Fluorinated heptacyclic carbazole-based ladder-type acceptors with aliphatic side chains for efficient fullerene-free organic solar cells†
Tsung-Wei
Chen
ab,
Yu-Tang
Hsiao
ab,
You-Wei
Lin
ab,
Chia-Chih
Chang
 ab,
Wei-Tsung
Chuang
ab,
Wei-Tsung
Chuang
 c,
Yongfang
Li
c,
Yongfang
Li
 def and
Chain-Shu
Hsu
def and
Chain-Shu
Hsu
 *ab
*ab
aDepartment of Applied Chemistry, National Chiao Tung University, 1001 University Rd., Hsinchu, 30010, Taiwan. E-mail: cshsu@mail.nctu.edu.tw
bCenter for Emergent Functional Matter Science, National Chiao Tung University, 1001 University Rd., Hsinchu, 30010, Taiwan
cNational Synchrotron Radiation Research Center, 101 Hsin-Ann Road, Hsinchu, 30076, Taiwan
dBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
eUniversity of Chinese Academy of Sciences, Beijing 100049, China
fLaboratory of Advanced Optoelectronic Materials, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu 215123, China
First published on 12th March 2019
Abstract
Molecular engineering of non-fullerene acceptors (NFAs) is a promising strategy to uncover new structure–property relationships and design principles for developing next-generation high performance n-type materials. Two dithienocyclopentacarbazole (DTC)-based NFAs, DTC(4R)-IC and DTC(4R)-4FIC, are synthesized to elucidate the effects of incorporating aliphatic side chains and 2-(5,6-difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (2FIC) end groups on the thermal and optoelectronic properties of these NFAs. An inverted organic solar cell architecture of ITO/ZnO/J71:NFA/MoO3/Ag is employed. By replacing the phenyl-based side chains with aliphatic side chains on the carbon bridges of the carbazole-based core, the device containing DTC(4R)-IC exhibits an enhanced PCE of 9.61%, a VOC of 0.94 V, a JSC of 16.44 mA cm−2, and a FF of 62.00%, whereas the device containing DTC(4Ph)-IC exhibits a PCE of 7.76%. The incorporation of 2FIC end groups affords DTC(4R)-4FIC featuring better device performance relative to DTC(4R)-IC, a more red-shifted absorption edge, and a broader absorption range upon fluorination. Notably, the device fabricated with DTC(4R)-4FIC affords a Voc of 0.82 V, a Jsc of 18.92 mA cm−2, a FF of 70.22% and a highest PCE of 10.89%, which is by far the highest PCE for NFA containing heptacyclic carbazole cores.
Introduction
Bulk-heterojunction (BHJ) organic solar cells (OSCs) have been regarded as a promising solution for obtaining renewable energy.1–3 Selecting compatible electron donors and electron acceptors that can form optimal morphologies is of critical importance to achieve high performance OSCs.4,5 In the past two decades, a significant amount of efforts has been focused on designing high performance p-type conjugated polymers to be used in conjunction with n-type fullerene derivatives, such as PC61BM and PC71BM, to afford high performance OSCs featuring photon conversion efficiencies (PCEs) over 10%.6 Although fullerene derivatives are promising n-type materials in photovoltaic devices,7–10 fullerene-based acceptors have several intrinsic drawbacks, such as high production cost, tedious purification, insufficient light absorbing capability in the visible region, and the need for additives or post-fabrication treatment to achieve higher PCEs. Furthermore, the energy levels of fullerene-based materials are difficult to modulate and thus limit the choices of p-type materials.To this end, NFAs have emerged as an alternative class of n-type materials in OSCs owing to their ease of synthesis, tunable energy levels, and broader absorption in comparison to various fullerene derivatives.11,12 Molecular engineering of NFAs has led to the realization of several strategies to obtain desirable energy levels as well as optimal morphologies featuring appropriate phase separation length scales; these strategies include optimization of molecular structures,13–25 manipulation of side chains, and alteration of end-capping acceptor structures.26–30 Our previous efforts in developing synthetic strategies for ladder-type conjugated polymers have provided a myriad of donor-type cores for tailoring the optoelectronic properties of NFAs.31–35 In particular, ladder-type multifused rings such as pentacyclic indacenodithiophene (IDT) and heptacyclic indacenodithieno[3,2-b]thiophene (IDTT) prove useful in constructing high performance NFAs, which afforded devices with efficiencies over 14%.36 Moreover, in combination with tandem cell architecture, the device efficiency of OSCs containing NFAs can further be improved to over 17%.37
