A light-tunable thermoresponsive supramolecular switch with reversible and complete “off–on”/“on–off” conversion†
Hao
Yao‡
a,
Zhen
Yang‡
a,
Xiaodong
Fan
*a,
Xin
Song
a,
Jia
He
a and
Wei
Tian
 *ab
*ab
aMOE Key Laboratory of Material Physics and Chemistry under Extraordinary Conditions, Shanxi Key Laboratory of Macromolecular Science and Technology, School of Science, Northwestern Polytechnical University, Xi’an, 710072, China. E-mail: happytw_3000@nwpu.edu.cn; xfand@126.com
bXi’an Institute for Biomedical Materials & Engineering, Northwestern Polytechnical University, Xi’an, 710072, China
First published on 10th April 2019
Abstract
Stimuli-responsive polymers have been widely used to design switchable materials and endow them with a “switch on” function with response characteristics. However, in some cases, the switch needs to be completely switched off even though the external environment changes in specific applications. Herein, we report the first example of a phototunable upper critical solution temperature transition of a binary/ternary supramolecular host–guest system for use as a thermoresponsive supramolecular switch for detecting temperature changes. The obtained supramolecular switch easily achieved reversible and complete “off–on”/“on–off” conversion of a temperature switch function under alternating UV/visible light irradiation. The smart supramolecular switch as a novel laser sensor device was used to detect temperature changes through an “off–on” or “on–off” mode. This study demonstrates an approach for constructing a new generation of smart materials or switchable materials based on supramolecular systems.
Introduction
Stimuli-responsive polymers, which are also known as “smart materials”, play important roles in a diverse range of applications, such as smart interfaces, controlled drug delivery systems, soft actuators, and molecular separation.1 These polymers can undergo changes in response to small external variations in environmental conditions, such as temperature, pH, light, electric or magnetic field.2 Therefore, these polymers have been widely used to design switchable materials with a “switch on” function with the previously mentioned response characteristics.3 However, in some cases, the switch must be completely switched off even though the external environment changes. For example, the body colour of chameleons can undergo reversible changes in response to small external variations. When danger approaches, chameleons will change their body colour as camouflage (switch on). However, their body colour will not change under safe conditions (switch off) even though they experience environmental changes. Unfortunately, a smart switch with a chameleon-like “on–off” function has not been previously designed. Alternatively, as a common stimuli-responsive polymer, thermoresponsive polymers with an upper critical solution temperature (UCST) in solution exhibit a miscibility gap at low temperature, and these polymers have been used to construct smart switches.4 Furthermore, the facile and dynamic reversible supramolecular interactions,5–9 such as host–guest interactions,6 have been incorporated into the backbone of thermoresponsive polymers to tune the reversible switch process. Nevertheless, the UCST transition-based supramolecular switches still only lie in an “on” state and cannot be completely switched off.Herein, we report the first example of a phototunable upper critical solution temperature transition of a binary/ternary supramolecular host–guest system for use as a thermoresponsive supramolecular switch for detecting temperature changes. Our strategy involves constructing a binary supramolecular system (β-cyclodextrin trimer (β-CD3)/double azobenzene-terminated polyethylene glycol oligomer (Azo-PEG-Azo)) based on the host–guest interaction between the β-CD and Azo moieties. This binary supramolecular system has a special switching device with a completely “off–on” mode, which can act as a detector under alternating UV/visible light irradiation. In the initial state, the sample solution temperature is increased above the UCST of the β-CD3/Azo-PEG-Azo supramolecular system (Scheme 1A). As the sample solution temperature decreases, a reversible UCST phase transition occurs, and the supramolecular switch remains in the “on” state (Scheme 1A-B and B-A). Furthermore, UV light and visible light irradiation can reversibly switch this phase transition (Scheme 1B-D and D-B). However, when the preliminary sample solution is irradiated with UV light above the UCST (Scheme 1A-C), the supramolecular switch can be completely held in the “off” state, resulting from the disappearance of the UCST transition even though the solution temperature is decreased further (Scheme 1C-D). With visible light irradiation, the “on” function of the supramolecular switch can be recovered again (Scheme 1C-A). Additionally, to confirm this supramolecular switching property, another ternary supramolecular system based on β-CD3/double naphthalene-terminated PEG (NP-PEG-NP)/single azobenzene-terminated polyethylene glycol monomethylether (mPEG-Azo) was constructed, and this system exhibited an “on–off” supramolecular switch function based on a similar UCST-type phase transition (Scheme S2, ESI†). Specifically, in the initial state, this system does not present a UCST transition when the sample solution temperature is decreased or increased (Scheme S1A-B and B-A, ESI†), so the supramolecular switch always remains in the “off” state. However, UV light irradiation (Scheme S1A-C, ESI†) can trigger the occurrence of the UCST transitions, and the supramolecular switch can be held in the “on” state (Scheme S1C-D and D-C, ESI†). Furthermore, with visible light irradiation, the “off” function of the supramolecular switch can be recovered again (Scheme S1C-B, ESI†).
