Biobased bismaleimide resins with high renewable carbon content, heat resistance and flame retardancy via a multi-functional phosphate from clove oil†
Jia-Tao
Miao
 ,
Li
Yuan
,
Li
Yuan
 ,
Guozheng
Liang
,
Guozheng
Liang
 * and
Aijuan
Gu
* and
Aijuan
Gu
 *
*
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, China. E-mail: lgzheng@suda.edu.cn; ajgu@suda.edu.cn; Fax: +86 51265880089; Tel: +86 51265880967
First published on 22nd October 2018
Abstract
To achieve sustainable development, it is important to keep a green consideration from the raw material source to the whole production process of developing biobased high performance thermosetting resins. Herein, a multi-functional phosphate (TAMPP) was synthesized from renewable eugenol through aqueous-phase synthesis, of which the renewable carbon content is as high as 100%. TAMPP was used to partly or totally replace petroleum-based 2,2′-diallylbisphenol A (DBA) for modifying 4,4′-bismaleimidodiphenylmethane (BDM), and then four bismaleimide (BMI) resins (BDTP or BTP) were developed. Compared with traditional DBA modified BDM resin (BD), BTP has better integrated properties including a higher renewable carbon content (45.0%), an approximately 70 °C higher glass transition temperature (Tg > 380 °C), excellent flame retardancy and good mechanical properties. These outstanding performances of BTP resin prove that the method proposed here is a sustainable and effective method to prepare a multi-functional biobased phosphate and high performance thermosetting resins for cutting-edge industries.
Introduction
Nowadays, using renewable biomass as a substitute for non-renewable petrochemical resources to develop biobased materials has attracted wide attention from academia and industry.1,2 Some types of polymers have been researched including thermosetting and thermoplastic resins.3–5 Among them, cured thermosetting resins cannot be fabricated again and are hard to recycle, so it is more meaningful to develop biobased thermosetting resins.High-performance thermosetting resins (HPTRs) have been the key basic materials of many cutting-edge fields including electronic information,6 electrical insulation,7 aerospace,8 new energy9 and so on. Bismaleimide resin (BMI) is a typical HPTR,10 and at present, 2,2′-diallyl bisphenol A (DBA) (Scheme 1) modified 4,4′-bismaleimidobenzodimethane (BDM) coded as BD is one of the best modified BMI resins.11 BD resin simultaneously possesses the advantages of a polyimide including high heat and radiation resistance as well as good processability such as low melting point, wide processing window and excellent solubility in non-toxic solvents. However, raw materials for synthesizing DBA come from petrochemical resources,12 and among them, the estrogen-like structure of bisphenol A is a potential threat to human health.13,14 To solve these problems, low-toxicity biomass is a good choice.
Eugenol can be extracted from renewable clove oil, its aromatic structure is beneficial for obtaining high thermal and mechanical properties in the final products;15 while reactive allyl and phenolic hydroxyl groups endow it with the ability to be polymerized or chemically modified.16 Taking eugenol as a raw material to prepare biobased BMI resins has been preliminarily reported in recent years. Dubois et al.17 prepared eugenol-based benzoxazine (E-pPDA) modified BDM resins, of which the glass transition temperature (Tg) ranged from 210 to 265 °C. Shibata et al.6,18,19 reported several eugenol-based allylphenyl compound modified BDM resins, of which Tg changed from 201 to over 400 °C, while the renewable carbon content varied from 47.8 to 12.7% as shown in Table S1, ESI.† Wu's group20 prepared a modified BDM resin using a phosphorus-containing allylphenyl compound that links eugenol molecules together with hexachlorocyclotriphosphazene, these BMI resins have excellent flame retardancy (a significant property for present HPTRs) and high Tg values (255–273 °C). More information on biobased eugenol derived BMI resins including renewable carbon contents and integrated performances in the literature is summarized in Table S1, ESI.†
Note that eugenol is used as a raw material in the synthesis of biobased allylphenyl compounds for BMI resins, but organic solvents are still ubiquitously used in the synthesis process. Their possible toxicity and the energy-consuming process to produce and dispose of organic solvents is bad for sustainable development.
Therefore, setting up a sustainable synthesis route to synthesize novel multi-functional allylphenyl compounds from renewable raw materials, and developing high performance thermosetting resins with ultra-high Tg, excellent flame retardancy, good mechanical properties and high renewable carbon content are important and urgent for the sustainable development of cutting-edge fields.
This paper reports biobased bismaleimide resins with a high renewable carbon content, heat resistance and flame retardancy, which were prepared through copolymerizing BDM with tris(4-allyl-2-methoxyphenyl) phosphate (TAMPP) derived from phosphorus oxychloride and renewable eugenol. Some attractive phenomena were observed, and the mechanisms behind them are discussed.
