High-quality ultralong copper sulphide nanowires for promising applications in high efficiency solar water evaporation†
Na
Li‡
ab,
Dandan
Yin‡
b,
Lingling
Xu‡
b,
Hongyang
Zhao
b,
Zhengqing
Liu
b and
Yaping
Du
 *ab
*ab
aTianjin Key Lab for Rare Earth Materials and Applications, Center for Rare Earth and Inorganic Functional Materials, School of Materials Science and Engineering & National Institute for Advanced Materials Nankai University, Tianjin 300350, China. E-mail: ypdu@nankai.edu.cn
bFrontier Institute of Science and Technology jointly with College of Science, Xi’an Jiaotong University, Xi'an Shaanxi Province 710054, China
First published on 18th December 2018
Abstract
Copper sulphide, with low cost and intrinsic near infrared (NIR) absorption, is an emerging and promising photothermal material that can provide high NIR photothermal conversion efficiency. Herein, we have successfully synthesized high-quality ultralong Cu2−xS nanowires (NWs) with an average diameter of ∼50 nm and length up to tens of micrometers. The synthetic strategy is based on the colloidal hot injection of precursors in a high-boiling solvent and in the presence of surfactant molecules that guide the nanowire growth. The as-harvested ultralong Cu2−xS NWs exhibit strong optical absorption in the NIR region and superior photothermal performance for solar water evaporation, yielding a solar thermal conversion efficiency of 89.9% under 8 kW m−2 solar illumination. Meanwhile, the theoretical calculation of the band structure reveals that the ultralong Cu2−xS NWs are hole-doped semiconductors with localized surface plasmon resonance (LSPR) from collective oscillation.
Introduction
With the intense development of modern technology, the increasing consumption of energy and resources subsequently causes serious environmental problems, especially water pollution and shortage of fresh water.1 Clean water supply is an extremely crucial issue that currently remains to be addressed in society.2 The ever-increasing need for fresh water relies on two well-established methodologies: membrane technology3 and distillation,4 wherein, water desalination with membranes requires high pressures and distillation requires extra energy consumption.5Solar energy is clean, renewable and sustainable. Solar desalinates water in artificial cells has been recently explored as a new strategy to obtain fresh water from high salinity water which can be used as a medium-scale or portable desalination unit.6 To date, abundant materials with light absorption have been explored as solar-thermal absorbers in solar thermal desalination apparatuses such as Au nanoparticles,7–9 polypyrrole,10–12 carbon nanoparticles,13 hollow carbon beads,14,15 graphene oxide,16–18 TiOx,19,20a Cu2SnSe3,20b Ti3C2,20c aluminum nanoparticles,21 and copper sulphide (Cu2−xS),22 to name a few. It is worth noting that among these materials, the light-to-heat conversion of gold and aluminum particles21 is due to localized surface plasmon resonance (LSPR) derived from collective oscillation of electrons, while for Cu2−xS it is derived from collective oscillation of holes.23,24 As a typical plasmon-enhanced material, Au nanoparticles can significantly increase the energy transfer efficiency and enhance light absorption. But utilization of Au nanoparticles in a large evaporation system is fairly expensive.8 Aluminum nanoparticle is a possible alternative for Au, however, the fabrication methods are complicated. Thus, there is an urgent demand for cost-effective and stable heat-localization layers that afford high steam-generation efficiency.
Copper sulphide (Cu2−xS), with low cost and intrinsic near infrared (NIR) absorption,25,26 has become an emerging and promising photothermal material as it can provide high NIR photothermal conversion efficiency.27–29 It is reported that anilite Cu7S4 (Cu1.75S) is the most stable structure along with the highest photo-thermal conversion efficiency among Cu2−xS structures and is a naturally hole-doped semiconductor.30
Herein, we report the preparation of high-quality ultralong Cu2−xS NWs with a uniform diameter of ∼50 nm and length up to tens of micrometers for efficient solar water evaporation (Scheme 1). The ultralong Cu2−xS NWs exhibit strong optical absorption in the NIR region and superior photothermal performance for the solar distillation of seawater, yielding a solar thermal conversion efficiency of 89.9% under 8 kW m−2 solar illumination. Meanwhile, the band structure of Cu2−xS has been investigated, demonstrating that Cu2−xS is a hole-doped semiconductor with LSPR from collective oscillation.