Results and discussion
The Azo-PEG-Azo and mPEG-Azo polymer precursors were first prepared, as shown in Scheme S1 (ESI†). Azo-PEG-Azo and mPEG-Azo were synthesized via a condensation reaction between 4-phenylazobenzoyl chloride and PEG/mPEG. The 1H NMR and FTIR spectra (Fig. S1–S4, ESI†) indicated that the condensation reaction was successfully completed. Additionally, β-CD3 and NP-PEG-NP were synthesized according to our previous work.10 The binary supramolecular system based on β-CD3/Azo-PEG-Azo was first constructed by dissolving β-CD3 and Azo-PEG-Azo in deionized water. The β-CD3/Azo-PEG-Azo supramolecular system was confirmed by 2D NOESY and isothermal titration calorimetry (ITC) measurements at 25 °C, as shown in Fig. S5 (ESI†). The 2D-NOESY NMR spectra implied that the protons of the Azo group were correlated with H-3 and H-5 located in the cavity of β-CD (Fig. S5A, ESI†). This result suggested the formation of inclusion complexes of β-CD3/Azo-PEG-Azo in the aqueous solution at 25 °C. ITC experiments were performed to directly measure the binding affinity between β-CD3 and Azo-PEG-Azo (Fig. S5B, ESI†). The data yielded a binding constant (Kt) of 3.95 × 103 M−1 and indicated a binding site number of 1, which suggested an inclusion relationship with a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between Azo and β-CD. In addition, the negative enthalpy of the inclusion complexation (−4.2 × 103 cal mol−1) indicated that the binding constant between the two substances increased when the solution temperature decreased.11 Moreover, the ternary supramolecular system based on β-CD3/NP-PEG-NP/mPEG-Azo was constructed by adding mPEG-Azo to the β-CD3/NP-PEG-NP solution, and the host–guest interaction was similarly confirmed by 2D NOESY spectra (Fig. S6, ESI†).
1 between Azo and β-CD. In addition, the negative enthalpy of the inclusion complexation (−4.2 × 103 cal mol−1) indicated that the binding constant between the two substances increased when the solution temperature decreased.11 Moreover, the ternary supramolecular system based on β-CD3/NP-PEG-NP/mPEG-Azo was constructed by adding mPEG-Azo to the β-CD3/NP-PEG-NP solution, and the host–guest interaction was similarly confirmed by 2D NOESY spectra (Fig. S6, ESI†).