Experimental
Materials
BDM and DBA (Scheme 1) were obtained from Northwestern Chemical Institute, China. TAMPP was synthesized from renewable eugenol through aqueous-phase synthesis by ourselves.21Preparation of resins
Keeping the molar ratio of imide to allyl double bond as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.86, BDM, DBA and TAMPP were mixed according to Table 1 at 145 °C for 30 min to obtain a clear liquid (prepolymer). Then the prepolymer was transferred to a preheated mold and degassed under vacuum at 150 °C for 30 min. After that, the mold was put into an oven for curing and postcuring using the procedures of 150 °C/2 h + 180 °C/2 h + 200 °C/2 h + 220 °C/2 h and 240 °C/4 h, successively.
0.86, BDM, DBA and TAMPP were mixed according to Table 1 at 145 °C for 30 min to obtain a clear liquid (prepolymer). Then the prepolymer was transferred to a preheated mold and degassed under vacuum at 150 °C for 30 min. After that, the mold was put into an oven for curing and postcuring using the procedures of 150 °C/2 h + 180 °C/2 h + 200 °C/2 h + 220 °C/2 h and 240 °C/4 h, successively.
Characterizations
Fourier transform infrared (FTIR) spectra were recorded between 600 to 4000 cm−1 on a Bruker Vertex 70 spectrometer (USA).The curing behavior of the resin was studied using a TA Instruments Q200 differential scanning calorimeter (DSC) made in the USA with a heating rate of 10 °C min−1 under a nitrogen atmosphere.
Densities were measured on an electronic density balance (Shanghai Sunny Hengping Scientific Instruments, China).
Water absorptions were tested at (100 ± 1) °C according to China standard GB/T 1034-2008.
Dynamic mechanical analyses (DMA) were carried on a TA Instruments Q800 (USA) in a multi-frequency-strain model at a heating rate of 3 °C min−1 under a nitrogen atmosphere. Each sample prepared had uniform dimensions of (35 ± 0.02) mm × (13 ± 0.02) mm × (3 ± 0.02) mm and was fixed in a single cantilever clamp. Other parameters include an oscillation frequency of 1.0 Hz, a deflection amplitude of oscillation of 20 μm, and a Poisson's ratio of 0.44. The peak temperature of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ–temperature plot was measured as Tg according to the International Electrotechnical Commission (IEC) standard: IEC 61006:2004.
δ–temperature plot was measured as Tg according to the International Electrotechnical Commission (IEC) standard: IEC 61006:2004.
Renewable carbon content (also called biobased content) is assigned as the “amount of biobased carbon in the material or product as a percent of the weight (mass) of the total organic carbon in the product”.22 Renewable carbon contents of the BMI resins were calculated according to eqn (1).
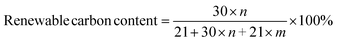 | (1) |
Thermogravimetric analyses (TGA) were performed on a TA Discovery apparatus (USA) under a nitrogen or air atmosphere, of which the gas flow rate was 10 mL min−1 and the heating rate was 10 °C min−1.
Flexural properties were evaluated using a three-point bending test conducted on an electronic universal testing machine (SUST, Zhuhai, China) at a speed of 2 mm min−1. The dimensions of each sample were (60 ± 0.02) mm × (15 ± 0.02) mm × (3.0 ± 0.02) mm. The test was performed with a span length of 44 mm. The data are reported as an average of five samples.
Limiting oxygen index (LOI) values of the samples with dimensions of (100 ± 0.02) mm × (6.5 ± 0.02) mm × (3 ± 0.02) mm were measured on a Stanton Redcraft Flame Meter (UK) following the test procedures stated in ASTM D2863/77.
The vertical burning (UL-94) tests were performed on a CZF-3 instrument (Jiangning Analysis Instrument Company, China) following the procedure described in ASTM D3801-2010, the dimensions of each sample were (125 ± 0.02) mm × (13 ± 0.02) mm × (3.0 ± 0.02) mm.
A cone calorimeter (FTTT007) from Fire Testing Technology Limited, UK, was used to investigate the flame retardancy according to ISO 5660. A cone shape heater provided an incident flux of 35 kW m−2 during the test. The dimensions of each sample were (100 ± 0.02) mm × (100 ± 0.02) mm × (3 ± 0.02) mm. The standard deviations of the measured data are within 5%.
TGA-IR spectra were recorded using a Netzsch TGA F1 thermogravimetric analyzer (Germany) connected with a Bruker TENSOR 27 FTIR spectrometer (Germany). Ten milligrams of each sample was heated from 30 to 800 °C under a nitrogen atmosphere with a heating rate of 10 °C min−1.