Rational design and efficient synthesis of novel NFAs and their structure-to-performance correlation are indispensable to unveil new design principles for developing highly efficient OSCs suitable for real-world applications. An NFA is usually composed of an A–D–A structure containing (a) a ladder-type core to ensure molecular planarity, (b) side chains as solubilizing groups that also impose steric hindrance to afford appropriate domain sizes, and (c) electron-withdrawing dye as end groups to strengthen intramolecular interaction and to facilitate charge transfer. Side-chain engineering is crucial for obtaining high performance active materials because steric hindrance imparted by side-chains can affect self-assembly behaviour in both solution and solid states as well as dictate the crystallinity of NFAs.38–46 Bo et al. compared the difference between phenyl side chains and alkyl side chains attached on the IDT core. The presence of phenyl side chains provides steric hindrance, which in turn would avoid the formation of large domain size.47 In contrast, alkyl side chains possess less hindered characteristics and retain the planarity of the molecule. Furthermore, the planarity of the molecule would ultimately facilitate charge transport and enhance π–π interaction, and thus result in improved Jsc and higher PCE from 6.48% for an IDT-based NFA containing phenyl side chains to 9.68% for the NFA containing aliphatic alkyl side chains. In addition, Baran and coworkers reported an IDTTIC derivative with even longer linear alkyl side chains, C16-IDTTIC, that exhibits a highest PCE of 11.2% when using PBDB-T as the donor polymer.48
We recently reported a heptacyclic carbazole-containing NFA, DTCCIC-C17 (abbreviated as DTC(4Ph)-IC), as a new NFA platform in 2017, affording a device efficiency of 9.5% when using PBDB-T as the donor.49 Besides featuring broad absorption in the range of 500–750 nm, the branched 1-octylnonyl group at the nitrogen center renders the corresponding NFA amorphous owing to the presence of the bulky substituent that is disruptive to intermolecular interaction. In this contribution, we synthesized novel heptacyclic carbazole-containing NFAs featuring aliphatic alkyl side chains and fluorinated IC units, DTC(4R)-IC and DTC(4R)-4FIC. We envisaged that the incorporation of alkyl substituents and fluorine atoms can lead to tighter packing of NFAs. Indeed, the newly synthesized DTC-based NFAs are more crystalline than previously reported DTC(4Ph)-IC. Since morphological control is crucial to obtaining high Voc, Jsc and FF, Zou et al. reported that the combination of a p-type polymer with relatively low crystallinity and an NFA with relatively high crystallinity can afford effective charge transport.50 Therefore, J71 was chosen as the p-type material because of its remarkable carrier mobilities and relatively low crystallinity in comparison to most of the other p-type conjugated polymers.51 In addition, J71 has the advantage of more blue-shifted absorption than the benzodithiophene-based conjugated polymers PBDB-T and PTB7, which is anticipated to provide more complementary absorption. We fabricated OSCs with an inverted structure consisting of ITO/ZnO/J71:NFA/MoO3/Ag, shown in Fig. 1(a). The chemical structures of J71 and the newly synthesized DTC derivatives are shown in Fig. 1(b and c). The devices fabricated with the non-fluorinated, octyl-functionalized DTC-based NFA DTC(4R)-IC displayed an enhanced PCE of 9.61%, with a Voc of 0.94 V, a Jsc of 16.44 mA cm−2, and a FF of 62.0%. Furthermore, J71:DTC(4R)-4FIC delivers a highest PCE of 10.89%, with a Voc of 0.82 V, a Jsc of 18.92 mA cm−2, and a FF of 70.22%.