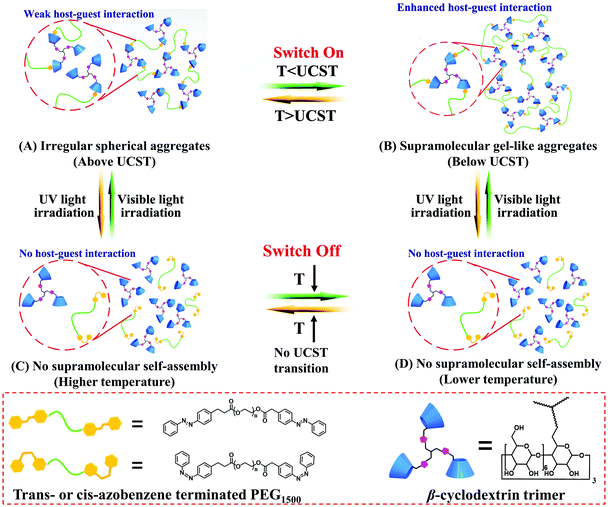
The UCST-type photoreversible supramolecular switching property of the binary supramolecular system (β-CD3/Azo-PEG-Azo) was investigated using turbidity measurements on a UV-Vis spectrometer. The cloud point (Tcloud) was determined as the temperature at 90% transmittance at 550 nm using the UV-Vis spectrometer. As shown in Fig. 1A, the β-CD3/Azo-PEG-Azo solution exhibited an evident UCST phase transition at Tcloud = 35.4 °C prior to UV light irradiation, and the transition process was reversible (Fig. S7A, ESI†). Interestingly, when the solution was exposed to UV light at a wavelength of 365 nm, Tcloud gradually decreased with an increase in the UV irradiation time from 0 to 45 min. Ultimately, the UCST transition completely disappeared at 45 min. Furthermore, by alternately irradiating the β-CD3/Azo-PEG-Azo solution with UV and visible light, the solution exhibited a UCST transition in the “off–on” mode, as shown in Fig. 1B. In addition, the change in Tcloud was only a 1 °C decrease from 35.4 to 34.4 °C after three cycles (Fig. 1C). The experimental results indicated that the UCST-type supramolecular switching property of the β-CD3/Azo-PEG-Azo system can be easily tuned in the complete “off–on” mode by alternating the irradiation between UV and visible light. Moreover, the concept of a photoreversible supramolecular switch was further confirmed in another ternary supramolecular system (i.e., β-CD3/NP-PEG-NP/mPEG-Azo) with a complete “on–off” mode. First, mPEG-Azo was added to the β-CD3/NP-PEG-NP solution at room temperature. The original turbid solution became clear in seconds without UV light irradiation due to the competitive inclusion complexation between β-CD/NP and β-CD/Azo. After being exposed to UV light from 0 to 60 min, the reversible UCST transition of the β-CD3/NP-PEG-NP/mPEG-Azo supramolecular system appeared, and Tcloud gradually increased to 32 °C (Fig. 1D and Fig. S7B, ESI†). By further alternating the UV and visible light, the β-CD3/NP-PEG-NP/mPEG-Azo supramolecular system exhibited a UCST transition in the complete “on–off” switch mode (Fig. 1E). Furthermore, the change in Tcloud was only a 1 °C decrease from 32 to 31 °C after three cycles (Fig. 1F). The UCST behaviour in both systems shows slight changes which can be negligible after repeating six times. Additionally, the effect of the thermal back reaction on the UCST behavior has been carefully investigated. The results showed that the turbidity–temperature curves for UCST behaviors slightly shifted (Fig. S7, ESI†). Therefore, UCST transition-based photoreversible supramolecular switches were successfully created with a complete “off–on” or “on–off” bidirectional mode using β-CD3/Azo-PEG-Azo binary and β-CD3/NP-PEG-NP/mPEG-Azo ternary supramolecular systems.