Results and discussion
Curing and swelling behaviors
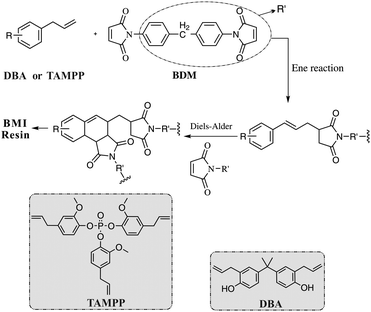
Up to now, as one of the most successful modified BMI resins, the BD resin has an outstanding integrated performance,10,23 so it was prepared and studied as a comparison for evaluating the new biobased BMI resins developed in this paper. Many studies have shown that the BD prepolymer has good processability, including a low melting point, wide processing window and excellent solubility in non-toxic solvents. These advantages are also found in BDTP and BTP resins. Specifically, all prepolymers with different content of TAMPP can also be dissolved in acetone, and their softening points are about 60 °C. The pot life at 130 °C of the BD and BTP prepolymers was tested (Fig. S1, ESI†), and it was found that the viscosities of both prepolymers increase with time but they have different rates of increase. The viscosity of BD increases from 0.1 to 1 Pa s within 134 min; while that of BTP increases from 0.09 to 1 Pa s within 215 min, so BTP resin has an 81 min longer pot life than BD, suggesting that the BTP resin has better processing features.BD, BDTP and BTP resins have similar reactive groups (allyl and imide groups), so they are expected to have similar curing mechanisms. The curing mechanism of BD resin has been intensively studied, and is believed to include “Ene” and “Diels–Alder” reactions between the imide and allyl groups (Scheme 2), together with homopolymerization of the double bonds in the imide groups.20
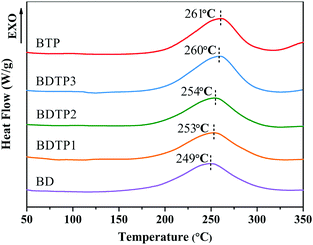
Fig. 1 shows DSC curves of the BD, BDTP and BTP prepolymers. It can be seen that each DSC curve displays a single exothermic peak corresponding to the curing reaction, the exothermic peaks of the BDTP and BTP systems appear at higher temperatures compared with that of BD prepolymer. The exothermic peak of the prepolymer with a higher content of TAMPP shifts toward higher temperatures; this is because TAMPP has a higher steric hindrance due to its trifunctional groups.
The BTP resin was cured following the procedures of 150 °C/2 h + 180 °C/2 h + 200 °C/2 h + 220 °C/2 h and 240 °C/4 h, successively. The FTIR spectrum of the cured BTP resin was recorded to confirm that under the curing process the above samples were all fully cured. As shown in Fig. S2 (ESI†), the characteristic peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond at 1639 cm−1 and maleimide framework at 719 cm−1 exist in the spectrum of the BTP prepolymer but are not found in the spectrum of the cured BTP resin, indicating that the resin has been cured completely, so the BD and BDTP resins were cured using the same curing procedure.
C bond at 1639 cm−1 and maleimide framework at 719 cm−1 exist in the spectrum of the BTP prepolymer but are not found in the spectrum of the cured BTP resin, indicating that the resin has been cured completely, so the BD and BDTP resins were cured using the same curing procedure.
The swelling experiment of the BTP resin was performed. As shown in Fig. S3 (ESI†), due to the high crosslinking structure of the BTP resin, the dimensions of the BTP resin did not change after swelling in THF at 25 °C for 24 h.
Thermal properties of the BMI resins
DMA is the most effective method for determining Tg of the thermosetting resins, either the storage modulus (E′), loss modulus or tan delta (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) can be used to define Tg. According to the IEC standard: IEC 61006:2004, Tg is determined as the peak of the tan
δ) can be used to define Tg. According to the IEC standard: IEC 61006:2004, Tg is determined as the peak of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve. On the other hand, the peak of tanδ is used to define Tg in all reports of biobased BMI resins, to make it easy to compare the value, we use the peak of tan
δ curve. On the other hand, the peak of tanδ is used to define Tg in all reports of biobased BMI resins, to make it easy to compare the value, we use the peak of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ to define Tg in this work.
δ to define Tg in this work.
