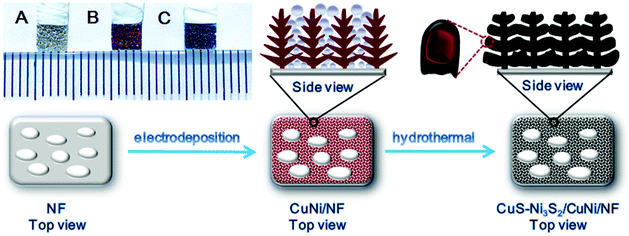
CuS–Ni3S2 grown in situ from three-dimensional porous bimetallic foam for efficient oxygen evolution†
Nan
Zhang
 *,
Ying
Gao
,
Yahui
Mei
,
Jian
Liu
,
Weiyu
Song
* and
Ying
Yu
*
*,
Ying
Gao
,
Yahui
Mei
,
Jian
Liu
,
Weiyu
Song
* and
Ying
Yu
*
State Key Laboratory of Heavy Oil Processing, College of Science, China University of Petroleum, Beijing, 102249, China. E-mail: zhangnan@cup.edu.cn; songwy@cup.edu.cn; yuying@cup.edu.cn
First published on 29th November 2018
Abstract
The development of efficient and stable electrocatalysts for the oxygen evolution reaction (OER), which is critical for the development of water splitting, has attracted great interest from many researchers. However, there have been few reports exhibiting satisfactory activity and durability under high current density (up to 1000 mA cm−2), which are significant for the practical production of water splitting. Herein, a novel electrocatalyst, CuS–Ni3S2, in situ grown from porous CuNi alloy supported on nickel foam (CuS–Ni3S2/CuNi/NF) was obtained via a facile two-step method, and it has achieved excellent OER performance in alkaline solution even under high current density. The electrocatalyst only needed low overpotentials of 337, 444 and 510 mV to reach the very high current densities of 100, 500, and 1000 mA cm−2, respectively, and it presented excellent durability at a relatively high current density of 100 mA cm−2 for 15 h. Despite the current density being normalized by the catalytic electrode's geometrical area, both the mass activity and turnover frequency (TOF) for each active site showed the superior catalytic activity of CuS–Ni3S2/CuNi/NF. With the assistance of theoretical calculations, the participation of CuS was demonstrated to contribute to the excellent OER performance, providing a promising composition for designing and synthesizing novel OER catalysts.
1. Introduction
The increase in serious environmental concerns coupled with the shortage of fossil fuel has sparked increased research pressure for exploring renewable and eco-friendly energy sources.1 Electrochemical water splitting is a clean technique for producing high-purity hydrogen, an ideal energy carrier with zero carbon emission and high energy density.2 However, its efficiency has been limited by the sluggish oxygen evolution reaction (OER) due to its complex four-electron transition process.3 Simultaneously, the application of the state-of-the-art OER catalysts RuO2 and IrO2 has been restricted by their low abundance and exorbitant prices.4Fortunately, non-noble metal-based materials such as transition-metal sulfides,5 oxides,6 nitrides7 and phosphides8 have been widely investigated as OER catalysts. Among these catalysts, Ni3S2 with attractive electrocatalytic behavior has been widely studied.9 Nonetheless, the OER catalytic activity has been further improved by synthesizing composites of Ni3S2 and other materials.10–12 In recent years, Cu has attracted significant interest from researchers for its high earth-abundance, low cost, high conductivity and extensive redox properties. For example, CuO/copper foam,13 CuO/FTO,14 [(TGG4−)Cu(II)–OH2]2−,15 and Co-doped CuO nanoarrays16 have been proved to be efficient electrocatalysts for the OER. As an important Cu-based material, CuS has been confirmed to be an excellent material in various electrochemical applications, such as CuS dendrite biosensor electrodes,17 hollow CuS nanosphere supercapacitors,18 and three dimensional (3D) hierarchical CuS microsphere aluminum-ion batteries.19 Nevertheless, the participation of CuS in OER electrocatalysts has been rarely reported and to the best of our knowledge, the CuS–Ni3S2 composite as an OER electrocatalyst has not been reported till date.
Furthermore, the structure and morphology of the electrocatalyst are essential to its OER catalytic activity.20 Generally, designing a self-supporting catalytic electrode with 3D porous structure has the following advantages: (1) the substrate's 3D porous architecture provides a high loading area for the catalyst, which leads to the tremendous exposures of catalytic active sites.21,22 (2) 3D porous architectures ensure the sufficient infiltration of electrolytes as well as the fast departure of oxygen bubbles, resulting in expedited reaction kinetics.23 (3) The interconnected conductive 3D skeletons construct plenty of electron transport highways, contributing to the high electron transfer efficiency.24 However, although commercial 3D nickel foam (NF) with low cost has been widely employed for synthesizing various OER catalysts,7,25,26 the above-mentioned merits can be further enlarged by designing an improved novel NF-based substrate with further increased porosity and loading capacity.
Herein, for the first time, the CuS–Ni3S2 composite supported on an improved 3D porous substrate (CuS–Ni3S2/CuNi/NF) as a high-performance catalyst for OER was synthesized via a facile two-step method. Compared with pristine NF, the CuNi alloy electrodeposited on NF (CuNi/NF) with the unique hierarchical porous structure and nanodendrite morphology not only allowed a higher mass transfer and a higher loading area but also constructed more delicate electron transport pathway networks. Afterward, the surface of the bimetallic alloy was sulfurized into the CuS–Ni3S2 layer via a hydrothermal method. This in situ growth strategy ensures a close contact between the catalytic phase and bimetallic substrate, which is favorable to the fast electron transfer and the stability of the catalytic electrode. Therefore, the novel catalytic electrode CuS–Ni3S2/CuNi/NF presented outstanding electrocatalytic performance with a low overpotentials of 337, 444 and 510 mV at very high current densities of 100, 500, 1000 mA cm−2, respectively, and a strong durability of 15 h at a high current density of 100 mA cm−2 in 1.0 M KOH solution. As far as we know, most works have focused on the catalytic performance within relative low current density (smaller than 200 mA cm−2),6,13,25 while practical application of water splitting needs electrocatalysts that can steadily achieve high current density at low overpotential. As a consequence, the high activity and durability of CuS–Ni3S2/CuNi/NF under high current density is the significant merit of excellent OER catalysts for practical water splitting.