A possible mechanism for this UCST-type photoreversible supramolecular switch was proposed. The most important factor that influences the effectiveness of the supramolecular switch is a tuneable host–guest interaction with photoresponse characteristics. Using the binary supramolecular system β-CD3/Azo-PEG-Azo as a typical example, irregular spherical aggregates were formed based on the weak host–guest interaction between β-CD3 and Azo-PEG-Azo above the UCST without UV light irradiation (Scheme 1A, magnified image). When the solution temperature decreased below the UCST, inclusion complexation between β-CD3 and Azo-PEG-Azo was enhanced.10 Therefore, a UCST phase separation occurred, resulting in the “on” state of the supramolecular switch (Scheme 1A-B) that was accompanied by self-assembly morphological transitions from irregular spherical aggregates to supramolecular cross-linked aggregates (Scheme 1B, magnified image). In contrast, when the temperature increased above the UCST again, the inclusion complex between β-CD3 and Azo-PEG-Azo was partially dissociated, and the morphology of the self-assembly reverted to the preliminary state (Scheme 1B-A). Furthermore, after irradiation with UV light above the UCST (Scheme 1A-C), the β-CD3/Azo-PEG-Azo supramolecular self-assemblies completely disassociated due to photoisomerization of the Azo from trans to cis12 (Scheme 1C, magnified image). Therefore, no UCST transition was observed as the solution temperature decreased, and the supramolecular switch remained entirely in the “off” state (Scheme 1C-D). Additionally, when the solution was exposed to visible light above the UCST, the weak inclusion interaction between Azo-PEG-Azo and β-CD3 was recovered, and the supramolecular switch could return to the “on” state (Scheme 1C-A and A-B).
In this case, the supramolecular switch exhibits a complete “off–on” function. The photoreversible supramolecular switch in the ternary β-CD3/NP-PEG-NP/mPEG-Azo system has a similar mechanism (Scheme S1, ESI†).
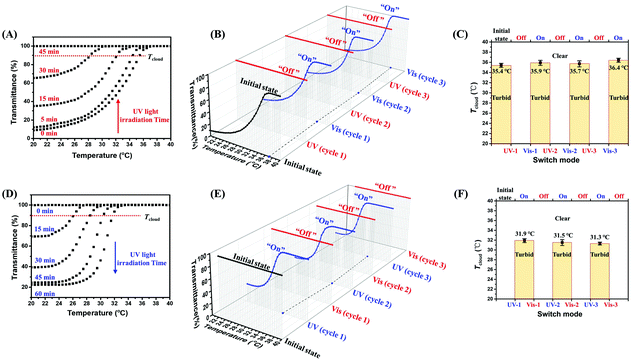
The proposed mechanism for the β-CD3/Azo-PEG-Azo binary supramolecular system was further confirmed by transmission electron microscopy (TEM) and dynamic light scattering (DLS) as well as UV-Vis, 1H NMR and 2D NOESY measurements. As shown in Fig. 2A, B and Fig. S8, S12 (ESI†), the TEM images show the reversible morphology transitions of the β-CD3/Azo-PEG-Azo self-assemblies from irregular spherical aggregates above the UCST to supramolecular cross-linked aggregates below the UCST without UV light irradiation. This behaviour may result from the significant temperature dependence of the β-CD inclusion complex, which has been reported in the literature.11 Additionally, the trend in the reversible size change of the β-CD3/Azo-PEG-Azo self-assemblies was confirmed by DLS, as shown in Fig. S9 (ESI†). Because the complexation process is an exothermic equilibration reaction, this process strongly depends on temperature. Herein, the negative enthalpy of the β-CD3/Azo-PEG-Azo inclusion complex (−4.2 × 103 cal mol−1) obtained from the ITC measurement (Fig. S5B, ESI†) indicated that the binding constant between the two substances increased when the solution temperature decreased. Therefore, the temperature-tuneable β-CD3/Azo-PEG-Azo host–guest interaction may lead to the formation of distinguishable microstructures at different temperatures, which would result in reversible UCST behaviour. Furthermore, the reversible photoinduced trans–cis isomerization of the Azo groups attached to the PEG chain during UV light irradiation was confirmed by UV-Vis and 1H NMR measurements. As shown in Fig. 2C, after being irradiated with UV light for 45 min, the characteristic absorption peak of trans-Azo at 326 nm decreased substantially and returned after being irradiated with visible light. This reversible process could be recycled at least three times by alternating the UV/visible light irradiation (inset of Fig. 2C). The 1H NMR spectra (Fig. 2D) indicated that the proton peak of cis-Azo increased markedly after UV irradiation and returned to the trans-Azo proton peaks after visible light irradiation. Furthermore, the cis/trans isomerization ratios for different irradiation times have been calculated by the ratios of the proton peaks between trans-azo and cis-azo determined by 1H NMR and UV-visible absorption spectra (Fig. S9A–C, ESI†). The result indicated that the trans-/cis-azo ratio was approximately 37/63 at the equilibrium point. Moreover, DLS was utilized to monitor the size change tendency when the irradiation time increased (Fig. S9D and E, ESI†). The 2D NOESY spectra of the β-CD3/Azo-PEG-Azo self-assemblies further indicated that the host–guest interactions between β-CD and Azo were dissociated and associated after alternately irradiating with UV and visible light (Fig. 2E, F and Fig. S10, ESI†). These results imply that the tuneable host–guest interaction between β-CD3 and Azo-PEG-Azo established the “on” function of the supramolecular switch based on reversible UCST transitions without UV light irradiation. Furthermore, the photoinduced dissociation and association of the β-CD3/Azo-PEG-Azo self-assemblies promoted a facile and controllable supramolecular switch in the completely “off–on” mode.