 | ||
| Scheme 1 Schematic illustration for the synthesis of ultralong Cu2−xS NWs and its application in solar water evaporation. | ||
Results and discussion
Characterization of the materials
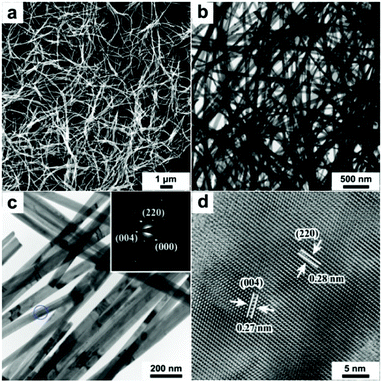
The colloidal hot injection method is used to synthesize high-quality ultralong Cu2−xS NWs (see Experimental section for details). The as-harvested Cu2−xS NWs are similar to the Cu1.75S compound with an orthorhombic phase (space group Pnma, JCPDS: 33-0489), as proved by powder X-ray diffraction (XRD) analysis (Fig. S1, ESI†), with the crystal lattice parameters calculated to be a = 7.906 Å, b = 7.822 Å, and c = 11.078 Å. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are used to determine the morphology of Cu2−xS NWs. SEM images show that the Cu2−xS NWs have lengths up to tens of micrometers (Fig. 1a). The low magnification TEM image in Fig. 1b and the high magnification TEM image in Fig. 1c clearly show that the nanowires have a uniform diameter of ∼50 nm. The selected area electron diffraction (SAED) pattern in the inset of Fig. 1c indicates that the nanowires are single-crystalline and can be labelled as orthorhombic-phase Cu1.75S, in accordance with the XRD result. The high-resolution transmission electron microscopy (HRTEM) image shown in Fig. 1d shows that the nanowires are single crystalline. The interplanar distance between the lattice fringes was ∼0.28 nm and ∼0.27 nm, corresponding to the (220) and (004) crystal planes of ultralong Cu2−xS NWs, respectively.X-ray photoelectron spectroscopy (XPS) was used to explore the chemical states of ultralong Cu2−xS NWs (Fig. S2, ESI†). Furthermore, we have conducted a series of experiments to optimize the reaction conditions for preparation of ultralong Cu2−xS NWs with high quality. In the current synthesis, the mole ratios of precursors and the reaction temperature were found to play crucial roles in the formation of high quality ultralong Cu2−xS NWs. For example, under the fixed experimental conditions, i.e. with solvent composition of oleylamine (10.0 ml), the reaction was fixed at 260 °C for 15 min, while varying the mole ratio of CuCl/NaDDTC to 1/1 (1.0 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 mmol), 1/2 (1.0 mmol
1.0 mmol), 1/2 (1.0 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 mmol), or 1/3 (1.0 mmol
2.0 mmol), or 1/3 (1.0 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0 mmol), ultralong Cu2−xS NWs with highly irregular nanoparticles were harvested (Fig. S3, ESI†). Besides the mole ratios of precursors, the reaction temperatures were essential for the preparation of high-quality ultralong Cu2−xS NWs. For instance, keeping other reaction conditions unchanged, we investigated the influence of temperature on the formation of ultralong Cu2−xS NWs. Obviously, with the reaction temperature decreased to 120 °C, 160 °C, 200 °C, and 240 °C, there were only severely aggregated nanorods with poor dispersibility, indicating the formation of high-quality ultralong Cu2−xS NWs at a relatively high temperature (Fig. S4, ESI†).
3.0 mmol), ultralong Cu2−xS NWs with highly irregular nanoparticles were harvested (Fig. S3, ESI†). Besides the mole ratios of precursors, the reaction temperatures were essential for the preparation of high-quality ultralong Cu2−xS NWs. For instance, keeping other reaction conditions unchanged, we investigated the influence of temperature on the formation of ultralong Cu2−xS NWs. Obviously, with the reaction temperature decreased to 120 °C, 160 °C, 200 °C, and 240 °C, there were only severely aggregated nanorods with poor dispersibility, indicating the formation of high-quality ultralong Cu2−xS NWs at a relatively high temperature (Fig. S4, ESI†).