2. Experimental section
2.1 Synthesis of CuNi /NF
First, in order to remove the oxidation layer on the surface, NF was cleaned in acetone, 1 M HCl and deionized water (DI water) successively for 10 min under ultrasonication, then dried by high purity nitrogen. 3D porous CuNi alloy was electrodeposited via the CHI 660e electrochemical workstation. NF (working area: 0.4 cm × 0.3 cm), graphite rod and Ag/AgCl (filled with saturated KCl solution) were used as the working electrode, counter electrode and reference electrode, respectively. The electrodeposition process was performed in an aqueous electrolyte (saturated with N2) containing CuSO4·5H2O (0.01 M), NiSO4·6H2O (0.5 M), and H2SO4 (1.5 M) at a constant current density of 1.8 A cm−2 for 90 s. After deposition, the electrode was thoroughly cleaned with DI water and immediately dried by nitrogen. For comparison, Ni/NF was synthesized by a similar process without CuSO4·5H2O.2.2 Synthesis of CuS–Ni3S2/CuNi/NF
The surface of the CuNi alloy was sulfurized to CuS–Ni3S2 by the hydrothermal process. The obtained CuNi/NF was submerged into the Teflon-lined stainless autoclave containing 30 mL of 0.01 M thiourea solution. In addition, 0.005 M and 0.02 M concentrations were respectively employed for contrast. The autoclave was sealed and maintained at 150 °C for 10 h. After that, the autoclave was cooled down to room temperature naturally. The synthesized CuS–Ni3S2/CuNi/NF was repeatedly washed with DI water and ethanol in sequence, and dried in nitrogen. For comparison, Ni/NF was sulfurized by the same method to obtain Ni3S2/Ni/NF. The loading masses of CuS–Ni3S2/CuNi/NF (16.7 mg cm−2), Ni3S2/Ni/NF (12.1 mg cm−2) and CuNi/NF (11.7 mg cm−2) were measured using an analytical balance.2.3 Characterization
Scanning electron microscope (SEM) images and their corresponding energy dispersive X-ray (EDX) spectra and EDX mapping images were obtained on a Quanta 200 FEI. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images, and their corresponding EDX spectra and EDX mapping images were obtained on an FEI TENAI G2 F20 at 200 kV. EDX Samples for TEM were prepared by ultrasonic vibration in ethanol, and then the suspension was dropped onto carbon-enhanced copper grids and dried in air. X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo Fisher K-Alpha instrument and XPS spectra were referenced to the C 1s peak (284.8 eV). X-ray diffraction (XRD) patterns were recorded in the range of 5–100° (2θ) on an X-ray diffractometer (Bruker, AXS D8) with Cu Kα radiation operated at 40 kV and 30 mA.2.4 Electrochemical measurements
Electrochemical measurements were performed with a standard three-electrode system on an electrochemical workstation CHI 660e in 1.0 M KOH solution. The Hg/HgO (1.0 M KOH) electrode and graphite rod were respectively used as the reference electrode and the counter electrode. The obtained catalytic electrode was directly used as the working electrode. All reported potentials were calibrated to the RHE, and were converted using the equation ERHE = EHg/HgO + 0.098 + 0.059 × pH (1.0 M KOH, pH = 14). The overpotential (η) was calculated using the following equation: η = ERHE − 1.23 V.In order to fabricate the IrO2 decorated working electrodes (abbreviated as IrO2), IrO2 was dispersed in a mixture solution of DI water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol
ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nafion = 10
Nafion = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via sonication to form a homogeneous ink. Then the IrO2 catalyst ink was drop-casted onto NF with loading mass of 5 mg cm−2 and 16.7 mg cm−2, respectively and dried in air at room temperature.
1 via sonication to form a homogeneous ink. Then the IrO2 catalyst ink was drop-casted onto NF with loading mass of 5 mg cm−2 and 16.7 mg cm−2, respectively and dried in air at room temperature.
For OER activity, cyclic voltammetry (CV) curves were recorded at the scan rate of 5 mV s−1. All CV curves were corrected for ohmic losses. The ohmic losses that constitute the series resistance (Rs) can be obtained from the electrochemical impedance spectroscopy (EIS) measurements. The correction was performed using the following equation: Ecorrected = E − IRs, where E is the potential and I is the current. The Nyquist plots for the electron transfer efficiency investigation were obtained by the EIS measurement recorded at the overpotential of 0.376 V in the frequency range of 100 kHz to 10 mHz. Chemical double-layer capacitance (Cdl) was acquired through CV curves conducted at the non-faradaic current region ranging from 0.574 to 0.674 V (vs. RHE) at various scan rates of 30, 50, 70, 90, 110, 130 and 150 mV s−1. Long-term durability tests were conducted by employing chronopotentiometric methods at 100 mA cm−2 for 15 h and cyclic voltammetry with the scan rate of 100 mV s−1 for 5000 cycles from 1.124 to 1.624 V (vs. RHE).
