The roles of thermally activated delayed fluorescence sensitizers for efficient red fluorescent organic light-emitting diodes with D–A–A type emitters†
Ya-Kun
Wang‡
 a,
Chen-Chao
Huang‡
a,
Sarvendra
Kumar
a,
Sheng-Fan
Wu
a,
Yi
Yuan
a,
Aziz Khan
a,
Zuo-Quan
Jiang
a,
Chen-Chao
Huang‡
a,
Sarvendra
Kumar
a,
Sheng-Fan
Wu
a,
Yi
Yuan
a,
Aziz Khan
a,
Zuo-Quan
Jiang
 *a,
Man-Keung
Fung
*a,
Man-Keung
Fung
 ab and
Liang-Sheng
Liao
ab and
Liang-Sheng
Liao
 *ab
*ab
aInstitute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, Suzhou 215123, P. R. China. E-mail: zqjiang@suda.edu.cn
bInstitute of Organic Optoelectronics (IOO), JITRI, Wujiang, Suzhou, Jiangsu 215211, P. R. China. E-mail: lsliao@suda.edu.cn; Fax: +86-512-65882846; Tel: +86-512-65880951
First published on 20th November 2018
Abstract
A thermally activated delayed fluorescence (TADF) sensitizer plays a key role in harvesting 100% excitons and transferring energy in TADF-assisted fluorescent organic light-emitting diodes (TAF-OLEDs). Despite its important roles, there have been few studies investigating how a TADF sensitizer affects the overall device performance and resonance energy transfer (RET) process in TAF-OLEDs. In this study, we investigate the effects of different parameters of sensitizers, i.e., spectra overlap and radiative constant (kr) on the device performances and RET process by developing two red emitters (OTPA-BT-CN and POZ-BT-CN) and choosing two TADF materials with similar photoluminescence spectra (4CzIPN and OSTFB). Mechanism studies reveal that kr of the sensitizer, rather than the overlap of sensitizer and fluorescent emitter, plays a more significant role in TAF-OLEDs. As a result, the device with higher kr of the sensitizer achieves a maximum external quantum efficiency (EQE) of 12.4% with an emission peak of 612 nm, which is higher by 2.0-fold compared with that of a 4CzIPN-based device (6.3%).
Introduction
Organic light-emitting diodes (OLEDs) have exhibited significant achievements in visible spectrum since the introduction of thermally activated delayed fluorescence (TADF), which has great potential in achieving 100% internal quantum efficiency (IQE) through reverse intersystem crossing (RISC) from the triplet (T1) to single (S1) state without using noble metals.1–7 Due to low cost and high external quantum efficiency (EQE), TADF materials have become promising candidates for the traditional phosphorescent materials. Until now, the efficiencies of blue and green TADF emitters have both surpassed 30%, whereas the development of red color lags behind relatively.8–11 There may be two reasons behind this fact: (1) the effect of energy-gap law, which dictates that the non-radiative constant will increase with red-shift in the emission spectra.12,13 (2) The design strategy contradiction of red or even near-infrared TADF emitters, which includes enhancing the donor/acceptor (D/A) interplay to achieve red emission and disturbing the highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO) overlap to achieve a balance.14In addition to directly adopting TADF as emitters, another strategy to harvest 100% excitons is TADF-assisted fluorescent OLEDs (TAF-OLEDs), which use TADF as the sensitizer and a fluorescent material as the emitter.15–17 Using this strategy, the two intrinsically contradictory parameters in red TADF emitters can be settled. By adopting this strategy, Adachi et al. reported full-color light emission (blue to red) and achieved impressive EQE of ∼10% by utilizing TADF sensitizers.15 Very recently, Duan et al. delivered superior EQEs of approximately 30% for green emitters through engineering of traditional hosts.16 Inspired by this fast-growing research topic, it is very important to investigate the roles of a sensitizer in tuning the performances of TAF-OLEDs.18
In this work, we study the function of TADF sensitizer in TAF-OLEDs by choosing two TADF emitters (4CzIPN and OSTFB) with similar photoluminescence spectra for two red emitters (POZ-BT-CN and OTPA-BT-CN). The new red emitters show an electroluminescence (EL) peak at 750 nm and good photoluminescence quantum yield (PLQY), which is suitable for the research of red TAF-OLEDs. As for the TADF sensitizers, commercially available 4CzIPN and novel spiro-based OSTFB are choosed due to their similar photoluminescence spectra. To demonstrate which parameter of TADF sensitizer is more important for increasing the overall device performance and resonance energy transfer (RET) process, the overlaps of the TADF sensitizer and red emitter and the radiative constant (kr) are compared. Furthermore, different types of devices are also fabricated to prove the phenomenon. As a result, the device based on OSTFB, which shows similar emission spectrum but larger kr, exhibits more favorable energy transfer and higher device performances. The 4CzIPN-based devices show clear emission from TADF sensitizer after changing its doping concentration from 10% to 50%. Consequently, the device performances based on OSTFB are higher (12.4%/10.1%) with an EL peak at 613/626 nm, whereas 4CzIPN-based devices show much inferior performances (6.3%/5.9%) with an EL peak at 610/623 nm.