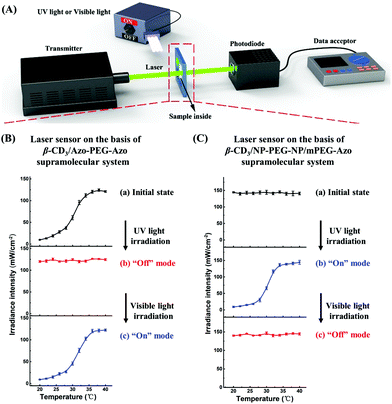
Finally, the UCST-type photoreversible supramolecular switch was considered for use as a controllable laser sensor to detect temperature changes. In particular, this smart supramolecular system has a special switching device with a complete “off–on” or “on–off” mode with detection achieved by alternating the UV/visible light irradiation. A device to detect temperature changes was constructed, as shown in Fig. 3A. The irradiance intensity was recorded with a photodiode after the laser light passed through the sample. The irradiance intensity of a laser can be effectively altered by the transmittance changes of the sample solution that are induced by temperature variations. Most importantly, we constructed a switching device surrounding the sample to tune the “on” or “off” mode of the detection function, which was controlled by UV/visible light.
The switching function of this laser sensor device was further confirmed using the β-CD3/Azo-PEG-Azo or β-CD3/NP-PEG-NP/mPEG-Azo supramolecular system. For β-CD3/Azo-PEG-Azo, the initial laser irradiance intensity was approximately 122 mW cm−2 at 40 °C. However, this intensity gradually decreased to 9 mW cm−2 as the temperature decreased to 20 °C. Therefore, the initial state of the optical transducer was in the “on” mode (Fig. 3B-a). Furthermore, the laser irradiance intensity passing through the sample was nearly unchanged after UV light irradiation even though the solution temperature decreased, indicating that the laser sensor was switched to the “off” mode (Fig. 3B-b). After visible light irradiation, the optical transducer was switched back to the “on” mode (Fig. 3B-c). Furthermore, when β-CD3/NP-PEG-NP/mPEG-Azo was used in the device (Fig. 3C), the initial state of the laser sensor state was the “off” mode because the initial laser irradiance intensity did not change when the solution temperature of the sample was varied without UV light irradiation (Fig. 3C-a). Moreover, the laser sensor was switched to the “on” mode after UV light irradiation, and the laser irradiance intensity changed from 140 to 9 mW cm−2 when the solution temperature decreased from 40 to 20 °C (Fig. 3C-b). Then, the laser sensor reverted to the “off” mode again after visible light irradiation (Fig. 3C-c). Therefore, this laser sensor device for detecting temperature changes can be easily and reversibly switched by alternating the UV/visible light irradiation.