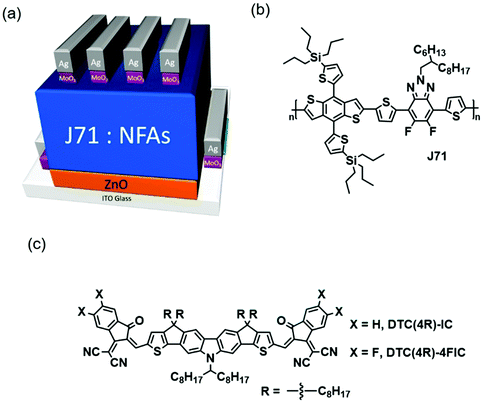 | ||
| Fig. 1 (a) Structure of the device; (b) chemical structure of J71; and (c) chemical structures of DTC(4R)-IC and DTC(4R)-4FIC. | ||
Results and discussion
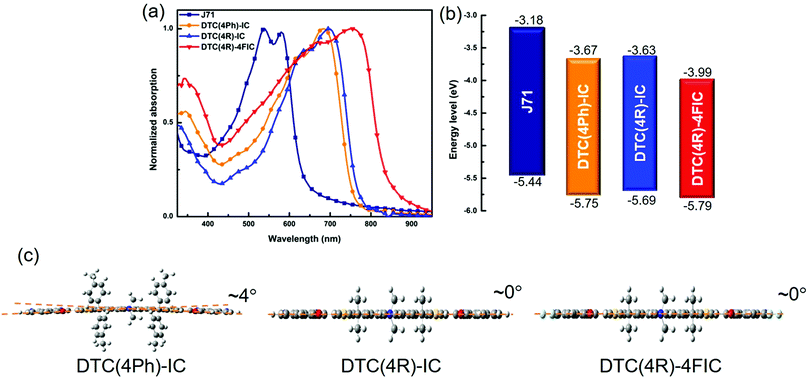
All the DTC-based NFAs have good solubility in chloroform and o-dichlorobenzene at room temperature. The detailed synthetic route is shown in Fig. S1 (ESI†). DTC(4R)-IC and DTC(4R)-4FIC possess high decomposition temperatures (Td) of 355 °C and 344 °C as characterized by thermogravimetric analysis (TGA) (Fig. S2, ESI†). The crystalline nature of DTC(4R)-IC and DTC(4R)-4FIC, confirmed by exothermic peaks observed in DSCs (Fig. S3, ESI†), is attributed to the presence of less sterically hindered alkyl side chains, which provides shorter intermolecular distance, compared with DTC(4Ph)-IC.Fig. 2 shows the absorption spectra and electronic energy levels (i.e., HOMO and LUMO energy levels) of the DTC-based NFAs. DTC(4Ph)-IC and DTC(4R)-IC exhibit strong absorption in the range of 600 nm to 800 nm, while DTC(4R)-4FIC shows absorption red-shifted to 900 nm. More importantly, these DTC-based NFAs exhibit complementary absorption to J71, which exhibits absorption in 400 nm to 600 nm. As summarized in Table 1, DTC(4Ph)-IC and DTC(4R)-IC exhibit extinction coefficients of 1.85 × 105 M−1 cm−1 and 1.90 × 105 M−1 cm−1, while DTC(4R)-4FIC possesses a highest extinction coefficient of 1.96 × 105 M−1 cm−1. The enhancement of the extinction coefficient of DTC(4R)-4FIC relative to the non-fluorinated derivative DTC(4R)-IC can be ascribed to the strengthened push–pull effect of the A–D–A configuration, which promotes ICT. The HOMO/LUMO level and bandgap are determined by cyclic voltammetry measurements, and the results are summarized in Table 1. The energy level diagram in the film state is presented in Fig. 2(b). The HOMO/LUMO levels are estimated to be −5.69/−3.63 eV for DTC(4R)-IC and −5.79/−3.99 eV for DTC(4R)-4FIC. J71 shows well-matched energy levels with DTC(4R)-IC and DTC(4R)-4FIC, ensuring efficient exciton transfer of the devices. The fluorinated IC moieties in DTC(4R)-4FIC are more electron-deficient, leading to down-shifted energy levels.
| NFA | λ solutionmax (nm) | λ filmmax (nm) | ε max [M−1 cm−1] | HOMO (eV) | LUMO (eV) | E CVg (eV) |
|---|---|---|---|---|---|---|
| a Extinction coefficient. | ||||||
| DTC(4Ph)-IC | 660 | 685 | 1.90 × 105 | −5.75 | −3.67 | 2.08 |
| DTC(4R)-IC | 670 | 695 | 1.85 × 105 | −5.69 | −3.63 | 2.06 |
| DTC(4R)-4FIC | 675 | 755 | 1.96 × 105 | −5.79 | −3.99 | 1.80 |
The side views of the NFAs generated by the DFT method at the B3LYP/6-31G level are shown in Fig. 2(c). DTC(4Ph)-IC, which contains phenyl side chains, possesses an ∼4° bending angle, while NFAs with alkyl side chains retain their planarity. To further elucidate the influence of having alkyl side chains instead of phenyl side chains on the electronic structures of the DTC-based NFAs, theoretical simulation of HOMO/LUMO electron distribution was carried out, as shown in Fig. S3 (ESI†). All NFAs show that the electrons gathered at the core in the HOMO diagrams, while the electron distribution accumulated at IC units in the LUMO diagrams.