Solar water evaporation performance
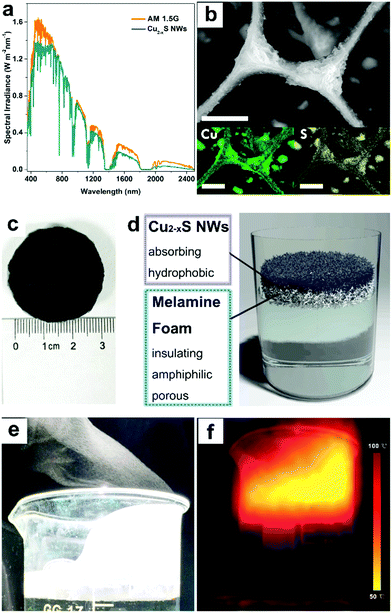
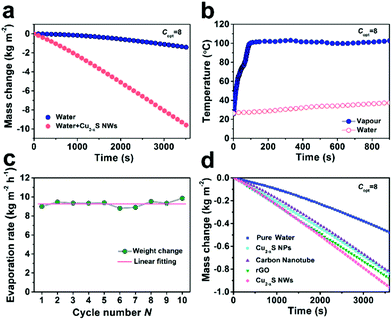
The optical properties of ultralong Cu2−xS NWs were characterized by ultraviolet-visible-near infrared (UV-Vis-NIR) spectroscopy, as shown in Fig. S5 (ESI†). The strong optical absorption appeared in the NIR region, suggesting that Cu2−xS NWs were suitable for application as solar absorbers.27,31 Meanwhile, the spectral irradiance of air mass 1.5 global (AM 1.5G) solar spectrum (yellow line) and the irradiance absorbed by ultralong Cu2−xS NWs (green line) in Fig. 2a verified the high photo-thermal transformation efficiency of the Cu2−xS NWs. Notably, in our work, the melamine foam layer is amphiphilic foam which can float on the water surface (Fig. S6, ESI†) and meanwhile can adhere to the oleylamine (OM)-coated ultralong Cu2−xS NWs (as validated by the Fourier transform infrared (FTIR) spectra in Fig. S7, ESI†) and formed a hydrophobic surface (Fig. S8, ESI†), which can provide vapour evaporation pathways. The ultralong Cu2−xS NWs with lengths up to tens of micrometers could wrap around the frames of melamine (Fig. 2b–d). From the SEM image and Energy Dispersive Spectrometer (EDS) elemental mapping of Cu and S elements (Fig. 2b), the ultralong Cu2−xS NW coating layer with rough surface structures could be clearly observed, confirming the uniform wrapping of the ultralong Cu2−xS NWs on melamine foam. Fig. 2e demonstrates a large amount of steam generated under 5 sun solar illumination. It is observed from Fig. 2f that the melamine foam with low thermal conductivity represses thermal conduction away from the heating zone.Under solar illumination, the evaporation rate of water with ultralong Cu2−xS NWs is evaluated by recording the mass change and the result is compared with pure water under solar illumination (300–2500 nm) at 8 kW m−2 (8 sun, Copt = 8). Fig. 3a shows that the evaporation rate with Cu2−xS NWs reaches ∼9.3 kg m−2 h−1 at Copt = 8, which is ∼6 times higher than that of pure water (∼1.5 kg m−2 h−1). The temperature near the surface of ultralong Cu2−xS NWs increased rapidly from room temperature to 100 °C as soon as the illumination was on, demonstrating the superior light-to-heat conversion efficiency of Cu2−xS NWs accompanied by the fast generation of water vapour. Meanwhile, the temperature of water below 1.5 cm remained at room temperature during 15 min of illumination, indicating excellent thermal-insulating property of melamine foam, and hence the localized heating zone in the proximity of the air–water interface (Fig. 3b).
