Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A cobalt-doped iron oxide nanozyme as a highly active peroxidase for renal tumor catalytic therapy†
Yixuan Wang a,
Hongjun Li*b,
Lihua Guoa,
Qi Jianga and
Feng Liua
a,
Hongjun Li*b,
Lihua Guoa,
Qi Jianga and
Feng Liua
aDepartment of Nephrology, China-Japan Union Hospital of Jilin University, Changchun, 130033, China
bThe Examination Center, China-Japan Union Hospital of Jilin University, Changchun, 130033, China. E-mail: lihongjun1960@126.com
First published on 17th June 2019
Abstract
The Fe3O4 nanozyme, the first reported nanozyme with intrinsic peroxidase-like activity, has been successfully employed for various diagnostic applications. However, only a few studies have been reported on the therapeutic applications of the Fe3O4 nanozyme partly due to its low affinity to the substrate H2O2. Herein, we report a new strategy for improving the peroxidase-like activity and affinity of the Fe3O4 nanozyme to H2O2 to generate reactive oxygen species (ROS) for kidney tumor catalytic therapy. We showed that cobalt-doped Fe3O4 (Co@Fe3O4) nanozymes possessed stronger peroxidase activity and a 100-fold higher affinity to H2O2 than the Fe3O4 nanozymes. The lysosome localization properties of Co@Fe3O4 enable Co@Fe3O4 to catalyze the decomposition of H2O2 at ultralow doses for the generation of ROS bursts to effectively kill human renal tumor cells both in vitro and in vivo. Moreover, our study provides the first evidence that the Co@Fe3O4 nanozyme is a powerful nanozyme for the generation of ROS bursts upon the addition of H2O2 at ultralow doses, presenting a potential novel avenue for tumor nanozyme catalytic therapy.
Introduction
Nanozymes are a class of nanomaterials with intrinsic enzyme-like activities.1–3 Over the last decade, a wide variety of nanomaterials have been reported to possess natural enzyme-like activities.1–5 The biochemical reactions catalyzed by these types of nanozymes exhibit similar enzymatic kinetics as in the case of natural enzymes. Nanozymes exhibit comparable enzymatic activity but with much higher stability and lower cost as compared to natural enzymes. In addition, their activities are tunable, and they can be easily integrated with nanosystems to achieve multifunctionality;6,7 therefore, nanozymes possess significant potential for a wide range of applications in biomedicine such as in immunoassays, biosensors, and antibacterial and antibiofilm agents.4,8,9As a classical magnetic nanomaterial, iron oxide (Fe3O4) nanoparticles are the first reported nanozyme with intrinsic peroxidase-like activity.10,11 Fe3O4 nanozymes with intrinsic magnetic properties have been extensively used for biological applications including magnetic resonance imaging, magnetic drug delivery, magnetic hyperthermia and magnetic separation.12–14 Based on its newly discovered catalytic properties, the Fe3O4 nanozyme can act as a multifunctional enzyme mimetic for versatile biomedical applications.12
Recently, significant efforts have been made to explore the feasibility of application of nanozymes in in vivo clinical diagnosis and therapy.9,15–18 As the first well-studied nanozyme, Fe3O4 nanozymes have already been evaluated in tumor catalytic therapy for catalyzing the decomposition of hydrogen peroxide to generate ROS.16,19,20 However, because of the low affinity of the Fe3O4 nanozymes to H2O2, Fe3O4 nanozyme-based catalytic therapy typically requires an additional high dose of H2O2 (approximately 10−3 to 10−4 M);19,20 this makes this nanozyme-based catalytic tumor therapy strategy unviable for practical application.
Some heterogeneous oxide nanomaterials, such as ZnFeO321 and NiFeO422, formed by iron and other metals have been reported to exhibit enhanced peroxidase-like behavior; this indicates that transition metal doping of Fe3O4 nanozymes may be an effective way to improve the enzymatic activity of these nanoenzymes;23 interestingly, Chen et al. have reported that Fe–Co bimetallic alloy nanoparticles also exhibit high peroxidase-like activity.24 Moreover, Vetr et al. have investigated the effect of transition metal (Co, Ni, and Zn) doping on the catalytic performance of Fe3O4 nanozymes. They have demonstrated that NiFe2O4 and ZnFe2O4 NPs exhibit lower catalytic activity as compared to CoFe2O4 NPs.25 Thus, doping of cobalt, a non-noble metal, into Fe3O4 nanozymes is a promising method to improve the peroxidase-like activity of Fe3O4 nanozymes; however, all these studies focus on the in vitro biosensing applications of metal-doped Fe3O4 nanozymes, and the applications of these nanozymes in tumor catalytic therapy have not been explored.