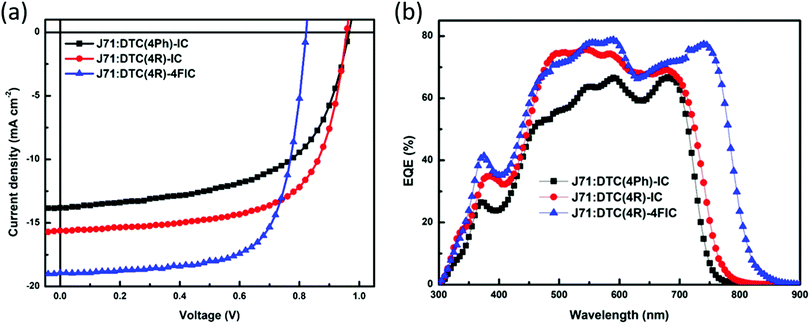
We fabricated OSCs with an inverted architecture of ITO/ZnO/active layer/MoO3/Ag using J71 and the DTC-based NFA blends as the active layers. 1-Chloronaphthalene (0.1 wt%) was selected as an additive to achieve an optimal morphology for this study because 1-chloronaphthalene was utilized to promote the face-on orientation and increase domain purity in a previous report.52 We found that 140 °C for 3 minutes are optimal thermal annealing conditions for obtaining high performance devices. Fig. 3 shows the J–V characteristics and external quantum efficiency (EQE) spectra of the optimized devices under simulated 100 mW cm−2 AM 1.5G illumination. The photovoltaic parameters are summarized in Table 2. The J71:DTC(4R)-IC device affords a PCE of 9.61%, a Voc of 0.94 V, a Jsc of 16.44 mA cm−2, and a FF of 62.00%. Both the Jsc and FF values of the DTC(4R)-IC based device are higher than those of the J71:DTC(4Ph)-IC device. The J71:DTC(4Ph)-IC device exhibits the lowest PCE of 7.76%, which corroborates with the fact that more planar NFAs often exhibit better photovoltaic performance than the non-planar derivatives. The highly planar structure of DTC(4R)-IC enables electrons to delocalize easily, leading to efficient charge transfer within the NFA domains. Moreover, the absorption range of DTC(4R)-IC is slightly red-shifted than that of DTC(4Ph)-IC, which is beneficial for harvesting more photons. We also synthesized the fluorinated NFA DTC(4R)-4FIC, where IC units were substituted with 2FIC units. The J71:DTC(4R)-4FIC device delivers a highest PCE of 10.89% with a Voc of 0.82 V, a Jsc of 18.92 mA cm−2, and a FF of 70.22%. The external quantum efficiency (EQE) measurements (Fig. 4(b)) are consistent with the results of the absorption spectra and Jsc obtained from the J–V curves (Table 1).
| Blend | V oc (V) | J sc (mA cm−2) | J sc (mA cm−2) | FF (%) | PCEb (%) | PCEc (%) |
|---|---|---|---|---|---|---|
| a Calculated by EQE measurements. b Highest PCE of over 10 devices. c Averaged PCE from over 10 devices. | ||||||
| J71:DTC(4Ph)-IC | 0.96 | 13.81 | 12.60 | 58.53 | 7.76 | 7.49 ± 0.15 |
| J71:DTC(4R)-IC | 0.94 | 16.44 | 15.06 | 62.00 | 9.61 | 9.39 ± 0.26 |
| J71:DTC(4R)-4FIC | 0.82 | 18.92 | 18.29 | 70.22 | 10.89 | 10.62 ± 0.14 |
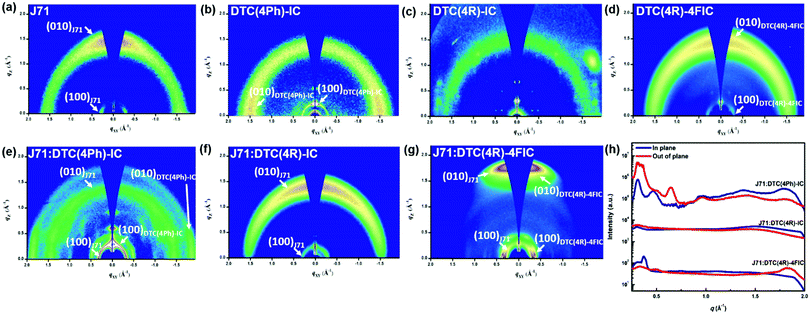
Since thin-film crystallization and phase separation play critical roles in charge carrier mobility and electron transfer for OSCs, we further studied the orientations of crystals in J71:DTC-based NFA blends by grazing incidence wide-angle X-ray scattering (GIWAXS) analysis. All the lattice parameters of the crystals are listed in Table S1 (ESI†) for the blended thin films of OSCs, and the neat films of J71 and DTC-based NFAs were also characterized, with the results summarized in Table S2 (ESI†). Fig. 4 shows GIWAXS patterns obtained for pristine films of J71 and the three DTC-based NFAs. Fig. S6 (ESI†) shows the corresponding 1D intensity profiles derived from the in-plane direction. The (010) diffraction, corresponding to the π–π stacking signal, determines the orientation of the thin film. In Fig. 4(a), the thin film of pristine J71 exhibits a stronger (010) diffraction along the out-of-plane direction in comparison to the in-plane direction, suggesting that J71 crystals preferred a face-on orientation. In contrast, the molecular stacking of DTC(4Ph)-IC crystals preferred an edge-on orientation, as identified by the stronger (010) diffraction at qXY of 1.46 Å−1 than that at qZ of 1.34 Å−1 (Fig. 4(b)). Moreover, the DTC(4R)-4FIC neat film shows a distinct face-on orientation in Fig. 4(d). The evolution of the orientation agrees with previous studies.48 Notably, these ladder-type DTC-based NFAs DTC(4R)-IC and DTC(4R)-4FIC exhibit highly ordered packing as observed in the scattering profiles of the corresponding neat films.