Materials and methods
General methods
All chemicals and reagents were used as received from commercial sources without further purification. THF and toluene used in synthetic routes were purified by PURE SOLV (Innovative Technology) purification system. 1H NMR and 13C NMR spectra were recorded using a Bruker 600 spectrometer at room temperature. Mass spectra were recorded using a Thermo ISQ mass spectrometer with a direct exposure probe. UV-Vis absorption spectra were recorded using a Perkin Elmer Lambda 750 spectrophotometer. PL spectra and phosphorescent spectra were recorded using a Hitachi F-4600 fluorescence spectrophotometer. Differential scanning calorimetry (DSC) was performed on a TA DSC 2010 unit at a heating rate of 10 °C min−1 in a nitrogen atmosphere. The glass transition temperatures (Tg) were determined from the second heating scan. Thermogravimetric analysis (TGA) was performed using a TA SDT 2960 instrument at a heating rate of 10 °C min−1 in a nitrogen atmosphere, and the temperature at 5% weight loss was used as the decomposition temperature (Td). Transient PL decays were measured using a single photon counting spectrometer from HORIBA Jobin Yvon. DFT calculations were performed using a B3LYP/6–31G(D) atomic basis set.Electrochemical property
Electrochemical measurements were obtained using a CHI600 voltammetric analyzer. A conventional three-electrode configuration consisting of a platinum working electrode, a Pt-wire counter electrode, and an Ag/AgCl reference electrode was used. The solvent in all measurements was CH2Cl2, and the supporting electrolyte was 0.1 M [Bu4N]PF6. Ferrocene was added as a calibrant after each set of measurements, and all potentials reported were quoted with reference to the ferrocene-ferrocenium (Fc/Fc+) couple at a scan rate of 100 mV s−1.Device fabrication process
OLEDs were fabricated on an ITO glass substrate layer (110 nm, 15 Ω square−1) under a base pressure of 3 × 10−6 Torr. The active area of each device is 0.09 cm2. The deposition rates and thicknesses of all materials were monitored with oscillating quartz crystals. The doping layer was deposited by using two different sensors to monitor the deposition rates of both the host and dopant materials. The deposition rate of the host was controlled at 0.2 nm s−1, and the deposition rate of the dopant was adjusted according to the volume ratio doped in the host materials. The electroluminescence (EL) and current density–voltage (J–V) characteristics of the devices were measured by a constant current source (Keithley 2400 Source Meter) combined with a photometer (Photo Research SpectraScan PR 655).Results and discussion
For the synthesis of these red emitters, a non-symmetric aldehyde-based benzo[c][1,2,5]thiadiazole intermediate was first obtained using commercially available starting materials.19,20 Also, 10-phenyl-10H-phenoxazine (POZ) donor moiety was constructed through the Ullman reaction followed by mono-bromination with N-bromosuccinimide (NBS). After boration and Suzuki–Miyaura coupling reactions, the red products OTPA-BT-CN and POZ-BT-CN could be facilely afforded with satisfactory yields. The detailed synthetic information is given in the ESI.† The thermal stability of the red emitters was investigated through differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). As shown in Fig. S1 (ESI†), owing to its more rigid donor property, POZ-BT-CN exhibited slightly higher Tg (90 °C) (glass transition temperature) and lower decomposition temperature (349 °C) (Td) (5% weight loss) than OTPA-BT-CN (67/359 °C).Single crystals of OTPA-BT-CN and POZ-BT-CN were analyzed by X-ray crystallography to illustrate the precise structures and packing modes. The diffraction data unambiguously confirmed the structures of OTPA-BT-CN and POZ-BT-CN (Fig. 1). Due to the relatively small torsion angle between the BT-CN moiety and the bridged benzene, a short intramolecular distance of approximately 2.5 Å was observed. Due to different donor groups, the two materials showed different crystal systems; POZ-BT-CN adopted a monoclinic system (P21/c space group), whereas OTPA-BT-CN possessed a triclinic system (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) group). Besides, different packing modes between OTPA-BT-CN and POZ-BT-CN were also observed. POZ-BT-CN demonstrated packing behavior with evident intermolecular interaction and a short plane–plane distance of ca. 3.40 Å between adjacent BT-CN moieties. In contrast, OTPA-BT-CN showed different packing behavior and there was no fixed plane–plane distance (Fig. 1c).