The average mass change is 9.29 kg m−2 and the ultralong Cu2−xS NWs display excellent cyclability, which does not deviate from the first run even after 10 cycles. In addition, we made a comparison between the state-of-art materials for solar vapor generation under 1 sun illumination. The evaporation rate of Cu2−xS NWs was the highest among Cu2−xS NPs, carbon nanotubes, and reduced graphene oxide (rGO), indicating the outstanding light-to-heat conversion capability (Fig. 3d and Fig. S9, S10 ESI†). As shown in Fig. S11 (ESI†), the optical absorption observed in the NIR region (700–1800 nm) of Cu2−xS NWs is much higher than that of Cu2−xS NPs. This means that the Cu2−xS NWs have lower optical losses, and thus show a better evaporation rate compared to Cu2−xS NPs.
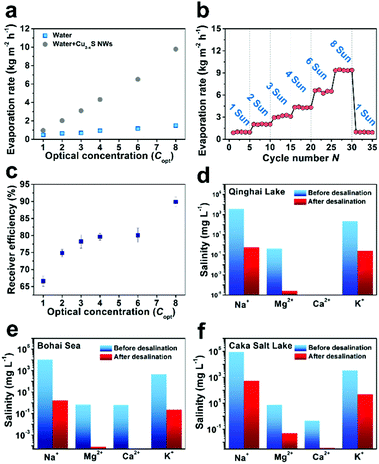
The evaporation rate as a function of irradiation concentrations is shown in Fig. 4a, the vapour generation with the Cu2−xS NWs sample is more efficient than with pure water at different irradiation concentrations. For instance, the evaporation rate of the Cu2−xS NWs reaches ∼0.95 kg m−2 h−1 at Copt = 1, which is 2.0 times that of pure water. When the solar concentration increased to Copt = 4, the evaporation rate of the ultralong Cu2−xS NWs reaches ∼4.31 kg m−2 h−1, which is 4.6 times that of pure water. Durability is one of the most important characteristics of solar water evaporation. Fig. 4b shows the cycling performance of the ultralong Cu2−xS NWs for a series of solar irradiance power densities. The ultralong Cu2−xS NWs demonstrate promising prospects in large-scale production and excellent recyclability. The experimental thermal efficiency (see ESI† for details) of solar water evaporation is shown in Fig. 4c with error bars standing for the standard deviation. The water evaporation efficiency of the ultralong Cu2−xS NWs reaches up to 89.9% under 8 sun illumination.
To methodically assess the influence of solar desalination of the ultralong Cu2−xS NWs, samples from the Caka Salt Lake (∼32.2 wt%), Bohai Sea (∼2.75 wt%), and Qinghai Lake (∼2.3 wt%) with different salinities were used and assayed by inductively coupled plasma optical emission spectroscopy (ICP-OES). It turns out that the concentrations of Na+ from the condensation of water are all markedly decreased in contrast to the concentrations before solar desalination, as shown in Fig. 4d–f. Meanwhile, the concentrations of the other three primary ions (Mg2+, Ca2+ and K+) before and after solar desalination are examined. Obviously, the concentrations of these three primary ions originally present in sea water are considerably reduced.
Simulation results
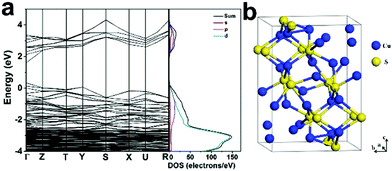
To understand the band features of Cu1.75S, we conducted density functional theory (DFT) calculations based on the Heyd–Scuseria–Ernzerhof (HSE) potential calculation method. As shown in Fig. 5a, from the projected and total electronic density of states (PDOS/DOS), the band gap of Cu1.75S is 1.27 eV at the Γ point and thus Cu1.75S is a naturally hole-doped semiconductor with LSPR from the collective oscillation of holes. As shown in the image of the Cu1.75S unit cell in Fig. 5b, the 4c and 8d Wyckoff sites were occupied by 28 Cu atoms, and the 4c Wyckoff sites were occupied by 16S atoms, indicating that it is doped with holes.Conclusions
In conclusion, we have developed an intriguing solar vapor generation device with high-quality ultralong Cu2−xS NWs with lengths up to tens of micrometers synthesized by an effective hot injection synthesis route. The calculated band structure of Cu1.75S demonstrated that Cu1.75S is a hole-doped semiconductor with LSPR from collective oscillation. The ultralong Cu2−xS NWs exhibit strong optical absorption in the NIR region and are suitable for application as solar absorbers. The ultralong Cu2−xS NWs absorb solar light, and confine the thermal energy to near the structure surface, yielding a solar thermal conversion efficiency of 89.9% under 8 kW m−2 solar illumination. Considering the increasing demand for recycling solar energy, our ultralong nanowire-based device opens a new avenue and is a promising candidates not only for solar-thermal steam generation but also for various potential applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Prof. Chunhua Yan for his kind suggestion and help. Research reported in this publication was supported by National Key R&D Program of China (2017YFA0208000) and the China National Funds for Excellent Young Scientists (grant no. 21522106). This work was also supported by the 111 Project (B18030) of China.References
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed.