Based on the (100) and (010) diffractions of the blended J71:DTC(4Ph)-IC film in Fig. 4(e), J71 and DTC(4Ph)-IC crystals still retain their innate face-on and edge-on orientations, respectively. In contrast, the GIWAXS pattern of the J71:DTC(4R)-IC film in Fig. 4(f) exhibits (100) diffractions for face-on crystals of J71 without discernable diffraction signals from DTC(4R)-IC. The lack of crystalline DTC(4R)-IC domains in the blended film can be attributed to the high miscibility with J71, suppressing the crystallization of DTC(4R)-IC. Fig. 4(g) shows two sets of in-plane (100) and out-of-plane (110) diffractions, indicating a dual face-on orientation for the J71:DTC(4R)-4FIC thin film. As such, these channels that are suitable for vertical transport of charge carriers are anticipated to improve device performance. Furthermore, the π–π stacking distance of 3.45 Å obtained from the (010) diffraction of the DTC(4R)-4FIC grains is significantly smaller than the distance of 4.3 Å in the amorphous DTC(4R)-IC grains, indicating stronger π–π interaction (Fig. 4(h)).
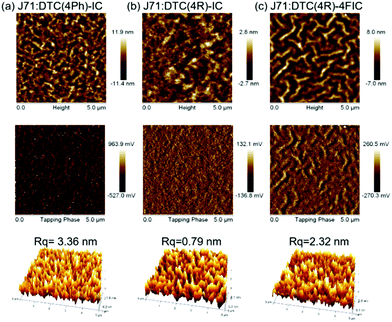
Owing to the significant relation between photovoltaic performance and phase separation, atomic force microscopy (AFM) was carried out to characterize the extent of phase separation. As shown in Fig. 5(a and c), both J71:DTC(4Ph)-IC and J71:DTC(4R)-4FIC are composed of nanoscale fibril-like interpenetrating networks, while in Fig. 5(b) J71:DTC(4R)-IC shows mixing phases, featuring the lowest Rq of 0.79 nm. The smooth J71:DTC(4R)-IC surface can be ascribed to the abovementioned amorphous character of DTC(4R)-IC and high J71-miscibility with DTC(4R)-IC. On the other hand, J71:DTC(4Ph)-IC shows an uneven surface with the highest Rq of 3.36 nm, which can, in part, be responsible for lower PCE.
To further distinguish the J71 grains and NFA grains, grazing incidence small-angle X-ray scattering (GISAXS) analysis is often utilized to detect microphase separated BHJ domains of OSCs, such as the binary blend of P3HT:PCBM.53 However, in our case, the phase-separated structural characteristics cannot be obtained from the GISAXS results (Fig. S7, ESI†) due to the indistinguishable electric density between J71 and NFA in these blends.