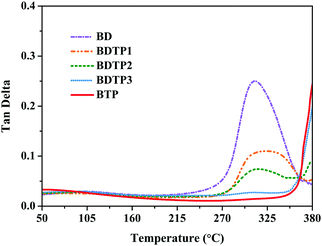
Fig. 2 shows the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ–temperature curves. Both the BD or BTP resins show only one symmetric peak, indicating that each resin has a uniform aggregation structure, whereas BDTP resins have two symmetric peaks, suggesting that they contain two kinds of aggregation structures. The whole peak of the tan
δ–temperature curves. Both the BD or BTP resins show only one symmetric peak, indicating that each resin has a uniform aggregation structure, whereas BDTP resins have two symmetric peaks, suggesting that they contain two kinds of aggregation structures. The whole peak of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ–temperature curve for BTP could not be obtained because the glass transition temperature was too high for the instrument. However, from the non-perfect plot, it is still reasonable to state that the Tg of the BTP resin is higher than 380 °C, more than 70 °C higher than that of the BD resin (311 °C). This statement can be confirmed by Tg′ (the peak of the loss modulus curve, Fig. S4, ESI†) of the BTP resin, which is as high as 365 °C, 77 °C higher than that of the BD resin (288 °C). These results prove that the BTP resin has good heat resistance, and can be used at higher temperatures because Tg is the highest end-use temperature for the thermosetting resin.24
δ–temperature curve for BTP could not be obtained because the glass transition temperature was too high for the instrument. However, from the non-perfect plot, it is still reasonable to state that the Tg of the BTP resin is higher than 380 °C, more than 70 °C higher than that of the BD resin (311 °C). This statement can be confirmed by Tg′ (the peak of the loss modulus curve, Fig. S4, ESI†) of the BTP resin, which is as high as 365 °C, 77 °C higher than that of the BD resin (288 °C). These results prove that the BTP resin has good heat resistance, and can be used at higher temperatures because Tg is the highest end-use temperature for the thermosetting resin.24
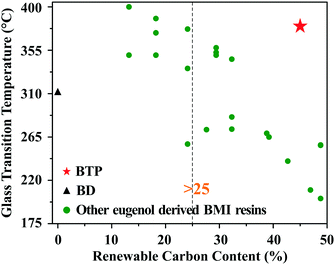
What is more, our results were compared to other biobased eugenol derived BMI resins. Fig. 3 gives Tg and the renewable carbon contents of BTP, BD and other eugenol derived BMI resins, which are also summarized in Table S1, ESI.† The United States Department of Agriculture (USDA) managed a biopreferred program to increase the purchase and use of biobased products. One of its two major parts is that biobased products containing at least 25% renewable carbon content (biobased content)25 can be certified, and a label can be displayed on the product to state that it is third party tested and has a verified renewable carbon content as well as the USDA certification about biobased products. A higher renewable carbon content indicates that the product contains more biobased units rather than petroleum-based units, and consequently, significantly reduces the consumption of petroleum resources and promotes the sustainable development of polymers. Fig. 3 shows that BTP is located in the upper right hand corner, meaning that BTP is a unique biobased resin that simultaneously has a high renewable carbon content (45.0%) and heat resistance (Tg > 380 °C). This advantage is attributed to the special network structure induced by the T-type trifunctional aromatic structure of TAMPP.
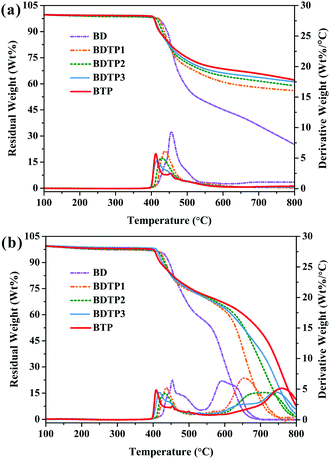
The thermal decomposition temperature (Tdi) often refers to the temperature when the weight loss is 5 wt% and can be detected using TGA techniques. Fig. 4 shows the TGA and DTG curves of the cured resins under nitrogen or air atmospheres. In nitrogen atmosphere (Fig. 4a), each BMI resin has a one-stage mass loss; BDTP and BTP resins have a lower Tdi than BD resin, and the resin with more TAMPP has a lower Tdi, this is a general trend of phosphorus-containing flame retardants.26,27 However, the decrease extent is not as large as that reported in literature,28 suggesting that the unique structure of TAMPP endows BMI resins with a more stable crosslinked network. Attractively, the Tdi of the BTP resin is as high as 412 °C, meeting the requirements of the HPTRs. Meanwhile, the char yield (Yc) at 800 °C of the BMI resin enlarges as the phosphorus content increases. The Yc values of the BDTP and BTP resins are about 2.3–2.5 times of that of the BD resin, respectively. A high Yc is favorable to obtain good flame retardancy.29
The Tdi values of the resins under air atmosphere present a similar trend to those under nitrogen atmosphere, while there are some differences. In detail, the degradation of each resin under air includes two stages (Fig. 4b). The first stage appears within the temperature range of 400–520 °C, corresponding to the degradation of the principal BMI networks, while the second one beyond 540 °C is due to the further thermal oxidative degradation of formed char. The addition of TAMPP significantly postpones the second step, and the corresponding peak of the DTG curves shifts to higher temperatures with the increase of phosphorus content. Specifically, BTP resin has the highest temperature at which the resin shows the biggest degradation rate (Tmax) (761 °C), while BD resin has the lowest Tmax (592 °C). On the other hand, at 700 °C, BD resin has almost completely degraded, and its Yc approaches zero, while BDTP and BTP resins still have residues. Specifically, there are about 50.4% and 7.6% residues for BTP resin at 700 °C and 800 °C, respectively, demonstrating that BDTP and BTP resins have obvious advantages in obtaining good flame retardancy. This is also confirmed by the following LOI, UL-94 and CONE tests.