group). Besides, different packing modes between OTPA-BT-CN and POZ-BT-CN were also observed. POZ-BT-CN demonstrated packing behavior with evident intermolecular interaction and a short plane–plane distance of ca. 3.40 Å between adjacent BT-CN moieties. In contrast, OTPA-BT-CN showed different packing behavior and there was no fixed plane–plane distance (Fig. 1c).
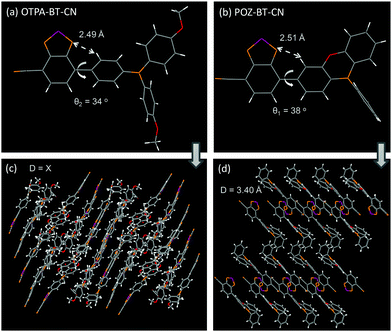 | ||
| Fig. 1 Single-crystal structures of (a) OTPA-BT-CN and (b) POZ-BT-CN. Packing modes of (c) OTPA-BT-CN and (d) POZ-BT-CN. | ||
To determine the ground and excited states of OTPA-BT-CN and POZ-BT-CN, density functional theory (DFT) and time-dependent DFT computational simulations at the B3LYP/6-31G(d) level were performed. Fig. 2 shows the distribution of the HOMO/LUMO of the ground and excited states. Due to the small repulsion force between the hydrogen–hydrogen interactions on the bridge, a small overlap between HOMO/LUMO on the central phenyl bridge is observed. This small overlap is actually beneficial because the probability of radiative transition can be facilitated by this orbital permeation, which in turn promotes PLQY.21 Both materials show similar HOMO and LUMO distributions when they are excited from ground states to excited states. From the DFT results, HOMO energy levels of 4.97/4.99 eV for OTPA-BT-CN and POZ-BT-CN are obtained. To verify the energy levels of these red emitters, cyclic voltammetry (CV) was employed to determine the HOMO energy levels. The diagram showing the obtained CV curves is given in Fig. S2 (ESI†), and the corresponding energy levels are displayed in Fig. S3 (ESI†). As calculated from the oxidation peak, a slightly lower HOMO energy for OTPA-BT-CN (−5.04 eV) than that for POZ-BT-CN (−5.07 eV) is observed. The LUMO energy levels of −2.98/−3.05 eV are also obtained for OTPA-BT-CN and POZ-BT-CN by combining their HOMO levels and band gaps.
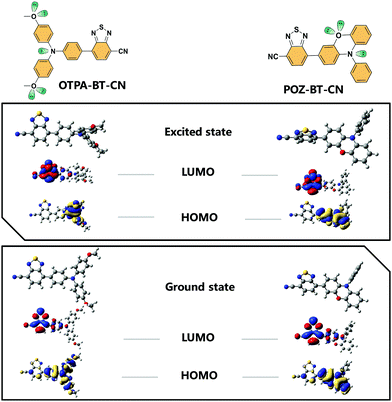 | ||
| Fig. 2 Molecular structures for OTPA-BT-CN and POZ-BT-CN. The HOMO/LUMO distribution in the ground state (below) and excited state (upper) with molecular geometry optimized. | ||
To verify the photophysical behaviors of OTPA-BT-CN and POZ-BT-CN, their photophysical behaviors were recorded. Absorption (Abs) spectra in dilute n-hexane (n-Hex) and toluene (Tol) were obtained to investigate the ground state properties. The absorption spectra (Fig. 3a) of OTPA-BT-CN and POZ-BT-CN show two kinds of peaks: a broad and structureless peak at approximately 500 nm and another sharp peak located below 320 nm. The charge transfer (CT) absorption at 500 nm can be ascribed to the transition from donor to acceptor moieties, whereas the higher-intensity absorption at approximately 320 nm is ascribed to the n–π* or π–π* transition of the donor centers.22–25 With increasing solvent polarity, the edge of the CT absorption red-shifted substantially due to its sensitivity to the environment.26 From the calculations for the absorption edge in Tol, band gaps of 2.06 and 2.02 eV are obtained for OTPA-BT-CN and POZ-BT-CN, respectively. Photoluminescence (PL) spectra of these red emitters are also obtained (Fig. 3). Compared to the emission peaks of OTPA-BT-CN in n-Hex (581 nm) and Tol (633 nm), the peaks of POZ-BT-CN exhibit redshifts of approximately 50 and 30 nm, respectively. In addition, the PL spectra of the pure film are also investigated, as shown in Fig. S4 (ESI†). Both OTPA-BT-CN and POZ-BT-CN show typical red emission spectra, with the spectra peaking over 750 nm and extending to 820 nm. Transient PL spectra in the same solvents were obtained to further investigate the excited state characteristics (Fig. 3b). The results indicate that after changing the solvent from n-Hex to Tol, OTPA-BT-CN and POZ-BT-CN exhibit the same behavior, that is, the lifespan decreases along with increasing solvent polarity. The sensitivity of OTPA-BT-CN to solvent polarity can be explained according to the energy gap law and the stronger interactions between solute and solvent.27–30 Besides, we also measure their photoluminescence quantum yields (in Tol), and both materials show high PLQYs of over 70%, with POZ-BT-CN (∼83%) exhibiting higher PLQY than OTPA-BT-CN (∼72%). All the photophysical and electrochemical data are summarized in Table 1.