2.5 Theoretical calculations
The generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof exchange–correlation functional correction was used to account for the dispersion interactions, and a 500 eV cutoff for the plane-wave basis set was employed to perform all the density functional theory (DFT) computations of the materials within the frame of the Vienna ab initio simulation package (VASP).27,28 The projector-augmented plane wave (PAW) method was used to describe the electron-ion interactions. 3 × 3 × 1 k-points were employed for the geometric optimization of the bulk Ni3S2 and four slab surfaces, of which two layers were fixed. Usually, the scheme developed by Nørskov et al. can be employed to gain insight into the OER, where the OER is assumed to involve four elementary reaction steps:29,30| H2O + * → *OH + H+ + e− | (A) |
| *HO → *O + H+ + e− | (B) |
| *O + H2O → *OOH + H+ + e− | (C) |
| *OOH → * + O2 + H+ + e− | (D) |
Herein, * and X* represent an adsorption site and an adsorbed X intermediate on the surface, respectively. The free energy of the OER is computed by the following equation:
| ΔG = ΔE + ΔZPE − TΔS |
The value of ΔE was obtained by the computation of geometrical structures. The values of ΔZPE and ΔS were determined by employing the computed vibrational frequencies and standard tables for the reactants and products in the gas phase. The dependence of enthalpy on temperature is not considered in the calculation. Moreover, an external bias U was imposed on each step by including the -eU term in the computation of reaction free energy. Consequently, the reaction free energy of each step could be expressed as follows:
| ΔGA = E(*OH) − E(*) – EH2O + 1/2EH2 + (ΔZPE − TΔS) − eU | (1) |
| ΔGB = E(*O) − E(*OH) + 1/2EH2 + (ΔZPE − TΔS) − eU | (2) |
| ΔGC = E(*OOH) − E(*O) − EH2O + 1/2EH2 + (ΔZPE − TΔS) − eU | (3) |
| ΔGD = E(*) − E(*OOH) + EO2 + 1/2EH2 + (ΔZPE − TΔS) − eU | (4) |
Herein, E (*), E (*OH), E (*O), and E(*OOH) are the computed DFT energies of the pure surface and the adsorbed surfaces with *OH, *O, and *OOH, respectively. EH2O, EH2 and EO2 are the computed energies for the sole H2O, H2 and O2 molecules, respectively. For the total reaction H2O → 1/2O2 + H2, the free-energy change was fixed at the experimental value of 2.46 eV per H2O molecule. Therefore, the Gibbs free energy of the reaction is 4.92 eV for each part of the oxygen generated. The difference between the corresponding minimum voltage needed for changing all the free-energy steps into downhill and the equilibrium potential (U = 1.23 V) is called the reaction overpotential.
3. Results and discussion
As shown in Scheme 1, the nanodendrite morphology of CuNi/NF was realized by the hydrogen bubbles generated and released from the cathode (NF) during the growth of deposited metal and functioned as a soft template.20,21 After the electrodeposition process, the silver NF turned reddish brown (photograph inset Scheme 1), which is attributed to the red color of Cu. Afterward, the as-prepared material underwent a sulfurization process by the hydrothermal method and became dark grey. This suggests that the surface layer of the bimetallic alloy was successfully converted into the CuS–Ni3S2 composite. Compared with the smooth surface of the pristine NF (Fig. S1†), Fig. S2A† exhibits the rough surface of the electrodeposited CuNi/NF, which uniformly and densely covered with numerous nanodendrites (shown in Fig. S2D† at higher magnifications). Moreover, as shown in Fig. S2B and C,† macropores of ∼50–100 nm in diameter on the plane surface of NF and larger macropores of ∼6–9 μm on the ridge of NF skeleton were uniquely constructed, which demonstrates the significantly increased porosity of the substrate. The EDX mapping images (Fig. S2E†) of a single nanodendrite revealed the uniform distribution of Ni and Cu in the CuNi nanodentrite, which attests to the bimetallic alloy's successful electrodeposition. Moreover, the SEM images of electrodeposited Ni/NF shown in Fig. S3† exhibit the flat layer of deposited Ni, with neither nanodendrite morphology nor 3D porous structures. Therefore, Cu has played an essential role in constructing the unique 3D structure of CuNi/NF with increased porosity, as well as increased loading capacity for the subsequently synthesized catalyst.After the sulfurization process, the 3D structure with increased porosity remained intact (Fig. 1A–D). Fig. 1A presents a homogeneous and dense loading of nanoparticles on the substrate. The piled up nanoparticles uniformly constructed macropores of ∼50–100 nm in diameter on the plane surface of NF (Fig. 1B and C), and the more stereoscopic macropores of ∼6–9 μm on the ridge of NF skeleton (Fig. 1D). However, the surface of sulfurized Ni/NF (Fig. S4A–C†) exhibited compactly and uniformly dispersed Ni3S2 nanohills on both the plane surface and the ridge of NF with negligibly increased porosity as compared with NF. This illustrates that the CuNi/NF substrate has successfully constructed the sophisticated and porous CuS–Ni3S2/CuNi/NF with extremely abundant active site exposure and sufficient space for electrolyte infusion as well as oxygen bubbles release during the OER process.
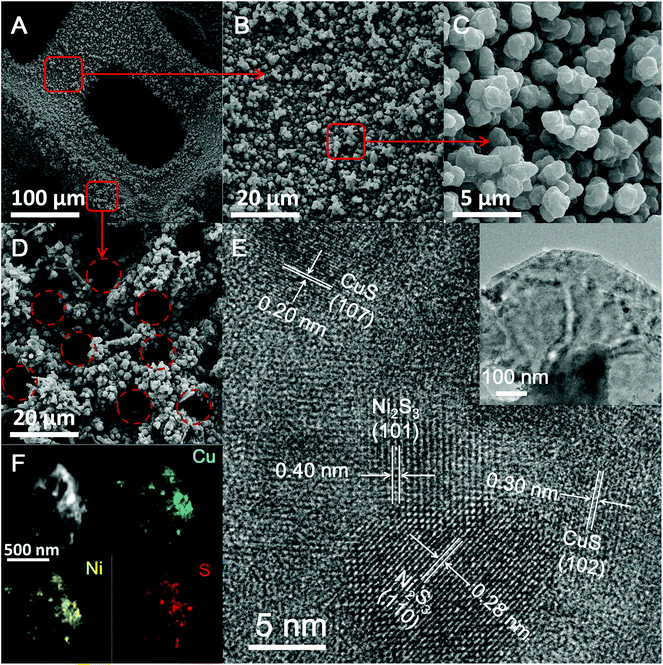 | ||
| Fig. 1 (A–D) SEM images of the 3D porous CuS–Ni3S2/CuNi/NF at different magnifications. (E) HRTEM and TEM (inset) images of CuS–Ni3S2 and (F) the EDX mapping images of the CuS–Ni3S2 nanolayer. | ||
CuS–Ni3S2 was peeled off from the CuS–Ni3S2/CuNi/NF electrode by the strong ultrasonication process, and the TEM image of CuS–Ni3S2 (the inset image of Fig. 1E) revealed its nanolayer structure. In the HRTEM images of CuS–Ni3S2 (Fig. 1E), four sets of lattice fringes can be observed. According to previous reports, the lattice fringe spacings of 0.20 and 0.30 nm correspond to the (107) and (102) crystal planes of CuS respectively,31,32 while 0.28 and 0.40 nm correspond to the (110) and (101) crystal planes of Ni3S2, respectively.33,34 EDX mapping images of the CuS–Ni3S2 nanolayer (Fig. 1F) and the CuS–Ni3S2/CuNi/NF electrode skeleton (Fig. S5†) indicate the homogeneous dispersion of Cu, Ni and S on both the catalyst film and throughout the catalytic electrode.