Flame retardancy and mechanisms of BMI resins
LOI and UL-94 grade are common indexes reflecting the flame retardancy of materials in applications. As shown in Table 2, all BDTP and BTP resins have higher LOI values than BD resin, as the content of TAMPP increases, LOI rises gradually from 30.7% of BD to 39.6% of BTP. A similar trend is also found in the UL-94 tests, specifically, the flame retardancy of the BD resin is UL-94 V-1 grade, while those of the BDTP and BTP resins belong to UL-94 V-0 grade. These results prove that TAMPP has a good ability to improve flame retardancy of BMI resins.| Resin | LOI (%) | UL94 Level | PHRR (kW m−2) | THR (mJ m−2) | EHC (mJ kg−1) | TSP (m2) |
|---|---|---|---|---|---|---|
| BD | 30.7 | V-1 | 247.3 | 53.6 | 17.8 | 7.77 |
| BDTP1 | 32.4 | V-0 | 118.6 | 44.8 | 17.5 | 4.13 |
| BDTP2 | 33.8 | V-0 | 113.4 | 35.3 | 13.0 | 3.66 |
| BDTP3 | 37.0 | V-0 | 91.3 | 22.0 | 11.6 | 3.24 |
| BTP | 39.6 | V-0 | 121.6 | 14.6 | 8.2 | 2.24 |
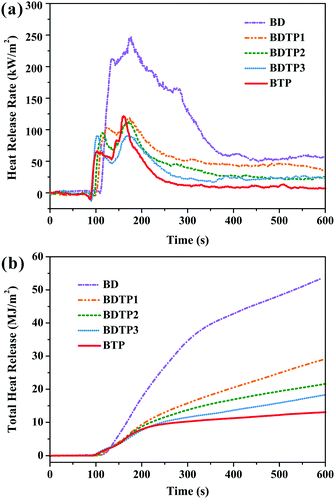
The CONE test is an effective tool to simulate the real combustion process of materials in a laboratory,30 and also provides information for revealing the flame retarding mechanism. Total heat release (THR) and heat release rate (HRR) including peak heat release rate (PHRR) are the most important parameters obtained in CONE tests, which provide a comprehensive insight into fire risks,31 the former indicates the danger of igniting materials, while the latter reflects the intensity of combustion.32 It can be seen from Fig. 5a that the HRR–time curve of the BD resin is very wide and the duration of the intense combustion is long. There are about three peaks throughout the entire process, and the PHRR reaches 247.3 kW m−2, reflecting that the carbon layers formed in different combustion stages are not able to prevent BD resin from further combustion. In contrast, the HRR–time curves of BDTP and BTP resins exhibit more narrow shapes with decreased peak intensities, meanwhile PHRR decreases by 50.8–63.1%. With the increase of the TAMPP content, THR decreases gradually by 16.4–72.9% compared to that of BD resin (Fig. 5b), and BTP resin has the lowest THR (14.55 mJ m−2), clearly demonstrating that TAMPP is an effective flame retardant for BDM resin.
The total smoke production (TSP) obtained in the CONE tests reflects the smoke release capacity of materials during combustion, so it is another important parameter for evaluating the fire risk of materials.33 As shown in Table 2, BTP resin has a TSP about 77.2% lower than BD resin. In fact, BTP resin also has a lower TSP than previously reported flame retardant BMI resins,34,35 clearly suggesting that BTP resin has good smoke suppression effects.
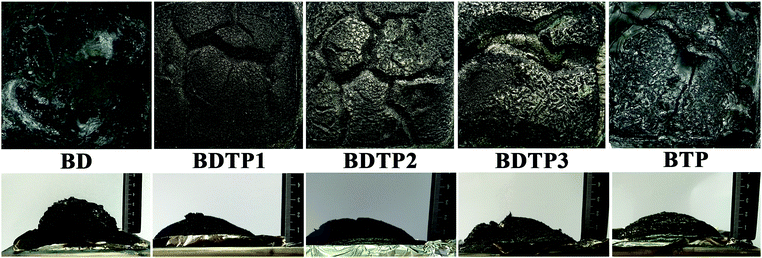
The ability to form char is one of the key factors that affect the flame retardancy of materials, so studying the morphology of residual chars is helpful to reveal the flame retarding mechanism. Fig. 6 shows digital photographs of residual chars of BD, BDTP and BTP resins after the CONE tests. The residual chars of both resins show obvious differences. For BD resin, the residual char composed of a layered sheet of carbon is fluffy and full of holes with an extremely low stability, indicating that its interior has been almost burned completely. On the contrary, the carbon residues of the BDTP and BTP resins are very dense and intact, and there are obviously fewer cracks, so the flame spread process can be hindered by the char on the burning surface in the combustion process, thereby enhancing the flame retardancy of the BDTP and BTP resins. These results show that there is a condensed phase flame retarding mechanism for BDTP and BTP resins as well.