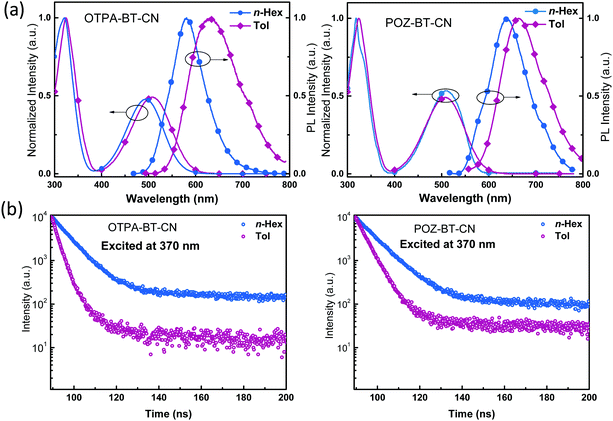 | ||
| Fig. 3 (a) Absorption and fluorescence spectra of OTPA-BT-CN and POZ-BT-CN in dilute hexane (n-Hex) and toluene (Tol). (b) Transient PL spectra in n-Hex and Tol. | ||
| Compound | Abs λmaxa [nm] | PL λmaxa [nm] | T g [°C] | T d [°C] | E g [eV] | HOMOe [eV] | LUMOf [eV] | PLQYg [%] |
|---|---|---|---|---|---|---|---|---|
| a Tested in toluene (Tol) solution at room temperature. b Glass transition temperature. c Decomposition temperature. d Bandgap energies calculated from the absorption edge in dilute CH2Cl2 solution (1 × 10−5 mol L−1). e HOMO energies: calculated from the onset of oxidation peak recorded by the cyclic voltammetry. f LUMO energies: speculated from the HOMO energy and band gap. g PLQY tested in Tol at room temperature. | ||||||||
| OTPA-BT-CN | 508 | 633 | 67 | 359 | 2.06 | −5.04 | −2.98 | 72 |
| POZ-BT-CN | 511 | 664 | 90 | 349 | 2.02 | −5.07 | −3.05 | 83 |
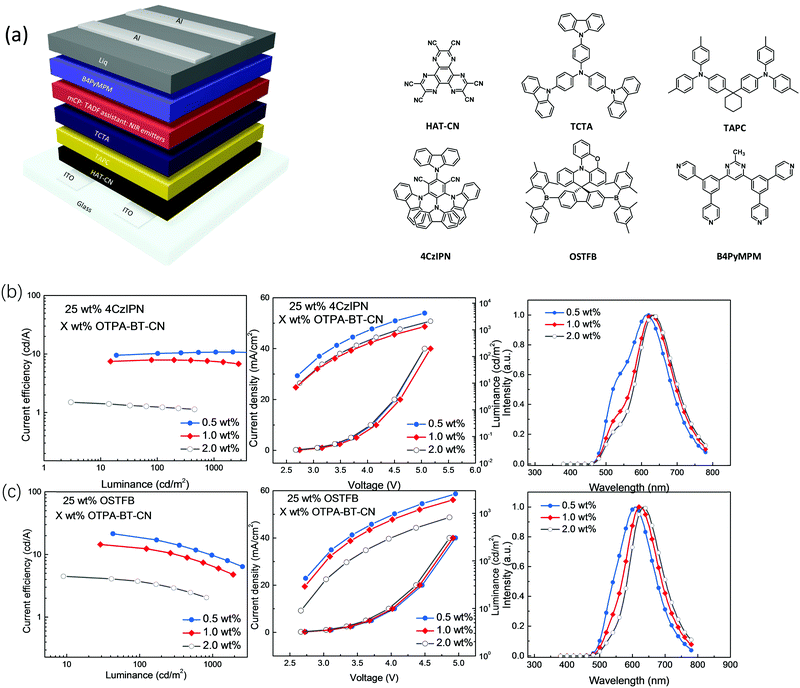
To investigate the different effects of different sensitizers on overall device performances and REF process, TAF-OLEDs were fabricated with the following configuration: ITO/1,4,5,8,9,11-hexaazatriphenylene-hexacarbonitrile (HAT-CN) (10 nm)/1,1-bis[4-[N,N-di(p-tolyl)amino]phenyl]cyclohexane (TAPC) (40 nm)/tris(4-(9H-carbazol-9-yl)phenyl)amine (TCTA) (10 nm)/mCP: 25 wt% sensitizer: X wt% red emitters (20 nm)/4,6-bis[3,5-(dipyrid-4-yl)phenyl]-2-methylpyrimidine (B4PyMPM (50 nm)). For TA-OLEDs, two kinds of sensitizer materials, 4CzIPN and OSTFB, were used to optimize the device performances and to investigate how different TADF sensitizers determine RET pathways and the overall device performances. In this regard, three laddered low doping ratios of 0.5, 1.0 and 2.0 wt% were adopted to ensure Förster energy transfer and avoid triplet–triplet annihilation (TTA) (Scheme S1, ESI†).31–33 The device diagram together with the molecular structures is shown in Fig. 4. In addition, all the device performances are shown in Table 2.
| Doping ratio (%) | Assistant dopant | Voltage@100 cd m−2 (V) | Max. CE (cd A−1) | Max. EQE (%) | λ EL,max (nm) |
|---|---|---|---|---|---|
| 0.5 | 4CzIPN | 2.4 | 10.8 | 6.3 | 610 |
| 1.0 | 4CzIPN | 2.4 | 7.9 | 5.9 | 623 |
| 2.0 | 4CzIPN | 2.4 | 1.5 | 1.2 | 630 |
| 0.5 | OSTFB | 3.0 | 21.5 | 12.4 | 612 |