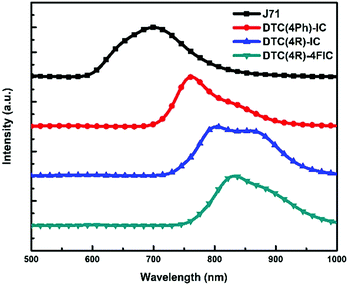
To probe the BHJ structure of the active layers, confocal PL mapping images at scan dimensions of 30 μm × 30 μm with 512 × 512 pixels were obtained for the three J71:DTC-based NFA blends. These PL spectra (Fig. 6) exhibit partially overlapped two peaks at approximately 640 nm/700 nm for J71, 760 nm/830 nm for DTC(4Ph)-IC, 800 nm/860 nm for DTC(4R)-IC, and 830 nm/890 nm for DTC(4R)-4FIC. The confocal PL mapping images reveal the distributions of J71 and NFAs in the blends, such that J71-rich domains are observed at a wavelength of 640 nm (Fig. 7a, c and e) and the NFA-rich domains are observed at their respective characteristic emission wavelengths (Fig. 7b, d and f). The J71:DTC(4Ph)-IC film presents the largest domain sizes with correlation lengths of Λ ∼ 6–8 μm. This suggests low miscibility of J71 and DTC(4Ph)-IC, leading to crystallization-induced phase separation. As shown in Fig. 7(c and d), no macroscopic phase separation was observed for the J71:DTC(4R)-IC film due to its high miscibility, which agrees well with the results obtained by AFM and GIWAXS that DTC(4R)-IC is incapable of forming its own domains. Nonetheless, obvious phase separation with small domain size (Λ ∼ 3–4 μm) was observed in the J71:DTC(4R)-4FIC film in Fig. 7(e and f). The presence of fluorine atoms in DTC(4R)-4FIC reduces its miscibility with J71. The J71:DTC(4R)-4FIC blend exhibits an appropriate distribution of domains that affords the best photovoltaic performance in J71:DTC-based systems.
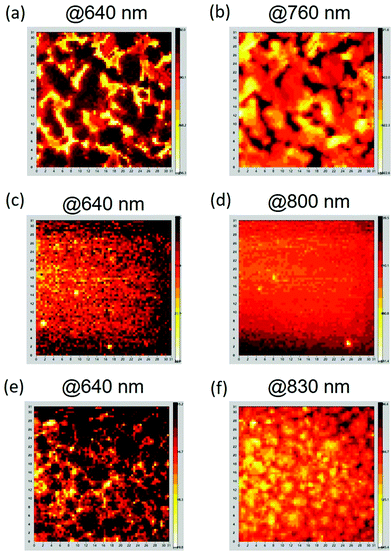 | ||
| Fig. 7 Confocal PL mapping images of (a and b) J71:DTC(4Ph)-IC, (c and d) J71:DTC(4R)-IC and (e and f) J71:DTC(4R)-4FIC. | ||
Conclusion
A series of novel heptacyclic carbazole-based NFAs featuring alkyl side chains and 2FIC end groups were designed and synthesized for photovoltaic application. These new NFAs display excellent solution processability and red-shifted absorption up to 900 nm. The presence of alkyl side chains, as opposed to the conventional phenyl groups, proves effective in further improving device performance. Fluorinated NFAs exhibit down-shifted molecular energy levels and broader absorption relative to their non-fluorinated counterparts. Detailed investigation of the structural characteristics with AFM, GIWAXS and confocal PL mapping show that appropriate phase separation length scales, a dual face-on orientation, and high domain crystallinity, as observed in the J71:DTC(4R)-4FIC system, can efficiently enhance photovoltaic performance in comparison with the other two cases. The J71:DTC(4R)-4FIC OSC device delivered a highest PCE of 10.89%. Our study provides a synergistic approach to modulate the optoelectronic properties of NFAs, which can be extended to other NFA systems so as to promote the commercial viability of fullerene-free OSCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by Ministry of Science and Technology, Taiwan (grant No. MOST107-3017-F009-003) and the Center for Emergent Functional Matter Science of National Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.References
- Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS PubMed.
- J. S. Wu, S. W. Cheng, Y. J. Cheng and C. S. Hsu, Chem. Soc. Rev., 2015, 44, 1113–1154 RSC.
- J. S. Wu, Y. J. Cheng, M. Dubosc, C. H. Hsieh, C. Y. Chang and C. S. Hsu, Chem. Commun., 2010, 46, 3259–3261 RSC.
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS PubMed.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- Y.-H. Chao, Y.-Y. Huang, J.-Y. Chang, S.-H. Peng, W.-Y. Tu, Y.-J. Cheng, J. Hou and C.-S. Hsu, J. Mater. Chem. A, 2015, 3, 20382–20388 RSC.
- Y. J. Cheng, S. W. Cheng, C. Y. Chang, W. S. Kao, M. H. Liao and C. S. Hsu, Chem. Commun., 2012, 48, 3203–3205 RSC.
- Y.-J. Cheng, C.-H. Hsieh, P.-J. Li and C.-S. Hsu, Adv. Funct. Mater., 2011, 21, 1723–1732 CrossRef CAS.
- Y.-Y. Lai, Y.-J. Cheng and C.-S. Hsu, Energy Environ. Sci., 2014, 7, 1866–1883 RSC.