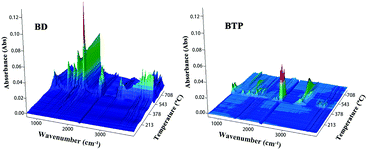
TGA-IR technology is often used to directly detect the gas produced during the degradation process, with which the mechanism of thermal degradation can be further investigated. BD and BTP resins are selected as the samples for investigation because each of them only contains one kind of allyl compound, and thus it is possible to find the respective role of DBA and TAMPP without confusion. Fig. 7 displays the 3D IR spectra of gaseous products of BD and BTP resins over the whole thermal degradation process. The intensity of the absorption peaks reflects the production of the degradation products for the samples with the same weights. As shown in Fig. 7, the products released by BTP resin are much fewer than that of BD resin.
Fig. 8 shows the IR spectra of the pyrolysis products of BD and BTP resins at the temperature with the maximum decomposition rate. Most pyrolysis products of BD resin produced during the test are organic gaseous and combustible, including various hydrocarbons (2800–3100 cm−1), carbonyl compounds (1716 cm−1), aromatic compounds (1616 and 1508 cm−1) and C–N (1174 cm−1); meanwhile water (3649 cm−1). CO2 (2351, 2310 and 669 cm−1) and CO (2180 cm−1) are also found. In contrast, the main pyrolysis product of BTP is CO2, and BTP has fewer combustible gaseous products than BD resin, this is beneficial to obtain good flame retardancy. At the same time, peaks reflecting P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1267 cm−1 and P–O–C at 1055 and 1033 cm−1
O at 1267 cm−1 and P–O–C at 1055 and 1033 cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 are also observed. Volatile phosphorus compounds can capture hydrogen radicals and thus play a flame retarding role in the gas phase.
31 are also observed. Volatile phosphorus compounds can capture hydrogen radicals and thus play a flame retarding role in the gas phase.
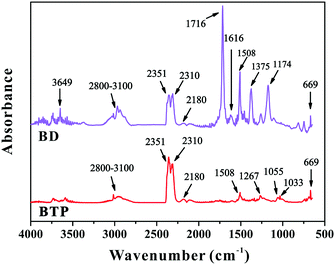 | ||
| Fig. 8 The FTIR spectra of the pyrolysis products of cured BD and BTP resins at the temperature with the maximum decomposition rate. | ||
The above studies prove that BTP resin shows an obviously higher flame retardancy than BD resin. In order to achieve this goal, both gas and condensed phase flame retarding effects play important roles.
Mechanical properties
The rigidity of resins is usually evaluated by flexural modulus and storage modulus (E′).36 As shown in Fig. 9a, BDTP and BTP resins have higher flexural moduli than BD resin, and more TAMPP leads to higher flexural modulus. BTP resin has the highest flexural modulus (3.95 ± 0.08 GPa), about 4.8% higher than that of BD resin. Considering the error bars, the increased ranges of the modulus are small; however, the average value reflects the general rule to some extent. In addition, the variation trend of the flexural modulus is consistent with those of the storage modulus and density below. A similar phenomenon also appears in E′ (Fig. 9b), that is, E′ at 50 °C gradually increases from 2404 MPa of BD resin to 2540 MPa of BTP resin as the content of TAMPP enlarges. It is known that both chemical structure and density affects E′ in the glassy state.36 The T-type trifunctional structure of TAMPP makes a shorter distance between crosslinked points. Besides, the densities of BDTP and BTP resins ranging from 1.272 to 1.297 g cm−3 are higher than that of BD resin (1.262 g cm−3) and increase as the TAMPP content increases, reflecting that TAMPP is good for improving E′ in the glassy state. Note that E′ of BD resin is only 316 MPa at 300 °C or 126 MPa at 350 °C, rather lower than the corresponding value of BTP resin (1406 MPa at 300 °C, or 1000 MPa at 350 °C). This result is meaningful for actual applications at higher temperatures.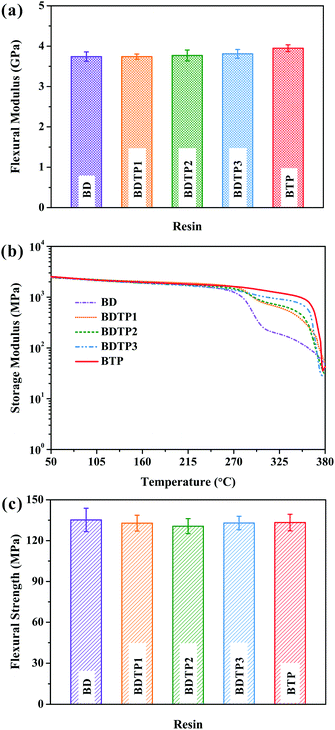 | ||
| Fig. 9 Flexural moduli (a), storage modulus–temperature curves from DMA tests (b), and flexural strengths (c) of different BMI resins. | ||
As shown in Fig. 9c, with the increase of the content of TAMPP, flexural strength tends to initially decease and then increase. BTP resin has a similar flexural strength to the BD resin. Note that for reported biobased eugenol derived BMI resins, BTP resin has higher flexural strength (Table S1, ESI†).