- L. F. Greenlee, D. F. Lawler, B. D. Freeman, B. Marrot and P. Moulin, Water Res., 2009, 43, 2317–2348 CrossRef CAS PubMed.
- X. Cao, X. Huang, P. Liang, K. Xiao, Y. Zhou, X. Zhang and B. E. Logan, Environ. Sci. Technol., 2009, 43, 7148–7152 CrossRef CAS PubMed.
- N. S. Lewis, Toward, Science, 2007, 315, 798–801 CrossRef CAS PubMed.
- (a) O. Neumann, A. S. Urban, J. Day, S. Lal, P. Nordlander and N. J. Halas, ACS Nano, 2012, 7, 42–49 CrossRef PubMed; (b) F. C. Meng, W. S. Hao, T. Yu, R. Feng, Y. M. Liu, F. Yu, P. Tao, W. Shang, J. B. Wu, C. Y. Song and T. Deng, J. Am. Chem. Soc., 2017, 139, 12362–12365 CrossRef CAS PubMed.
- Z. H. Wang, Y. M. Liu, P. Tao, Q. C. Shen, N. Yi, F. Y. Zhang, Q. L. Liu, C. Y. Song, D. Zhang, W. Shang and T. Deng, Small, 2014, 10, 3234–3239 CrossRef CAS PubMed.
- D. W. Zhao, H. Z. Duan, S. T. Yu, Y. Zhang, J. Q. He, X. J. Quan, P. Tao, W. Shang, J. B. Wu, C. Y. Song and T. Deng, Sci. Rep., 2015, 5, 17276 CrossRef CAS PubMed.
- P. F. Li, B. A. Liu, Y. Z. Ni, K. K. Liew, J. Sze, S. Chen and S. Shen, Adv. Mater., 2015, 27, 4585–4591 CrossRef CAS PubMed.
- L. B. Zhang, B. Tang, J. B. Wu, R. Y. Li and P. Wang, Adv. Mater., 2015, 27, 4889–4894 CrossRef CAS PubMed.
- G. Ni, G. Li, S. V. Boriskina, H. X. Li, W. L. Yang, T. J. Zhang and G. Chen, Nat. Energy, 2016, 1, 16126 CrossRef CAS.
- (a) Y. Zeng, J. F. Yao, B. A. Horri, K. Wang, Y. Z. Wu, D. Li and H. T. Wang, Energy Environ. Sci., 2011, 4, 4074 RSC; (b) P. H. Yang, K. Liu, Q. Chen, J. Li, J. J. Duan, G. B. Xue, Z. S. Xu, W. K. Xie and J. Zhou, Energy Environ. Sci., 2017, 10, 1923–1927 RSC.
- H. Ghasemi, G. Ni, A. M. Marconnet, J. Loomis, S. Yerci, N. Miljkovic and G. Chen, Nat. Commun., 2014, 5, 4449 CrossRef CAS PubMed.
- Q. S. Jiang, L. M. Tian, K. K. Liu, S. Tadepalli, R. Raliya, P. Biswas, R. R. Naik and S. Singamaneni, Adv. Mater., 2016, 28, 9400–9407 CrossRef CAS PubMed.
- (a) X. Q. Li, W. C. Xu, M. Y. Tang, L. Zhou, B. Zhu, S. N. Zhu and J. Zhu, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13953–13958 CrossRef CAS PubMed; (b) G. B. Xue, Y. Xu, T. P. Ding, J. Li, J. Yin, W. W. Fei, Y. Z. Cao, J. Yu, L. Y. Yuan, L. Gong, J. Chen, S. Z. Deng, J. W. Zhou and L. Guo, Nat. Nanotechnol., 2017, 12, 317–322 CrossRef CAS PubMed.