| 1.0 | OSTFB | 3.0 | 14.5 | 10.1 | 626 |
| 2.0 | OSTFB | 3.4 | 4.5 | 4.4 | 638 |
Fig. 4b and c demonstrate the device characteristics with 25 wt% 4CzIPN (Fig. 4b) and OSTFB (Fig. 4c) as the sensitizers and OTPA-BT-CN (0.5, 1.0 and 2.0 wt%) as the emitter. For the 4CzIPN-based TAF device, reduced efficiency is observed as the doping ratio is increased. A current efficiency (CE) of 10.8 cd A−1 and EQE of 6.3% are achieved for the 0.5 wt% OTPA-BT-CN-based device. A slightly lower performance with much more redshifted spectra is observed for 1.0 wt% OTPA-BT-CN-based devices. In contrast, EQE decreases drastically to 1.2% when the doping ratio is increased to 2.0 wt%. For the 4CzIPN-sensitized device based on OTPA-BT-CN, a clear emission peak originating from 4CzIPN is observed for all doping ratios, which is beyond our expectation. To confirm this phenomenon and to test the optimized doping ratio, comparison devices using different concentrations of 4CzIPN (from 10 wt% to 50 wt%) are fabricated. As the results indicate (Fig. S5 in ESI†), the emission peak of 4CzIPN is stronger when the doping ratio is below 25 wt%, whereas the efficiency is drastically reduced at higher concentrations (>30 wt%). This finding suggests that the optimized concentration for 4CzIPN is approximately 25 wt%.
For direct comparison, devices adopting 25 wt% OSTFB as the sensitizer with the same ladder doping ratios of the emitter (0.5, 1.0 and 2.0 wt%) and configuration were also fabricated (Fig. 4c). Unlike the observations for 4CzIPN as the sensitizer, the energy transfer from the OSTFB sensitizer to OTPA-BT-CN is complete. Furthermore, the device performance with OSTFB as a sensitizer is much higher than that of the 4CzIPN-based devices. With the same trend, the 0.5 wt% OTPA-BT-CN device achieves the highest performance with CE of over 21 cd A−1 and EQE of around 12%. In addition, EQE of the OSTFB-based device is almost two times higher than that of the 4CzIPN-based device. As the OTPA-BT-CN concentration increases, reduced EQEs of 10.1% and 4.4% are observed for 1.0 and 2.0 wt% red OLEDs, respectively. This reduced efficiency is due to the enhanced probability for Dexter energy transfer from the sensitizer to the emitter, which is a short-range, multi-polar interaction determined by the dopant concentration.