Conclusions
In summary, we have demonstrated a UCST-type photoreversible supramolecular switch using a binary (β-CD3/Azo-PEG-Azo) or ternary (β-CD3/NP-PEG-NP/mPEG-Azo) supramolecular system based on a host–guest interaction between β-CD and Azo moieties. For the β-CD3/Azo-PEG-Azo supramolecular system, the supramolecular switch remained in the “on” state based on a reversible UCST phase transition without UV light irradiation. However, the supramolecular switch can be completely held in the “off” state with UV light irradiation. With visible light irradiation, the “on” function of the supramolecular switch was recovered. The β-CD3/NP-PEG-NP/mPEG-Azo supramolecular system exhibited an “on–off” supramolecular switch function based on a similar UCST-type phase transition. To the best of our knowledge, a UCST-type photoreversible supramolecular switch with complete “off–on”/“on–off” modes has not been previously reported. This supramolecular switch was utilized as a laser sensor device for detecting temperature changes with a smart photocontrolled “off–on”/“on–off” mode. This type of supramolecular switch may provide a new method for preparing smart materials and fabricating switch devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the National Science Foundation of China (No. 21674086) and the Natural Science Basic Research Plan in Shaanxi Province of China (2018JZ2003). W. T. thanks Prof. Dong Mao and Jiwei Zhang (Northwestern Polytechnical University) for providing the transmitter, photodiode, and data acceptor.Notes and references
- (a) M. A. Stuart, W. T. Huck, J. Genzer, M. Muller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed; (b) D. Roy, J. N. Cambre and B. S. Sumerlin, Prog. Polym. Sci., 2010, 35, 278–301 CrossRef CAS; (c) F. Xia and L. Jiang, Adv. Mater., 2008, 20, 2842–2858 CrossRef CAS; (d) Y. S. Kim, R. Tamate, A. M. Akimoto and R. Yoshida, Mater. Horiz., 2017, 4, 38–54 RSC; (e) E. B. Murphy and F. Wudl, Prog. Polym. Sci., 2010, 35, 223–251 CrossRef CAS; (f) H. Yang, B. Yuan, X. Zhang and O. A. Scherman, Acc. Chem. Res., 2014, 47, 2106–2115 CrossRef CAS PubMed.
- (a) S. J. Jeon, A. W. Hauser and R. C. Hayward, Acc. Chem. Res., 2017, 50, 161–169 CrossRef CAS PubMed; (b) D. H. Gracias, Curr. Opin. Chem. Eng., 2013, 2, 112–119 CrossRef; (c) A. Cangialosi, C. Yoon, J. Liu, Q. Huang, J. Guo, T. D. Guyen, D. H. Gracias and R. Schulman, Science, 2017, 357, 1126–1130 CrossRef CAS PubMed; (d) F. Ilievski, A. D. Mazzeo, R. F. Shepherd, X. Chen and G. M. Whitesides, Angew. Chem., Int. Ed., 2011, 50, 1890–1895 CrossRef CAS PubMed; (e) J. F. Xu, Y. Z. Chen, D. Wu, L. Z. Wu, C. H. Tung and Q. Z. Yang, Angew. Chem., Int. Ed., 2013, 52, 9738–9742 CrossRef CAS PubMed; (f) Y. Wang, J.-F. Xu, Y. Z. Chen, L. Y. Niu, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chem. Commun., 2014, 50, 7001–7003 RSC; (g) S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905 CrossRef CAS PubMed; (h) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed.
- (a) S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed; (b) D. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 532–546 RSC; (c) R. Klajn, J. F. Stoddart and B. A. Grzybowski, Chem. Soc. Rev., 2010, 39, 2203–2237 RSC; (d) C. Karavasili and D. G. Fatouros, Drug Discovery Today, 2016, 21, 157–166 CrossRef CAS PubMed; (e) R. Verma, R. R. Adhikary and R. Banerjee, Lab Chip, 2016, 16, 1978–1992 RSC; (f) Y. W. Yang, Y. L. Sun and N. Song, Acc. Chem. Res., 2014, 47, 1950–1960 CrossRef CAS PubMed; (g) L. Chen, H. Bai, J.-F. Xu, S. Wang and X. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 13950–13957 CrossRef CAS PubMed; (h) Y. Jiao, W. L. Li, J. F. Xu, G. Wang, J. Li, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2016, 55, 8933–8937 CrossRef CAS PubMed; (i) K. Liu, Y. Liu, Y. Yao, H. Yuan, S. Wang, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2013, 52, 8285–8289 CrossRef CAS PubMed.