- L. B. Zhang, R. Y. Li, B. Tang and P. Wang, Nanoscale, 2016, 8, 14600–14607 RSC.
- G. Wang, Y. Fu, X. F. Ma, W. B. Pi, D. W. Liu and X. B. Wang, Carbon, 2017, 114, 117–124 CrossRef CAS.
- J. Wang, Y. Y. Li, L. Deng, N. N. Wei, Y. K. Weng, S. Dong, D. P. Qi, J. Qiu, X. D. Chen and T. Wu, Adv. Mater., 2016, 1603730 Search PubMed.
- (a) M. M. Ye, J. Jia, Z. J. Wu, C. X. Qian, R. Chen, P. G. O'Brien, W. Sun, Y. C. Dong and G. A. Ozin, Adv. Energy Mater., 2016, 1601811 Search PubMed; (b) Y. W. Yang, H. Y. Zhao, Z. Y. Yin, J. Zhao, X. Yin, N. Li, D. Yin, Y. Li, B. Lei, Y. P. Du and W. X. Que, Mater. Horiz., 2018, 5, 1143–1150 RSC; (c) J. Q. Zhao, Y. W. Yang, C. H. Yang, Y. P. Tian, Y. Han, J. Liu, X. T. Yin and W. X. Que, J. Mater. Chem. A, 2018, 6, 16196–16204 RSC.
- L. Zhou, Y. L. Tan, J. Y. Wang, W. C. Xu, Y. Yuan, W. S. Cai, S. N. Zhu and J. Zhu, Nat. Photonics, 2016, 10, 393–398 CrossRef CAS.
- C. B. Zhang, C. Yan, Z. J. Xue, W. Yu, Y. D. Xie and T. Wang, Small, 2016, 12, 5320–5328 CrossRef CAS PubMed.
- S. W. Hsu, K. On and A. R. Tao, J. Am. Chem. Soc., 2011, 133, 19072–19075 CrossRef CAS PubMed.
- J. M. Luther, P. K. Jain, T. Ewers and A. P. Alivisatos, Nat. Mater., 2011, 10, 361–366 CrossRef CAS PubMed.
- Q. W. Tian, M. H. Tang, Y. G. Sun, R. J. Zou, Z. G. Chen, M. F. Zhu, S. P. Yang, J. L. Wang and J. Q. Hu, Adv. Mater., 2011, 23, 3542–3547 CrossRef CAS PubMed.
- J. B. Cui, R. Y. Jiang, S. Xu, G. F. Hu and L. Y. Wang, Small, 2015, 11, 4183–4190 CrossRef CAS PubMed.
- J. Mou, C. B. Liu, P. Li, Y. Chen, H. X. Xu, C. Y. Wei, L. Song, J. L. Shi and H. R. Chen, Biomaterials, 2015, 57, 12–21 CrossRef CAS PubMed.
- M. Zhou, J. J. Li, S. Liang, A. K. Sood, D. Liang and C. Li, ACS Nano, 2015, 9, 7085–7096 CrossRef CAS PubMed.
- H. L. Chen, M. L. Song, J. Tang, G. F. Hu, S. Y. Xu, Z. D. Guo, N. N. Li, J. B. Cui, X. Z. Zhang, X. Y. Chen and L. Y. Wang, ACS Nano, 2016, 10, 1355–1362 CrossRef CAS PubMed.
- Q. Xu, B. Huang, Y. F. Zhao, Y. F. Yan, R. Noufi and S. H. Wei, Appl. Phys. Lett., 2012, 100, 061906 CrossRef.
- M. Zhou, R. Zhang, M. Huang, W. Lu, S. L. Song, M. P. Melancon, M. Tian, D. Liang and C. Li, J. Am. Chem. Soc., 2010, 132, 15351–15358 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, XRD, XPS, FTIR data and TEM images. See DOI: 10.1039/c8qm00549d |
| ‡ N. Li, D. D. Yin and L. L. Xu contributed equally to this work. |
| This journal is © the Partner Organisations 2019 |