The composition of CuS–Ni3S2/CuNi/NF was further demonstrated by XPS spectra and XRD patterns. The two strong peaks in the Cu 2p region (Fig. 2A) at 932.1 and 952.1 eV, respectively, correspond to Cu 2p3/2 and Cu 2p1/2 of Cu2+, as previously reported.17,35 In the Ni 2p spectrum (Fig. 2B), peaks at 855.5 and 873.6 eV correspond to Ni 2p3/2 and Ni 2p1/2 of Ni3S2, respectively, and peaks at 861.5 and 879.2 eV can be assigned to the shakeup-type peaks.25,36 It is worth noting that the Ni 2p3/2 signal of Ni3S2 in CuS–Ni3S2/CuNi/NF exhibits a positive shift of about 0.1 eV relative to that in Ni3S2/Ni/NF, which suggests the existence of electronic interactions between Ni3S2 and CuS, and the existence of the coupling interfaces.37 Moreover, the binding energies of S 2p (Fig. 2C) appearing at 161.9 and 163.0 eV can be assigned to S 2p3/2 and S 2p1/2, thus indicating the presence of S2−.38,39 The peak at 168.8 eV corresponds to SO42−, which may have been formed due to the oxidation of the surface of the sulfide composite.40
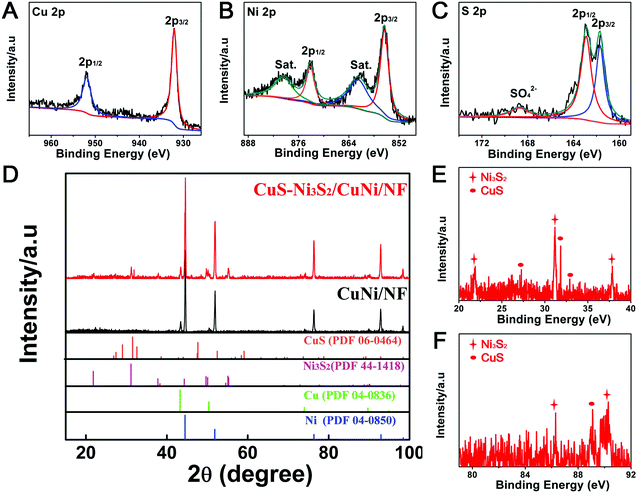 | ||
| Fig. 2 XPS spectra in (A) Cu 2p, (B) Ni 2p and (C) S 2p regions of CuS–Ni3S2/CuNi/NF; (D–F) XRD patterns of CuNi/NF and CuS–Ni3S2/CuNi/NF. | ||
As shown in the XRD patterns of CuNi/NF (Fig. 2D), the diffraction peaks at 43.3°, 50.4°, 74.1° and 89.9° can be assigned to Cu (PDF#04-0836) and the diffraction peaks at 44.5°, 51.8°, 76.4°, and 92.9° can be attributed to Ni (PDF#04-0850). In the XRD pattern of CuS–Ni3S2/CuNi/NF, the observed diffraction peaks at 27.7°, 31.8°, 32.8°, 59.3° and 88.9° can be assigned to the CuS phase (PDF#06-0464), and the peaks at 21.7°, 31.1°, 37.8°, 50.1°, 55.3°, 86.3° and 90.4° can be attributed to the Ni3S2 phase (PDF#44-1418). In comparison, the characteristic peaks of CuNi/NF remain after the sulfurization process, illustrating the existence of the CuNi metal core under the sulfide phase.
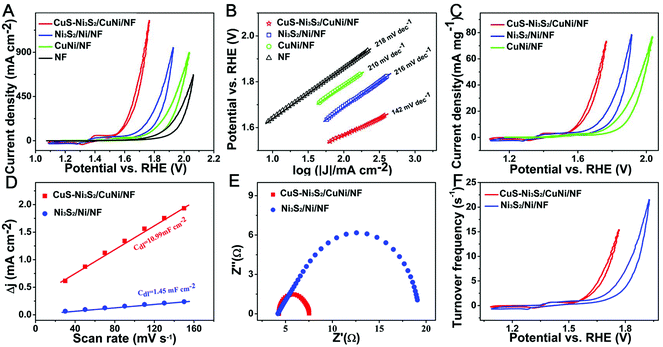
In order to explore the effect of thiourea concentration on the catalyst synthesis and the corresponding OER performance, 0.02, 0.01 and 0.005 M thiourea were respectively employed to sulfurize CuNi/NF. The electrochemical OER performances of the different sulfurization products are shown in Fig. S6.† To achieve the current density of 200 mA cm−2, CuS–Ni3S2/CuNi/NF (synthesized by 0.01 M thiourea) needs the lowest overpotential of 380 mV compared with that required for CuS–Ni3S2/CuNi/NF-0.02 M (400 mV) and CuS–Ni3S2/CuNi/NF-0.005 M (390 mV), suggesting that 0.01 M is the optimal thiourea concentration for the synthesis of the superior OER electrocatalyst. Fig. S7† shows the morphologies of CuS–Ni3S2/CuNi/NF-0.02 M and CuS–Ni3S2/CuNi/NF-0.005 M. Compared with the above-described morphology of CuS–Ni3S2/CuNi/NF (Fig. 1A–D), the pores and cavities on the ridge of the CuS–Ni3S2/CuNi/NF-0.02 M skeleton (Fig. S7A–C†) collapsed, while the pores disappeared on the plane surface of CuS–Ni3S2/CuNi/NF-0.005 M; both lost plenty of exposed active sites. As a consequence, the superior OER performance of CuS–Ni3S2/CuNi/NF has arisen from its unique morphology with high porosity. Hereafter, all CuS–Ni3S2/CuNi/NF mentioned throughout the manuscript were obtained via 0.01 M thiourea.