- (a) D. Wang, Y. Jin, X. Zhu and D. Yan, Prog. Polym. Sci., 2017, 64, 114–153 CrossRef CAS; (b) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC; (c) X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971–1981 CrossRef CAS PubMed; (d) J. Niskanen and H. Tenhu, Polym. Chem., 2017, 8, 220–232 RSC; (e) J. Seuring and S. Agarwal, Macromol. Rapid Commun., 2012, 33, 1898–1920 CrossRef CAS PubMed; (f) J. Seuring and S. Agarwal, ACS Macro Lett., 2013, 2, 597–600 CrossRef CAS; (g) Q. Zhang and R. Hoogenboom, Prog. Polym. Sci., 2015, 48, 122–142 CrossRef CAS; (h) D. Roy, W. L. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 RSC.
- (a) E. Karjalainen, V. Aseyev and H. Tenhu, Macromolecules, 2014, 47, 2103–2111 CrossRef CAS; (b) J. Yin, J. Hu, G. Zhang and S. Liu, Langmuir, 2014, 30, 2551–2558 CrossRef CAS PubMed; (c) X. Jia, D. Chen and M. Jiang, Chem. Commun., 2006, 1736–1738 RSC; (d) F. A. Plamper, A. Schmalz, M. Ballauff and A. H. E. Müller, J. Am. Chem. Soc., 2007, 129, 14538–14539 CrossRef CAS PubMed; (e) K. Wu, L. Shi, W. Zhang, Y. An, X. Zhang, Z. Li and X. Zhu, Langmuir, 2006, 22, 1474–1477 CrossRef CAS PubMed.
- (a) O. Kretschmann, S. W. Choi, M. Miyauchi, I. Tomatsu, A. Harada and H. Ritter, Angew. Chem., Int. Ed., 2006, 45, 4361–4365 CrossRef PubMed; (b) O. Kretschmann, C. Steffens and H. Ritter, Angew. Chem., Int. Ed., 2007, 46, 2708–2711 CrossRef CAS PubMed; (c) T. Ogoshi, K. Kida and T. A. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20146–20150 CrossRef CAS PubMed; (d) T. Ogoshi, R. Shiga and T. A. Yamagishi, J. Am. Chem. Soc., 2012, 134, 4577–4580 CrossRef CAS PubMed; (e) S. Amajjahe, S. Choi, M. Munteanu and H. Ritter, Angew. Chem., Int. Ed., 2008, 47, 3435–3437 CrossRef CAS PubMed; (f) X. Ji, J. Chen, X. Chi and F. Huang, ACS Macro Lett., 2014, 3, 110–113 CrossRef CAS.
- S. Amemori, K. Kokado and K. Sada, Angew. Chem., Int. Ed., 2013, 52, 4174–4178 CrossRef CAS PubMed.
- S. Amemori, K. Kokado and K. Sada, J. Am. Chem. Soc., 2012, 134, 8344–8347 CrossRef CAS PubMed.
- A. Das and S. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 1092–1097 CrossRef CAS PubMed.
- Z. Yang, X. Fan, W. Tian, D. Wang, H. Zhang and Y. Bai, Langmuir, 2014, 30, 7319–7326 CrossRef CAS PubMed.
- Y. Liu, B. Han, B. Li, Y. Zhang, P. Zhao, Y. Chen, T. Wada and Y. Inoue, J. Org. Chem., 1998, 63, 1444–1454 CrossRef CAS.
- H. Yao, M. Qi, Y. Liu and W. Tian, Chem. – Eur. J., 2016, 22, 8508–8519 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, characterization and other materials. See DOI: 10.1039/c9qm00141g |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2019 |