y. Huang, Adv. Mater., 2014, 26, 6622–6628 CrossRef CAS.
- L. Hu and Q. Chen, Nanoscale, 2014, 6, 1236–1257 RSC.
- D. Luo, Y. P. Deng, X. Wang, G. Li, J. Wu, J. Fu, W. Lei, R. Liang, Y. Liu, Y. Ding, A. Yu and Z. Chen, ACS Nano, 2017, 11, 11521–11530 CrossRef CAS.
- L. Zhang, H. B. Wu and X. W. Lou, J. Am. Chem. Soc., 2013, 135, 10664–10672 CrossRef CAS PubMed.
- Q. Wang, R. Zou, W. Xia, J. Ma, B. Qiu, A. Mahmood, R. Zhao, Y. Yang, D. Xia and Q. Xu, Small, 2015, 11, 2511–2517 CrossRef CAS.
- P. G. Bruce, B. Scrosati and J.-M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS.
- S. L. P. Poizot, S. Grugeon, L. Dupont and J.-M. Tarascon, Nature, 2000, 47, 496 CrossRef.
- L. Kong, J. Zhu, W. Shuang and X.-H. Bu, Adv. Energy Mater., 2018, 1801515 CrossRef.
- P. Zhang, B. Y. Guan, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2017, 56, 7141–7145 CrossRef CAS.
- L. Kong, C. C. Xie, H. Gu, C. P. Wang, X. Zhou, J. Liu, Z. Zhou, Z. Y. Li, J. Zhu and X. H. Bu, Small, 2018, 14, 1800639 CrossRef.
- H. Wang, Z. Chen, Y. Liu, H. Xu, L. Cao, H. Qing and R. Wu, J. Mater. Chem. A, 2017, 5, 23221–23227 RSC.
- N. Mahmood, C. Zhang and Y. Hou, Small, 2013, 9, 1321–1328 CrossRef CAS.
- M. Pang, A. J. Cairns, Y. Liu, Y. Belmabkhout, H. C. Zeng and M. Eddaoudi, J. Am. Chem. Soc., 2013, 135, 10234–10237 CrossRef CAS.
- S. Wang, X. Wang, L. Li and R. C. Advincula, J. Org. Chem., 2004, 69, 9073–9084 CrossRef CAS.
- Q. Ma, H. Song, Q. Zhuang, J. Liu, Z. Zhang, C. Mao, H. Peng, G. Li and K. Chen, Chem. Eng. J., 2018, 338, 726–733 CrossRef CAS.
- H. Pang, W. Sun, L.-P. Lv, F. Jin and Y. Wang, J. Mater. Chem. A, 2016, 4, 19179–19188 RSC.
- G. Zou, C. Wang, H. Hou, C. Wang, X. Qiu and X. Ji, Small, 2017, 13, 1700762 CrossRef.
- C. Wu, Y. Zhang, D. Dong, H. Xie and J. Li, Nanoscale, 2017, 9, 12432–12440 RSC.
- J. Yang, X. Zhou, D. Wu, X. Zhao and Z. Zhou, Adv. Mater., 2017, 29, 1604108 CrossRef PubMed.
- P. Zhang, F. Sun, Z. Xiang, Z. Shen, J. Yun and D. Cao, Energy Environ. Sci., 2014, 7, 442–450 RSC.
- F. Zheng, Y. Yang and Q. Chen, Nat. Commun., 2014, 5, 5261 CrossRef CAS PubMed.
- S. Fu, C. Zhu, J. Song, S. Feng, D. Du, M. H. Engelhard, D. Xiao, D. Li and Y. Lin, ACS Appl. Mater. Interfaces, 2017, 9, 36755–36761 CrossRef CAS.
- X. Gao, B. Wang, Y. Zhang, H. Liu, H. Liu, H. Wu and S. Dou, Energy Storage Mater., 2019, 16, 46–55 CrossRef.
- D. H. Liu, W. H. Li, Y. P. Zheng, Z. Cui, X. Yan, D. S. Liu, J. Wang, Y. Zhang, H. Y. Lu, F. Y. Bai, J. Z. Guo and X. L. Wu, Adv. Mater., 2014, 26, 6025–6030 CrossRef.
- L. Li, S. Peng, N. Bucher, H.-Y. Chen, N. Shen, A. Nagasubramanian, E. Eldho, S. Hartung, S. Ramakrishna and M. Srinivasan, Nano Energy, 2017, 37, 81–89 CrossRef CAS.
- J. Liu, Y. Wen, Y. Wang, P. A. van Aken, J. Maier and Y. Yu, Adv. Mater., 2014, 26, 6025–6030 CrossRef CAS.
- L. Fei, Q. Lin, B. Yuan, G. Chen, P. Xie, Y. Li, Y. Xu, S. Deng, S. Smirnov and H. Luo, ACS Appl. Mater. Interfaces, 2013, 5, 5330–5335 CrossRef CAS.
- H. Hu, J. Zhang, B. Guan and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 9514–9518 CrossRef CAS.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- G. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS.
- D. Wang, J. Yang, X. Li, D. Geng, R. Li, M. Cai, T.-K. Sham and X. Sun, Energy Environ. Sci., 2013, 6, 2900–2906 RSC.
- X. C. Chen, W. Wei, W. Lv, F. Y. Su, Y. B. He, B. Li, F. Kang and Q. H. Yang, Chem. Commun., 2012, 48, 5904–5906 RSC.
- M. Zhong, D. Yang, C. Xie, Z. Zhang, Z. Zhou and X.-H. Bu, Small, 2016, 12, 5564–5571 CrossRef CAS.
- C. Xu, Y. Zeng, X. Rui, N. Xiao, J. Zhu, W. Zhang, J. Chen, W. Liu, H. Tan, H.-H. Hng and Q. Yan, ACS Nano, 2012, 6, 4713–4721 CrossRef CAS.
- Y. Xu, W. Li, F. Zhang, X. Zhang, W. Zhang, C.-S. Lee and Y. Tang, J. Mater. Chem. A, 2016, 4, 3697–3703 RSC.
- X. Wei, W. Li, J. A. Shi, L. Gu and Y. Yu, ACS Appl. Mater. Interfaces, 2015, 7, 27804–27809 CrossRef CAS.
- F. Zhang, C. Wang, G. Huang, D. Yin and L. Wang, J. Power Sources, 2016, 328, 56–64 CrossRef CAS.
- M. Huang, A. Xu, H. Duan and S. Wu, J. Mater. Chem. A, 2018, 6, 7155–7161 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: SEM image of Fe-MOF with adding different amount of Tween-85, N2 sorption/desorption isotherms and pore size distribution curve, XRD pattern and SEM images of Fe1−xS, Nyquist plots of the Fe1−xS/NSC and Fe1−xS electrodes, table of contents for electrochemical performance comparison, SEM image of the Fe1−xS/NSC electrode after cycles. See DOI: 10.1039/c8qi00910d |
| This journal is © the Partner Organisations 2019 |