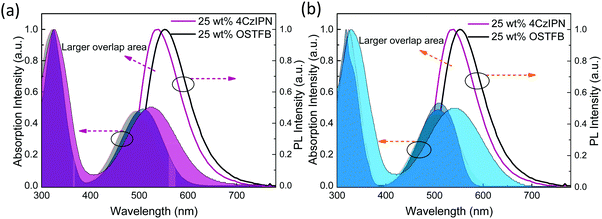
For complete energy transfer, it is generally believed that the overlap between the host and the emitter should be as large as possible to maximize the transfer efficiency.16 In TAF-OLEDs, excitons generated on the traditional host are transferred to the sensitizer first, which converts all triplet excitons into singlet excitons by RISC; then, 100% of the singlet excitons are expected to transfer to the emitters for light emission (Fig. 5). As there is no emission peak originating from the mCP host, the energy transfer of the first state is effective. Hence, the absorption of red emitters and the PL spectra of the sensitizers were investigated to explore the aforementioned behaviors. Fig. 6a and b illustrate the PL spectra of 25 wt%-doped 4CzIPN and OSTFB films and the absorption spectra of OTPA-BT-CN and POZ-BT-CN. As the results show, 4CzIPN exhibits a larger amount of overlap than OSTFB both in solution and in the film, which is contrary to the device behavior (the 4CzIPN-sensitized device shows peaks from 4CzIPN, whereas OSTFB-based device does not exhibit any peaks).
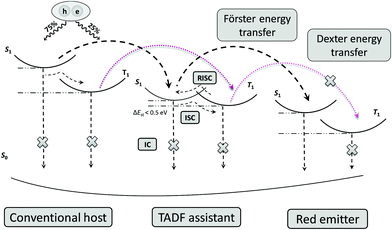 | ||
| Fig. 5 Energy transfer modes for a TAF-OLED system. ISC and RISC denote intersystem crossing and reverse intersystem crossing, respectively. | ||
To explore the behavior underlying this phenomenon, long-range energy transfer (Förster’ mechanism) parameters are investigated. The energy transfer rate from energy donor (D) to acceptor (A) for Förster energy transfer,34 according to literature, can be formulated as follows:
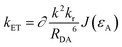 | (1) |
Here, kET represents the rate constant for Förster transfer, ∂ and k2 can be seen as constants, εA is the absorption coefficient of the guest, J denotes the spectra overlap between absorption of the guest and fluorescence of the host, R represents the distance between D/A, which can be simplified as the doping ratio, and kD* represents the radiative constant for D. As the doping ratio and εA are the same, the main factor can be the radiative constant (kr). To determine the effect of kD*, some parameters should be clarified:
| kr = 1/τ0 | (2) |
Here, τ0 denotes the natural lifetime data for the energy donor part; τ0 can be obtained according to the following equations:
| τ/τ0 = kf/(kf + kn) = φf | (3) |
| τ = τ0 × φf | (4) |
Here, τ is the actual lifetime data and φf denotes the PLQY of 4CzIPN and OSTFB. As τ can be obtained through measurement of the transient PL and PLQY using an integrating sphere, kr can be determined according to the above formulae. As the results show (Fig. S6 in ESI†), OSTFB displays a slightly lower average lifetime compared with 4CzIPN in the mCP-doped film. As for the PLQYs, OSTFB (67%) shows a relatively higher value than 4CzIPN (61%), resulting in OSTFB showing a higher kr value (0.79/0.35 for OSTFB/4CzIPN).
Based on the above discussion, OSTFB-based TAF-OLEDs were also fabricated (Table S1 and Fig. S7, ESI†). As expected, the 0.5 wt% POZ-BT-CN-based device shows the highest performance, with EQE of 10.1% and an EL peak at approximately 620 nm. Although the best EQE is slightly lower, the peak emission of 0.5 wt% POZ-BT-CN-based device clearly redshifts. Furthermore, 1.0 wt% POZ-BT-CN also achieves superior EQE of approximately 10% even when the EL peak is located at 637 nm. Our results not only reveal the important parameters of the sensitizer in TAF-OLEDs, but also provide an efficient way to improve the performances of red fluorescent OLEDs by choosing suitable sensitizers.