OER activities of NF, CuNi/NF and Ni3S2/Ni/NF were measured for comparison. As shown in Fig. 3A, the redox peaks at ∼1.4 V before the oxygen evolution of CuS–Ni3S2/CuNi/NF originated from the electron-transfer between Ni2+ and Ni3+ species.36 CuS–Ni3S2/CuNi/NF requires the overpotential of 337 mV to reach the current density of 100 mA cm−2, which is much lower than that of Ni3S2/Ni/NF (465 mV), CuNi/NF (555 mV) and NF (631 mV). It only needs 444 and 510 mV to reach 500 and 1000 mA cm−2, respectively, which is much smaller than that of the most recently reported non-noble metal-based OER catalysts at such high current densities (Table S1†). Additionally, the reaction kinetics of the above-studied electrodes were revealed by Tafel plots as shown in Fig. 3B. The relatively large Tafel slope, 142 mV dec−1 of CuS–Ni3S2/CuNi/NF can be attributed to the high oxidation current peak, which cannot be separated well from the OER catalytic current, as observed in previous reports.25,41 Nonetheless, the Tafel slope of CuS–Ni3S2/CuNi/NF (142 mV dec−1) is significantly smaller than that of Ni3S2/Ni/NF (216 mV dec−1), CuNi/NF (210 mV dec−1), and the pure NF (218 mV dec−1), attesting to the much faster OER kinetics of CuS–Ni3S2/CuNi/NF.42
To compare the OER activity of CuS–Ni3S2/CuNi/NF with the benchmark OER catalyst IrO2, two IrO2 decorated electrodes with different loading masses have been further investigated as shown in Fig. S8.† When the loading mass of IrO2 (16.7 mg cm−2) and CuS–Ni3S2/CuNi/NF were the same, CuS–Ni3S2/CuNi/NF achieved a comparable OER activity with IrO2, and the catalytic activity of CuS–Ni3S2/CuNi/NF even exceeded that of IrO2 at the high potential range. However, it is too expensive to employ such a high loading mass of the noble metal-based benchmark catalyst IrO2 in real applications. Thus, the loading mass of IrO2 was decreased to 5 mg cm−2, which is still higher than that of IrO2 in other reports.43,44 In this case, CuS–Ni3S2/CuNi/NF obviously outperforms IrO2, which confirms its outstanding electrocatalytic behavior. On the other hand, it is worth mentioning that compared with both IrO2 decorated electrodes, CuS–Ni3S2/CuNi/NF can reach very high current densities of 500 and 1000 mA cm−2 with a steady CV curve, without the serious shaking caused by oxygen release. This may have resulted from the unique 3D structure with increased porosity, which led to a fast oxygen release process.
In order to comprehensively evaluate the intrinsic OER performances of CuS–Ni3S2/CuNi/NF, Ni3S2/Ni/NF, and CuNi/NF,45 their mass activities were examined and are shown in Fig. 3C. To reach the current density of 10 mA mg−1, CuS–Ni3S2/CuNi/NF needs a lower overpotential of 368 mV than that of Ni3S2/Ni/NF (481 mV) and CuNi/NF (568 mV). Therefore, CuS–Ni3S2/CuNi/NF exhibited a superior catalytic activity measured by the current density normalized by both the geometrical area and the loading mass, and the excellent performance of CuS–Ni3S2/CuNi/NF may be ascribed to the unique 3D porous structure of the catalytic electrode and the synergetic effect of CuS and Ni3S2 in the sulfide composite.
To further attest to the above estimation, the Cdl values of CuS–Ni3S2/CuNi/NF and Ni3S2/Ni/NF were then evaluated to uncover the actual quantities of catalytic sites of electrocatalysts as the electrochemical surface area (ECSA) is proportional to the Cdl.46 CV curves were collected in the non-faradaic current region of 0.574 to 0.674 V in 1.0 M KOH of the scan rate from 30 to 150 mV s−1 (Fig. S9†).47 The corresponding Cdl values of CuS–Ni3S2/CuNi/NF and Ni3S2/Ni/NF electrodes can be calculated by the slope extracted from the linear relationship of the current density against the increasing scan rates. As shown in Fig. 3D, Cdl of CuS–Ni3S2/CuNi/NF is 10.99 mF cm−2, about 7 times that of the Cdl of Ni3S2/Ni/NF (1.45 mF cm−2), which indicates the much higher ECSA of CuS–Ni3S2/CuNi/NF as compared to Ni3S2/Ni/NF; this can be ascribed to the following. Firstly, compared with the much flatter morphology of Ni3S2/Ni/NF, which may conceal many active sites, the 3D porous morphologies constructed by the piled up nanoparticles of CuS–Ni3S2/CuNi/NF have realized the exposure of the numerous active sites. Secondly, the numerous pores of CuS–Ni3S2/CuNi/NF helped the electrolyte to infuse into the catalytic electrode and sufficiently contact the exposed active sites, which also contributed to the larger ECSA. Thirdly, coupling the interfaces of the CuS phase and Ni3S2 phase may boost more new active sites. Furthermore, the electrode kinetics of the OER was investigated by the Nyquist plots. As shown in Fig. 3E, the charge transfer resistance (Rct) of CuS–Ni3S2/CuNi/NF (3.2 Ω) is much smaller than that of Ni3S2/Ni/NF (14.8 Ω), which indicates the fast electron transfer between the active sites on the surface of CuS–Ni3S2/CuNi/NF and the electrolyte. This may be attributed to tremendous delicate high-efficiency electronic transmission pathways constructed by the 3D interconnected porous CuNi skeleton of CuS–Ni3S2/CuNi/NF.