- Q. Wang, S. Zhang, B. Xu, S. Li, B. Yang, W. Yuan and J. Hou, J. Phys. Chem. C, 2017, 121, 4825–4833 CrossRef CAS.
- Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed.
- Q. An, W. Gao, F. Zhang, J. Wang, M. Zhang, K. Wu, X. Ma, Z. Hu, C. Jiao and C. Yang, J. Mater. Chem. A, 2018, 6, 2468–2475 RSC.
- W. Bai, X. Xu, Q. Li, Y. Xu and Q. Peng, Small Methods, 2018, 2, 1700373 CrossRef.
- Q. Cao, W. Xiong, H. Chen, G. Cai, G. Wang, L. Zheng and Y. Sun, J. Mater. Chem. A, 2017, 5, 7451–7461 RSC.
- M. Chang, Y. Wang, N. Qiu, Y.-Q.-Q. Yi, X. Wan, C. Li and Y. Chen, Chin. J. Chem., 2017, 35, 1687–1692 CrossRef CAS.
- H. Fan, T. Vergote, S. Xu, S. Chen, C. Yang and X. Zhu, Mater. Chem. Front., 2018, 2, 760–767 RSC.
- R. Hou, M. Li, S. Feng, Y. Liu, L. Wu, Z. Bi, X. Xu, W. Ma and Z. Bo, J. Mater. Chem. A, 2018, 6, 3724–3729 RSC.
- S. Li, L. Zhan, F. Liu, J. Ren, M. Shi, C. Z. Li, T. P. Russell and H. Chen, Adv. Mater., 2018, 30, 1705208 CrossRef PubMed.
- T. Li, H. Zhang, Z. Xiao, J. J. Rech, H. Niu, W. You and L. Ding, Mater. Chem. Front., 2018, 2, 700–703 RSC.
- W. Liu, J. Zhang, Z. Zhou, D. Zhang, Y. Zhang, S. Xu and X. Zhu, Adv. Mater., 2018, 30, 1800403 CrossRef PubMed.
- Y. Ma, M. Zhang, Y. Yan, J. Xin, T. Wang, W. Ma, C. Tang and Q. Zheng, Chem. Mater., 2017, 29, 7942–7952 CrossRef CAS.
- J. Sun, X. Ma, Z. Zhang, J. Yu, J. Zhou, X. Yin, L. Yang, R. Geng, R. Zhu, F. Zhang and W. Tang, Adv. Mater., 2018, 30, 1707150 CrossRef PubMed.
- W. Wu, G. Zhang, X. Xu, S. Wang, Y. Li and Q. Peng, Adv. Funct. Mater., 2018, 28, 1707493 CrossRef.
- G. Zhang, G. Yang, H. Yan, J. H. Kim, H. Ade, W. Wu, X. Xu, Y. Duan and Q. Peng, Adv. Mater., 2017, 29, 1606054 CrossRef PubMed.
- T. Li, S. Dai, Z. Ke, L. Yang, J. Wang, C. Yan, W. Ma and X. Zhan, Adv. Mater., 2018, 30, 1705969 CrossRef PubMed.
- J. Wang, W. Wang, X. Wang, Y. Wu, Q. Zhang, C. Yan, W. Ma, W. You and X. Zhan, Adv. Mater., 2017, 29, 1702125 CrossRef PubMed.
- N. Qiu, H. Zhang, X. Wan, C. Li, X. Ke, H. Feng, B. Kan, H. Zhang, Q. Zhang, Y. Lu and Y. Chen, Adv. Mater., 2017, 29, 1604964 CrossRef PubMed.
- X. Shi, X. Liao, K. Gao, L. Zuo, J. Chen, J. Zhao, F. Liu, Y. Chen and A. K. Y. Jen, Adv. Funct. Mater., 2018, 28, 1802324 CrossRef.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148–7151 CrossRef CAS PubMed.
- C. Y. Chang, Y. J. Cheng, S. H. Hung, J. S. Wu, W. S. Kao, C. H. Lee and C. S. Hsu, Adv. Mater., 2012, 24, 549–553 CrossRef CAS PubMed.
- H.-H. Chang, C.-E. Tsai, Y.-Y. Lai, W.-W. Liang, S.-L. Hsu, C.-S. Hsu and Y.-J. Cheng, Macromolecules, 2013, 46, 7715–7726 CrossRef CAS.
- Y.-L. Chen, C.-Y. Chang, Y.-J. Cheng and C.-S. Hsu, Chem. Mater., 2012, 24, 3964–3971 CrossRef CAS.