Conclusion
To summarize, we developed two red emitters with high photoluminescence quantum yields of over 70% and electroluminescence (EL) peaks of around 750 nm and utilized them to investigate the exact roles of the sensitizer in thermally activated delayed fluorescence (TADF)-assisted fluorescent organic light-emitting diodes (TAF-OLEDs). To study the exact parameter of the sensitizer, two TADF materials with similar EL spectra but different systems are chosen, and the overlaps of TADF sensitizers and red emitters and the radiative constant (kr) are also compared. Mechanism studies and different types of devices reveal that. of the sensitizer, rather than the overlap of sensitizer and fluorescent emitter, plays a more significant role in TAF-OLEDs. In this regard, the device based on OSTFB, which shows a similar emission spectrum but larger kr, exhibits both redshifted EL spectra and higher device performances. Consequently, almost two-time higher EQEs of 12.4%/10.1% with an EL peak at 613/626 nm for OSTFB-based devices compared to those for 4CzIPN-based devices (6.3%/5.9% with an EL peak at 610/623 nm) are achieved.Conflicts of interest
There is no conflict of interest between all authors.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (Grant No. 61575136, 21572152 and 51873139) and the National Key R&D Program of China (Grant No. 2016YFB0400700). This project is also funded by the Collaborative Innovation Center of Suzhou Nano Science and Technology (Nano-CIC), by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), by the “111” Project of the State Administration of Foreign Experts Affairs of China, and by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX18_2491).Notes and references
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- J. W. Sun, J.-H. Lee, C.-K. Moon, K.-H. Kim, H. Shin and J.-J. Kim, Adv. Mater., 2014, 26, 5684–5688 CrossRef CAS PubMed.
- J. Lee, N. Aizawa, M. Numata, C. Adachi and T. Yasuda, Adv. Mater., 2017, 29, 1604856 CrossRef PubMed.
- D. Zhang, L. Duan, C. Li, Y. Li, H. Li, D. Zhang and Y. Qiu, Adv. Mater., 2014, 26, 5050–5055 CrossRef CAS PubMed.
- Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Adv. Mater., 2014, 26, 7931–7958 CrossRef CAS PubMed.
- X. Cai, B. Gao, X.-L. Li, Y. Cao and S.-J. Su, Adv. Funct. Mater., 2016, 26, 8042–8052 CrossRef CAS.
- K.-C. Pan, S.-W. Li, Y.-Y. Ho, Y.-J. Shiu, W.-L. Tsai, M. Jiao, W.-K. Lee, C.-C. Wu, C.-L. Chung, T. Chatterjee, Y.-S. Li, K.-T. Wong, H.-C. Hu, C.-C. Chen and M.-T. Lee, Adv. Funct. Mater., 2016, 26, 7560–7571 CrossRef CAS.
- T.-A. Lin, T. Chatterjee, W.-L. Tsai, W.-K. Lee, M.-J. Wu, M. Jiao, K.-C. Pan, C.-L. Yi, C.-L. Chung, K.-T. Wong and C.-C. Wu, Adv. Mater., 2016, 28, 6976–6983 CrossRef CAS PubMed.
- T.-L. Wu, M.-J. Huang, C.-C. Lin, P.-Y. Huang, T.-Y. Chou, R.-W. Chen-Cheng, H.-W. Lin, R.-S. Liu and C.-H. Cheng, Nat. Photonics, 2018, 12, 235–240 CrossRef CAS.
- Q. Zhang, H. Kuwabara, W. J. Potscavage, S. Huang, Y. Hatae, T. Shibata and C. Adachi, J. Am. Chem. Soc., 2014, 136, 18070–18081 CrossRef CAS PubMed.
- K. R. Graham, Y. Yang, J. R. Sommer, A. H. Shelton, K. S. Schanze, J. Xue and J. R. Reynolds, Chem. Mater., 2011, 23, 5305–5312 CrossRef CAS.
- L. Yao, S. Zhang, R. Wang, W. Li, F. Shen, B. Yang and Y. Ma, Angew. Chem., Int. Ed., 2014, 53, 2119–2123 CrossRef CAS PubMed.
- P. Murto, A. Minotto, A. Zampetti, X. Xu, M. R. Andersson, F. Cacialli and E. Wang, Adv. Opt. Mater., 2016, 4, 2068–2076 CrossRef CAS.
- Y. Yuan, Y. Hu, Y.-X. Zhang, J.-D. Lin, Y.-K. Wang, Z.-Q. Jiang, L.-S. Liao and S.-T. Lee, Adv. Funct. Mater., 2017, 27, 1700986 CrossRef.