The calculation of the turnover frequency (TOF) for each active site makes it possible to compare the intrinsic activity of CuS–Ni3S2/CuNi/NF with other different catalytic electrodes. The number of active sites was quantified by the electrochemical method, referring to the previous literature.48,49 Fig. S10† shows the CV curves of CuS–Ni3S2/CuNi/NF in the region of 0.25 to 0.85 V at pH 7, and the integrated charge over the whole potential range should be proportional to the total number of active sites. Detailed descriptions of the calculations of the quantity of active sites and the corresponding TOF are presented in Fig. S10.†Fig. 3F presents the CV curves normalized by the quantity of active sites, and expressed in terms of TOF. To obtain the TOF value of 5 s−1, Ni3S2/Ni/NF required the overpotential of 534 mV, while CuS–Ni3S2/CuNi/NF only needed a much lower overpotential of 425 mV. This demonstrated the superior intrinsic activity of the catalytic active phase, CuS–Ni3S2, as compared to the individual Ni3S2 phase. The intrinsically enhanced activity may be attributed to the participation of CuS in the CuS–Ni3S2 composite.
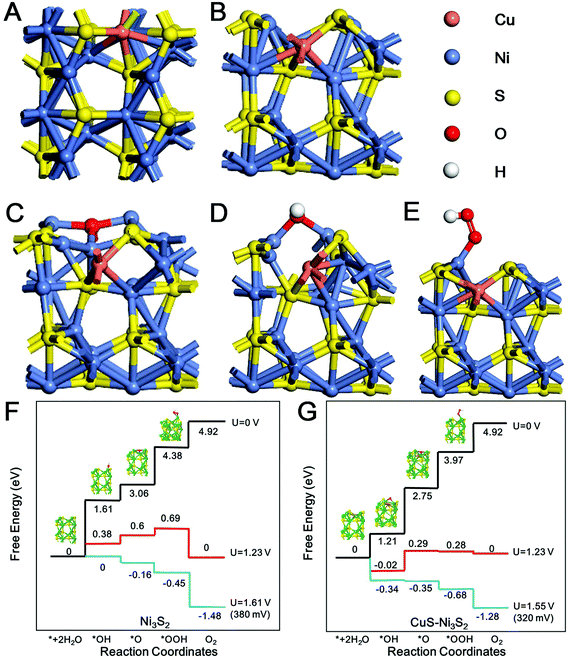
In order to prove the above experimental phenomenon, theoretical calculations focused on the influence of CuS on the OER catalytic activity of CuS–Ni3S2 were conducted, and the functional theory (DFT) was used to calculate the Gibbs free energy (ΔG) of the OER. In the coupling interfaces between CuS and Ni3S2, Cu atoms substituting for Ni atoms in the Ni3S2 phase were rationally constructed and the top view and side view of the CuS–Ni3S2 interface theoretical model are shown in Fig. 4A and B. According to the lowest chemisorption free energy, Ni sites acted as the adsorption active sites of *OH, *O and *OOH intermediates during the OER in both the Ni3S2 surface and CuS–Ni3S2 interfaces, and the adsorption configurations on the CuS–Ni3S2 interface are shown in Fig. 4C–E. Thus, all the reaction steps discussed below took place on Ni sites, and we focused on the effect of the participation of CuS on Ni sites in CuS–Ni3S2 interfaces. As shown in Fig. 4F and G, the free energy diagrams of OER for the Ni3S2 surface and the CuS–Ni3S2 interface exhibited a four-step reaction involving three intermediates of *OH, *O and *OOH. Firstly, when no bias was applied (U = 0 V), all of the steps on the Ni3S2 surface and CuS–Ni3S2 interface were found to move uphill (ΔG > 0). Until the equilibrium potential (U = 1.23 V) for OER was applied, ΔG(*OOH→*+O2) of Ni3S2 became negative, while on the CuS–Ni3S2 interface, ΔG(*+H2O→*OH), ΔG(*O→*OOH) and ΔG(*OOH→*+O2) simultaneously became negative values. These three spontaneous reaction steps could lead to an easier OER process, and according to the only positive ΔG value for CuS–Ni3S2, *OH + OH− → *O + H2O should be the rate-determining step of CuS–Ni3S2 for the OER. Moreover, compared with 0 eV ΔG(*+H2O→*OH) of Ni3S2, the negative ΔG(*+H2O→*OH) value −0.02 eV of CuS–Ni3S2 implies the more effective adsorption in the first catalytic step, which could easily trigger the subsequent reaction steps. Finally, we employed the minimum bias to change all the steps into downhill (ΔG < 0), and the difference between the above-mentioned minimum bias and the equilibrium potential (U = 1.23 V) for the OER is exactly the onset overpotential of the catalyst for the OER.9 With the participation of CuS, the required overpotential value decreased from 380 mV of Ni3S2 to 320 mV of CuS–Ni3S2, which is consistent with the experimental data shown in Fig. 3A. Moreover, the ΔG(*+H2O→*OH) value is −0.34 eV for CuS–Ni3S2, which is much lower than 0 eV ΔG(*+H2O→*OH) of Ni3S2, demonstrating the more obviously thermodynamically favored catalytic reaction step with the assistance of CuS. Therefore, the participation of CuS effectively enhanced the Ni sites’ OER catalytic activity of Ni3S2 by decreasing both the ΔG of the intermediates and the overpotential needed to drive the OER. Additionally, in order to further explore the influence of the participation of CuS in the electron conductivity of the catalytic material, the density of states (DOS) for CuS–Ni3S2 and pristine Ni3S2 were calculated. As shown in Fig. S11,† DOS of CuS–Ni3S2 and pristine Ni3S2 catalyst are continuous around the Fermi level, suggesting their intrinsic metallic characters. Remarkably, the DOS of CuS–Ni3S2 near the Fermi level is stronger than that of pristine Ni3S2, indicating that the electrical behavior of CuS–Ni3S2, especially the concentration of charge carrier and the electronic conductivity, have been further enhanced by the participation of CuS. The improvement of the electron transfer efficiency proved by DOS theoretical calculation is consistent with the experimental data shown in the Nyquist plots in Fig. 3E.