- Y.-J. Cheng, J.-S. Wu, P.-I. Shih, C.-Y. Chang, P.-C. Jwo, W.-S. Kao and C.-S. Hsu, Chem. Mater., 2011, 23, 2361–2369 CrossRef CAS.
- J.-S. Wu, Y.-J. Cheng, T.-Y. Lin, C.-Y. Chang, P.-I. Shih and C.-S. Hsu, Adv. Funct. Mater., 2012, 22, 1711–1722 CrossRef CAS.
- S. Li, L. Ye, W. Zhao, H. Yan, B. Yang, D. Liu, W. Li, H. Ade and J. Hou, J. Am. Chem. Soc., 2018, 140, 7159–7167 CrossRef CAS PubMed.
- L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chen, Science, 2018, 361, 1094 CrossRef CAS PubMed.
- M. Chang, Y. Wang, Y.-Q.-Q. Yi, X. Ke, X. Wan, C. Li and Y. Chen, J. Mater. Chem. A, 2018, 6, 8586–8594 RSC.
- W. Gao, Q. An, R. Ming, D. Xie, K. Wu, Z. Luo, Y. Zou, F. Zhang and C. Yang, Adv. Funct. Mater., 2017, 27, 1702194 CrossRef.
- Y. Li, L. Zhong, B. Gautam, H.-J. Bin, J.-D. Lin, F.-P. Wu, Z. Zhang, Z.-Q. Jiang, Z.-G. Zhang, K. Gundogdu, Y. Li and L.-S. Liao, Energy Environ. Sci., 2017, 10, 1610–1620 RSC.
- X. Liu, B. Xie, C. Duan, Z. Wang, B. Fan, K. Zhang, B. Lin, F. J. M. Colberts, W. Ma, R. A. J. Janssen, F. Huang and Y. Cao, J. Mater. Chem. A, 2018, 6, 395–403 RSC.
- Z. Luo, C. Sun, S. Chen, Z. G. Zhang, K. Wu, B. Qiu, C. Yang, Y. Li and C. Yang, Adv. Energy Mater., 2018, 8, 1800856 CrossRef.
- W. Su, Q. Fan, X. Guo, J. Chen, Y. Wang, X. Wang, P. Dai, C. Ye, X. Bao, W. Ma, M. Zhang and Y. Li, J. Mater. Chem. A, 2018, 6, 7988–7996 RSC.
- Y. Yang, Z. G. Zhang, H. Bin, S. Chen, L. Gao, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 15011–15018 CrossRef CAS PubMed.
- H. Yao, Y. Cui, R. Yu, B. Gao, H. Zhang and J. Hou, Angew. Chem., Int. Ed., 2017, 56, 3045–3049 CrossRef CAS PubMed.
- Z. Zhang, M. Li, Y. Liu, J. Zhang, S. Feng, X. Xu, J. Song and Z. Bo, J. Mater. Chem. A, 2017, 5, 7776–7783 RSC.
- S. Feng, C. Zhang, Y. Liu, Z. Bi, Z. Zhang, X. Xu, W. Ma and Z. Bo, Adv. Mater., 2017, 29, 1703527 CrossRef PubMed.
- X. Song, N. Gasparini, M. M. Nahid, H. Chen, S. M. Macphee, W. Zhang, V. Norman, C. Zhu, D. Bryant, H. Ade, I. McCulloch and D. Baran, Adv. Funct. Mater., 2018, 28, 1802895 CrossRef.
- Y. T. Hsiao, C. H. Li, S. L. Chang, S. Heo, K. Tajima, Y. J. Cheng and C. S. Hsu, ACS Appl. Mater. Interfaces, 2017, 9, 42035–42042 CrossRef CAS PubMed.
- J. Yuan, Y. Liu, C. Zhu, P. Shen, M. Wan, L. Feng and Y. Zou, Acta Phys.-Chim. Sin., 2018, 34, 1272–1278 Search PubMed.
- H. Bin, L. Gao, Z. G. Zhang, Y. Yang, Y. Zhang, C. Zhang, S. Chen, L. Xue, C. Yang, M. Xiao and Y. Li, Nat. Commun., 2016, 7, 13651 CrossRef CAS PubMed.
- X. Song, N. Gasparini, L. Ye, H. Yao, J. Hou, H. Ade and D. Baran, ACS Energy Lett., 2018, 3, 669–676 CrossRef CAS.
- W. R. Wu, U. S. Jeng, C. J. Su, K. H. Wei, M. S. Su, M. Y. Chiu, C. Y. Chen, W. B. Su, C. H. Su and A. C. Su, ACS Nano, 2011, 5, 6233–6243 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qm00005d |
| This journal is © the Partner Organisations 2019 |