Water absorption
Generally, the introduction of phosphorus into resins may cause higher water absorption,37 so it is necessary to evaluate the water absorptions of new BMI resins reported herein. As shown in Table 3, after immersion in water at 100 °C for 100 h, the water absorptions of BDTP and BTP resins are only slightly higher than that of BD resin. This is because BDTP and BTP resin have more compact crosslinking structures due to the trifunctional structure of TAMPP, which offsets the negative influence of phosphorus.| Resin | BD | BDTP1 | BDTP2 | BDTP3 | BTP |
|---|---|---|---|---|---|
| Water absorption (%) | 2.78 | 2.81 | 2.97 | 2.91 | 2.86 |
Conclusions
A biobased phosphate (TAMPP) with a high renewable carbon content (100%) and reactive allyl groups was facilely synthesized through an environmentally friendly method based on renewable eugenol. Renewable clove oil was taken as the raw material, while water was used as an eco-friendly solvent, coinciding with the demands of sustainable development. In addition, compared with traditional BD resin, BTP resin in which DBA was completely replaced, simultaneously shows a high renewable carbon content (45.0%) and extremely high heat resistance (Tg > 380 °C), the combined advantage of which is the best among eugenol-modified bismaleimide resins ever reported so far. At the same time, BTP resin has excellent flame retardancy and good mechanical properties. The outstanding integrated performances of BTP resin originate from the unique T-type trifunctional chemical structure of TAMPP and its effect on the crosslinked structure of BDM. On the other hand, as a phosphate, TAMPP can also be used as a flame retardant for vinyl resins, cellulose resins, natural and synthetic rubbers and additives for gasoline and lubricants, showing great potential for replacing petroleum-based phosphate.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (51873135), Key Major Program of Natural Science Fundamental Research Project of Jiangsu Colleges and Universities (18KJA430013), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China, and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYLX16_0120), China.Notes and references
- J. Zhang, J. Wu, J. Yu, X. Zhang, J. He and J. Zhang, Mater. Chem. Front., 2017, 1, 1273–1290 RSC.
- G.-Z. Yin, X.-M. Yang, Z. Zhou and Q.-F. Li, Mater. Chem. Front., 2018, 2, 544–553 RSC.
- C. Zhang, T. F. Garrison, S. A. Madbouly and M. R. Kessler, Prog. Polym. Sci., 2017, 71, 91–143 CrossRef CAS.
- R. Auvergne, S. Caillol, G. David, B. Boutevin and J.-P. Pascault, Chem. Rev., 2014, 114, 1082–1115 CrossRef CAS PubMed.
- A. Scholz, R. L. Lewis, M. A. Bachan, A. L. Stewart and R. L. Quirino, Mater. Chem. Front., 2017, 1, 1795–1803 RSC.
- M. Shibata, N. Tetramoto, A. Imada, M. Neda and S. Sugimoto, React. Funct. Polym., 2013, 73, 1086–1095 CrossRef CAS.
- X. Zeng, S. Yu, R. Sun and J.-B. Xu, Composites, Part A, 2015, 73, 260–268 CrossRef CAS.
- J. J. King, M. Chaudhari and S. Zahir, 29th SAMPE Symp, 1984, 392–408 CAS.
- Y. C. Jiao, L. Yuan, G. Z. Liang and A. J. Gu, J. Phys. Chem. C, 2014, 118, 24091–24101 CrossRef CAS.
- R. J. Iredale, C. Ward and I. Hamerton, Prog. Polym. Sci., 2017, 69, 1–21 CrossRef CAS.
- Y. Xiong, F. Y. C. Boey and S. K. Rath, J. Appl. Polym. Sci., 2003, 90, 2229–2240 CrossRef CAS.
- S. Zahir, M. A. Chaudhari and J. King, Makromol. Chem., Macromol. Symp., 1989, 25, 141–154 CrossRef CAS.
- M. V. Maffini, B. S. Rubin, C. Sonnenschein and A. M. Soto, Mol. Cell. Endocrinol., 2006, 254–255, 179–186 CrossRef CAS PubMed.
- M.-Y. Chen, M. Ike and M. Fujita, Environ. Toxicol., 2002, 17, 80–86 CrossRef CAS PubMed.