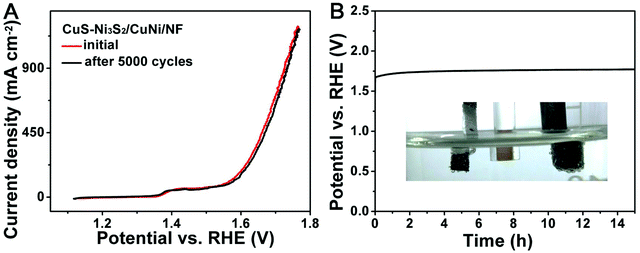
The durability is another important criterion for an electrocatalyst for the OER. The long-term stability of the catalytic electrode was firstly explored by 5000 continuous CV cycles. As shown in Fig. 5A, the polarization plot after continuously working for 5000 cycles nearly overlaps with the initial polarization plot, demonstrating the excellent stability of CuS–Ni3S2/CuNi/NF. Besides, chronopotentiometry at a relatively high constant current density of 100 mA cm−2 was carried out and is shown in Fig. 5B. Over a period of 15 h, the potential to attain a high current density of 100 mA cm−2 exhibited only slight decay. The robustness may be due to the in situ growth and close contact between the sulfide composite and bimetallic substrate, which leads to the stable binding strength between them. During the long-term stability test, steady tiny bubbles were generated and released from the surface of the catalytic electrode during the entire chronopotentiometry process and can be observed from the inset photograph in Fig. 5B and Video S1.† As shown in Fig. S12A and B,† the SEM images of CuS–Ni3S2/CuNi/NF after the 15 h duration test illustrate that the morphology of CuS–Ni3S2/CuNi/NF remained almost the same after the vigorous OER process in the harsh reaction environment, implying the robustness of this catalytic electrode.
4. Conclusion
In summary, we have successfully synthesized a novel OER catalyst, CuS–Ni3S2/CuNi/NF, and explored the positive role CuS has played in this Ni3S2-based electrocatalyst during the OER process. This 3D OER catalytic electrode preserved its CuNi/NF porous skeleton both as the high-efficiency electron transport pathway networks and the template for CuS–Ni3S2/CuNi/NF. Consequently, the 3D porous morphology of CuS–Ni3S2/CuNi/NF realized the maximum exposure of active sites and fast mass transfer during the OER process. Moreover, the in situ growth of the sulfide composite nanolayer from the bimetallic alloy guaranteed the fast electron transfer and excellent stability of the catalytic electrode. Based on the above aspects, the catalytic electrode has achieved excellent OER activity and stability at high current density, even greater than 1000 mA cm−2. Despite the activity measured by the current density normalized by the geometrical area, CuS–Ni3S2/CuNi/NF also exhibited superior mass activity. Moreover, the higher TOF of CuS–Ni3S2/CuNi/NF compared with that of Ni3S2/Ni/NF indicated the improved intrinsic activity of the catalytic active phase by the participation of CuS. Theoretical calculations have demonstrated that CuS can effectively enhance the Ni sites’ OER catalytic activity of Ni3S2, and also improve the electronic conductivity of Ni3S2. As a consequence, although CuS has been rarely studied as an OER catalyst, this work has proved that it may be promising for use in the design of novel OER catalysts for further improved catalytic performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are most grateful to the talents start scientific research funds of China University of Petroleum, Beijing (No. 2462016YJRC022).References
- B. Chang, S. Hao, Z. X. Ye and Y. C. Yang, Chem. Commun., 2018, 54, 2393–2396 RSC.
- J. X. Feng, S. H. Ye, H. Xu, Y. X. Tong and G. R. Li, Adv. Mater., 2016, 28, 4698–4703 CrossRef CAS PubMed.
- J. X. Feng, H. Xu, Y. T. Dong, S. H. Ye, Y. X. Tong and G. R. Li, Angew. Chem., Int. Ed., 2016, 55, 3694–3698 CrossRef CAS.
- H. Zhong, X. Cheng, H. Xu, L. Li, D. Li, P. Tang, N. Alonso-Vante and Y. Feng, Electrochim. Acta, 2017, 258, 554–560 CrossRef CAS.
- S. Fu, J. Song, C. Zhu, G.-L. Xu, K. Amine, C. Sun, X. Li, M. H. Engelhard, D. Du and Y. Lin, Nano Energy, 2018, 44, 319–326 CrossRef CAS.
- X. Li, X. Du, X. Ma, Z. Wang, X. Hao, A. Abudula, A. Yoshida and G. Guan, Electrochim. Acta, 2017, 250, 77–83 CrossRef CAS.
- Y. Wang, B. Zhang, W. Pan, H. Ma and J. Zhang, ChemSusChem, 2017, 10, 4170–4177 CrossRef CAS.
- J. H. Hao, W. S. Yang, Z. P. Huang and C. Zhang, Adv. Mater. Interfaces, 2016, 3, 8 Search PubMed.
- L. L. Feng, G. Yu, Y. Wu, G. D. Li, H. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS.
- X. Zhong, J. Tang, J. Wang, M. Shao, J. Chai, S. Wang, M. Yang, Y. Yang, N. Wang, S. Wang, B. Xu and H. Pan, Electrochim. Acta, 2018, 269, 55–61 CrossRef.
- X. Du, Z. Yang, Y. Li, Y. Gong and M. Zhao, J. Mater. Chem. A, 2018, 6, 6938–6946 RSC.
- N. Cheng, Q. Liu, A. M. Asiri, W. Xing and X. Sun, J. Mater. Chem. A, 2015, 3, 23207–23212 RSC.
- C. Lu, J. Wang, S. Czioska, H. Dong and Z. Chen, J. Phys. Chem. C, 2017, 121, 25875–25881 CrossRef CAS.
- X. Liu, S. Cui, Z. Sun, Y. Ren, X. Zhang and P. Du, J. Phys. Chem. C, 2016, 120, 831–840 CrossRef CAS.
- M. T. Zhang, Z. Chen, P. Kang and T. J. Meyer, J. Am. Chem. Soc., 2013, 135, 2048–2051 CrossRef CAS.