- H. Nakanotani, T. Higuchi, T. Furukawa, K. Masui, K. Morimoto, M. Numata, H. Tanaka, Y. Sagara, T. Yasuda and C. Adachi, Nat. Commun., 2014, 5, 4016–4022 CrossRef CAS PubMed.
- D. Zhang, X. Song, M. Cai and L. Duan, Adv. Mater., 2018, 30, 1705250 CrossRef PubMed.
- Y. Takahiko, N. Hajime, H. Shigeo, H. Toru and A. Chihaya, Appl. Phys. Express, 2017, 10, 074101 CrossRef.
- H. Ahn, J. H. Jeong, J. Song, J. Y. Lee and J. H. Kwon, ACS Appl. Mater. Interfaces, 2018, 10, 10246–10253 CrossRef PubMed.
- Y.-K. Wang, S.-F. Wu, S.-H. Li, Y. Yuan, F.-P. Wu, S. Kumar, Z.-Q. Jiang, M.-K. Fung and L.-S. Liao, Adv. Opt. Mater., 2017, 5, 1700566 CrossRef.
- S. Holliday, R. S. Ashraf, C. B. Nielsen, M. Kirkus, J. A. Röhr, C.-H. Tan, E. Collado-Fregoso, A.-C. Knall, J. R. Durrant, J. Nelson and I. McCulloch, J. Am. Chem. Soc., 2015, 137, 898–904 CrossRef CAS PubMed.
- W.-C. Chen, Y. Yuan, S.-F. Ni, Q.-X. Tong, F.-L. Wong and C.-S. Lee, Chem. Sci., 2017, 8, 3599–3608 RSC.
- Y.-K. Wang, Y.-L. Deng, X.-Y. Liu, X.-D. Yuan, Z.-Q. Jiang and L.-S. Liao, Dyes Pigm., 2015, 122, 6–12 CrossRef CAS.
- M. Romain, D. Tondelier, B. Geffroy, A. Shirinskaya, O. Jeannin, J. Rault-Berthelot and C. Poriel, Chem. Commun., 2015, 51, 1313–1315 RSC.
- Y.-K. Wang, S.-F. Wu, Y. Yuan, S.-H. Li, M.-K. Fung, L.-S. Liao and Z.-Q. Jiang, Org. Lett., 2017, 19, 3155–3158 CrossRef CAS PubMed.
- C. Wang, X.-L. Li, Y. Gao, L. Wang, S. Zhang, L. Zhao, P. Lu, B. Yang, S.-J. Su and Y. Ma, Adv. Opt. Mater., 2017, 5, 1700441 CrossRef.
- C. Poriel, J. Rault-Berthelot, S. Thiery, C. Quinton, O. Jeannin, U. Biapo, D. Tondelier and B. Geffroy, Chem. – Eur. J., 2016, 22, 17930–17935 CrossRef CAS PubMed.
- X. Lv, W. Zhang, D. Ding, C. Han, Z. Huang, S. Xiang, Q. Zhang, H. Xu and L. Wang, Adv. Opt. Mater., 2018, 6, 1800165 CrossRef.
- J. Xue, Q. Liang, Y. Zhang, R. Zhang, L. Duan and J. Qiao, Adv. Funct. Mater., 2017, 27, 1703283 CrossRef.
- T. Liu, L. Zhu, C. Zhong, G. Xie, S. Gong, J. Fang, D. Ma and C. Yang, Adv. Funct. Mater., 2017, 27, 1606384 CrossRef.
- S. Lee, K.-H. Kim, D. Limbach, Y.-S. Park and J.-J. Kim, Adv. Funct. Mater., 2013, 23, 4105–4110 CrossRef CAS.
- Y.-K. Wang, S.-H. Li, S.-F. Wu, C.-C. Huang, S. Kumar, Z.-Q. Jiang, M.-K. Fung and L.-S. Liao, Adv. Funct. Mater., 2018, 28, 1706228 CrossRef.
- S.-F. Wu, S.-H. Li, Y.-K. Wang, C.-C. Huang, Q. Sun, J.-J. Liang, L.-S. Liao and M.-K. Fung, Adv. Funct. Mater., 2017, 27, 1701314 CrossRef.
- H. Yang, Q. Liang, C. Han, J. Zhang and H. Xu, Adv. Mater., 2017, 29, 1700553 CrossRef PubMed.
- B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence: Principles and Applications, John Wiley & Sons, Weinheim, 2012 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1828817 and 1828818 for POZ-BT-CN and OTPA-BT-CN. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8qm00442k |
| ‡ Ya-Kun Wang and Chen-Chao Huang contribute equally to this work. |
| This journal is © the Partner Organisations 2019 |