- J.-T. Miao, L. Yuan, Q. B. Guan, G. Z. Liang and A. J. Gu, ACS Sustainable Chem. Eng., 2017, 5, 7003–7011 CrossRef CAS.
- J. Wan, J. Zhao, B. Gan, C. Li, J. Molina-Aldareguia, Y. Zhao, Y.-T. Pan and D.-Y. Wang, ACS Sustainable Chem. Eng., 2016, 4, 2869–2880 CrossRef CAS.
- L. Dumas, L. Bonnaud, M. Olivier, M. Poorteman and P. Dubois, Eur. Polym. J., 2015, 67, 494–502 CrossRef CAS.
- M. Shibata, T. Shimasaki, H. Satoh, M. Iwai and M. Neda, React. Funct. Polym., 2015, 97, 69–76 CrossRef CAS.
- M. Neda, K. Okinaga and M. Shibata, Mater. Chem. Phys., 2014, 148, 319–327 CrossRef CAS.
- X. Zhang, R. Akram, S. Zhang, H. Ma, Z. Wu and D. Wu, React. Funct. Polym., 2017, 113, 77–84 CrossRef CAS.
- J.-T. Miao, L. Yuan, Q. B. Guan, G. Z. Liang and A. J. Gu, ACS Sustainable Chem. Eng., 2018, 6, 7902–7909 CrossRef CAS.
- X. Pan, P. Sengupta and D. C. Webster, Biomacromolecules, 2011, 12, 2416–2428 CrossRef CAS PubMed.
- T. Iijima, N. Yuasa and M. Tomoi, Polym. Int., 1999, 48, 587–592 CrossRef CAS.
- X. X. Chen, G. Z. Liang, A. J. Gu and L. Yuan, Ind. Eng. Chem. Res., 2015, 54, 1806–1815 CrossRef CAS.
- USDA, The USDA Certified Biobased Product Label on a Product, https://www.biopreferred.gov/BioPreferred/faces/Welcome.xhtml.
- I.-D. Carja, D. Serbezeanu, T. Vlad-Bubulac, C. Hamciuc, A. Coroaba, G. Lisa, C. G. Lopez, M. F. Soriano, V. F. Perez and M. D. Romero Sanchez, J. Mater. Chem. A, 2014, 2, 16230–16241 RSC.
- G. You, Z. Cheng, Y. Tang and H. He, Ind. Eng. Chem. Res., 2015, 54, 7309–7319 CrossRef CAS.
- X. X. Chen, A. J. Gu, G. Z. Liang, L. Yuan, D. X. Zhuo and J.-T. Hu, Polym. Degrad. Stab., 2012, 97, 698–706 CrossRef CAS.
- Q. Luo, Y. Yuan, C. Dong, S. Liu and J. Zhao, RSC Adv., 2015, 5, 68476–68484 RSC.
- K. Shang, J.-C. Yang, Z.-J. Cao, W. Liao, Y.-Z. Wang and D. A. Schiraldi, ACS Appl. Mater. Interfaces, 2017, 9, 22985–22993 CrossRef CAS PubMed.
- X. Zhao, F. R. Guerrero, J. Llorca and D.-Y. Wang, ACS Sustainable Chem. Eng., 2016, 4, 202–209 CrossRef CAS.
- B. Schartel, M. Bartholmai and U. Knoll, Polym. Degrad. Stab., 2005, 88, 540–547 CrossRef CAS.
- Y.-T. Pan, L. Zhang, X. Zhao and D.-Y. Wang, Chem. Sci., 2017, 8, 3399–3409 RSC.
- T. T. Cao, L. Yuan, A. J. Gu and G. Z. Liang, Polym. Degrad. Stab., 2015, 121, 157–170 CrossRef CAS.
- Y. J. Zhu, L. Yuan, G. Z. Liang and A. J. Gu, Polym. Degrad. Stab., 2015, 118, 33–44 CrossRef CAS.
- E. D. Hernandez, A. W. Bassett, J. M. Sadler, J. J. La Scala and J. F. Stanzione, ACS Sustainable Chem. Eng., 2016, 4, 4328–4339 CrossRef CAS.
- P. M. Hergenrother, C. M. Thompson, J. G. Smith, J. W. Connell, J. A. Hinkley, R. E. Lyon and R. Moulton, Polymer, 2005, 46, 5012–5024 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 gives viscosity–time curves (at 130 °C) of BD and BTP prepolymers. Fig. S2 gives FTIR spectra of BDM, TAMPP, BTP prepolymer and cured BTP resin. Fig. S3 gives digital photos of BTP resin before and after swelling in THF at 25 °C for 24 h. Fig. S4 gives loss modulus–temperature plots of BMI resins from DMA tests. Table S1 summarizes renewable carbon contents and integrated performances of biobased eugenol derived BMI resins in the literature. See DOI: 10.1039/c8qm00443a |
| This journal is © the Partner Organisations 2019 |