- X. L. Xiong, C. You, Z. Liu, A. M. Asiri and X. P. Sun, ACS Sustainable Chem. Eng., 2018, 6, 2883–2887 CrossRef CAS.
- W. B. Kim, S. H. Lee, M. Cho and Y. Lee, Sens. Actuators, B, 2017, 249, 161–167 CrossRef CAS.
- H. Heydari, S. E. Moosavifard, S. Elyasi and M. Shahraki, Appl. Surf. Sci., 2017, 394, 425–430 CrossRef CAS.
- S. Wang, S. Jiao, J. Wang, H. S. Chen, D. Tian, H. Lei and D. N. Fang, ACS Nano, 2017, 11, 469–477 CrossRef CAS PubMed.
- B. J. Plowman, L. A. Jones and S. K. Bhargava, Chem. Commun., 2015, 51, 4331–4346 RSC.
- H. Xu, J.-X. Feng, Y.-X. Tong and G.-R. Li, ACS Catal., 2016, 7, 986–991 CrossRef.
- W. Wang, S. You, X. Gong, D. Qi, B. K. Chandran, L. Bi, F. Cui and X. Chen, Adv. Mater., 2016, 28, 270–275 CrossRef CAS.
- H. Ashassi-Sorkhabi and P. La'le Badakhshan, Appl. Surf. Sci., 2017, 419, 165–176 CrossRef CAS.
- L. Wei, K. Goh, O. Birer, H. E. Karahan, J. Chang, S. Zhai, X. Chen and Y. Chen, Nanoscale, 2017, 9, 4401–4408 RSC.
- Y. Gong, Z. Xu, H. Pan, Y. Lin, Z. Yang and X. Du, J. Mater. Chem. A, 2018, 6, 5098–5106 RSC.
- W. J. Zhou, X. J. Wu, X. H. Cao, X. Huang, C. L. Tan, J. Tian, H. Liu, J. Y. Wang and H. Zhang, Energy Environ. Sci., 2013, 6, 2921–2924 RSC.
- A. J. Misquitta, B. Jeziorski and K. Szalewicz, Phys. Rev. Lett., 2003, 91, 033201 CrossRef PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- I. C. Man, H.-Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- J. Rossmeisl, Z. W. Qu, H. Zhu, G. J. Kroes and J. K. Nørskov, J. Electroanal. Chem., 2007, 607, 83–89 CrossRef CAS.
- L. Zhang, Y. Guo, A. Iqbal, B. Li, D. Gong, W. Liu, K. Iqbal, W. Liu and W. Qin, Int. J. Hydrogen Energy, 2018, 43, 1251–1260 CrossRef CAS.
- F. Li, J. Wu, Q. Qin, Z. Li and X. Huang, Powder Technol., 2010, 198, 267–274 CrossRef CAS.
- H. Liu, X. Ma, Y. Rao, Y. Liu, J. Liu, L. Wang and M. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 10890–10897 CrossRef CAS PubMed.
- N. Tronganh, Y. Gao, W. Jiang, H. Tao, S. Wang, B. Zhao, Y. Jiang, Z. Chen and Z. Jiao, Appl. Surf. Sci., 2018, 439, 386–393 CrossRef CAS.
- K.-J. Huang, J.-Z. Zhang, Y. Liu and Y.-M. Liu, Int. J. Hydrogen Energy, 2015, 40, 10158–10167 CrossRef CAS.
- M. Al-Mamun, H. J. Yin, P. R. Liu, X. T. Su, H. M. Zhang, H. G. Yang, D. Wang, Z. Y. Tang, Y. Wang and H. J. Zhao, Nano Res., 2017, 10, 3522–3533 CrossRef CAS.
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. H. Dong, S. H. Liu, X. D. Zhuang and X. L. Feng, Angew. Chem., Int. Ed., 2016, 55, 6702–6707 CrossRef CAS PubMed.
- Q. Liu, L. S. Xie, Z. Liu, G. Du, A. M. Asiri and X. P. Sun, Chem. Commun., 2017, 53, 12446–12449 RSC.
- Z. Li and Z. Zhang, Nano Res., 2018, 11, 1530–1540 CrossRef CAS.
- Y. Yang, K. Zhang, H. Lin, X. Li, H. C. Chan, L. Yang and Q. Gao, ACS Catal., 2017, 7, 2357–2366 CrossRef CAS.
- S. Qu, J. Huang, J. Yu, G. Chen, W. Hu, M. Yin, R. Zhang, S. Chu and C. Li, ACS Appl. Mater. Interfaces, 2017, 9, 29660–29668 CrossRef CAS.
- S. Zhao, M. Li, M. Han, D. Xu, J. Yang, Y. Lin, N.-E. Shi, Y. Lu, R. Yang, B. Liu, Z. Dai and J. Bao, Adv. Funct. Mater., 2018, 28, 1706018 CrossRef.
- S. Anantharaj, K. Karthick, M. Venkatesh, TVSV. Simha, AS. Salunke, L. Ma, H. Liang and S. Kundu, Nano Energy, 2017, 39, 30–43 CrossRef CAS.
- H. Wang, T. Zhou, P. Li, Z. Cao, W. Xi, Y. Zhao and Y. Ding, ACS Sustainable Chem. Eng., 2017, 6, 380–388 CrossRef.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069–8097 CrossRef CAS.
- C. Luan, G. Liu, Y. Liu, L. Yu, Y. Wang, Y. Xiao, H. Qiao, X. Dai and X. Zhang, ACS Nano, 2018, 12, 3875–3885 CrossRef CAS PubMed.
- D. Merki, H. Vrubel, L. Rovelli, S. Fierro and X. L. Hu, Chem. Sci., 2012, 3, 2515–2525 RSC.
- D. Merki, S. Fierro, H. Vrubel and X. Hu, Chem. Sci., 2011, 2, 1262–1267 RSC.
- J. Tian, Q. Liu, A. M. Asiri and X. Sun, J. Am. Chem. Soc., 2014, 136, 7587–7590 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qi01148f |
| This journal is © the Partner Organisations 2019 |