In this study, we demonstrated that doping of Co into Fe3O4 nanozymes (Co@Fe3O4) resulted in not only excellent peroxidase-like activity, but also a 100-fold higher affinity of Co@Fe3O4 to H2O2 than that in the case of Fe3O4 nanozymes. By employing Co@Fe3O4 nanozymes, we successfully achieved effective antitumor activity with the addition of an ultralow dose (10 nM) of H2O2 both in vitro and in vivo. This study provides a promising strategy to enhance the peroxidase-like activity of the Fe3O4 nanozyme and achieves the purpose of Fe3O4 nanozyme based-renal tumor catalytic therapy.
Materials and methods
Materials
Chemicals and materials were supplied by Sigma-Aldrich (St. Louis, MO) unless otherwise specified.Synthesis and characterization of the Fe3O4 and Co@Fe3O4 nanozymes
The Fe3O4 nanozymes and Co-doped Fe3O4 nanozymes were synthesized according to the solvothermal method reported in the literature10,26 with some modifications. Briefly, for the Fe3O4 nanozymes, FeCl3·6H2O (0.82 g) was dissolved in 40 mL ethylene glycol. When the solution became clear, NaAc (3.6 g) was added under continuous vigorous stirring for 30 min. The mixture was sonicated for 10 min, then transferred to a 50 mL Teflon-lined stainless-steel autoclave and reacted at 200 °C for 12 h. After the reaction was completed, the autoclave was cooled down to room temperature. Then, the products obtained were washed several times with ethanol and dried at 60 °C.The Co@Fe3O4 nanozymes were also synthesized using the same procedure but extra Co(NO3)3·6H2O (0.82 g) was added to the reaction system.
The morphology and structure of the Fe3O4 and Co@Fe3O4 nanozymes were characterized by transmission electron microscopy (TEM, JEOL JEM-1400 120 kV), scanning electron microscopy (SEM, Zeiss Supra55) and dynamic light scattering (DLS, DynaPro Titan). Energy dispersive X-ray spectroscopy (EDX) of the Fe3O4 and Co@Fe3O4 nanozymes was conducted using the Tecnai G2 F30 instrument. X-ray diffraction (XRD) measurements were performed using the X'Pert pro Philips X-ray powder diffractometer. X-ray photoelectron spectroscopy (XPS) was performed by the ESCALab220i-XL high-performance electron spectrometer with a monochromatic Al Kα source.
Kinetic analysis of the Fe3O4 and Co@Fe3O4 nanozymes
The kinetic parameters of the Fe3O4 and Co@Fe3O4 nanozymes were determined by monitoring the absorbance change at 652 nm using the iMark™ Microplate Reader (Bio-Rad, USA) in the time course mode at room temperature. Kinetic assays were carried out using the Fe3O4 nanozymes (0.2 μg) or Co@Fe3O4 nanozymes (0.2 μg) in a 100 μL of reaction buffer (0.2 M NaAc buffer, pH 4.5) in the presence of H2O2 and TMB. The kinetic analysis of Fe3O4 and Co@Fe3O4 with H2O2 as the substrate was performed by varying the concentrations of H2O2 with 0.8 mM TMB and vice versa. The absorbance (652 nm) changes were calculated relative to the changes in the molar concentration of TMB using the molar absorption coefficient of 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 for the TMB-derived oxidation products according to the Beer–Lambert law.27 All the measurements were performed at least in triplicate, and the values were then averaged. The results are provided as mean ± the standard deviation (SD). The Michaelis–Menten constant was calculated using the Lineweaver–Burk plots of the double reciprocal of the Michaelis–Menten equation ν = Vmax × [S]/(KM + [S]) by GraphPad Prism 6.02 (GraphPad Software), where ν is the initial velocity, Vmax is the maximal reaction velocity, [S] is the concentration of the substrate and KM is the Michaelis–Menten constant.
000 M−1 cm−1 for the TMB-derived oxidation products according to the Beer–Lambert law.27 All the measurements were performed at least in triplicate, and the values were then averaged. The results are provided as mean ± the standard deviation (SD). The Michaelis–Menten constant was calculated using the Lineweaver–Burk plots of the double reciprocal of the Michaelis–Menten equation ν = Vmax × [S]/(KM + [S]) by GraphPad Prism 6.02 (GraphPad Software), where ν is the initial velocity, Vmax is the maximal reaction velocity, [S] is the concentration of the substrate and KM is the Michaelis–Menten constant.
ESR spectroscopy measurements
The ESR measurements were carried out using a Bruker electron spin resonance (ESR) spectrometer (A300-10/12, Germany) at ambient temperature. Herein, fifty microliter aliquots of the control or sample solutions were put in glass capillary tubes with the internal diameters of 1 mm and sealed. The capillary tubes were then inserted into the ESR cavity, and the spectra were obtained at selected times. The instrument settings are as follows: 1 G field modulation, 100 G scan range, and a 20 mW microwave power for the detection of spin adducts using spin traps. The spin trap BMPO was employed to verify the formation of hydroxyl radicals (OH˙) during the degradation of H2O2 in the presence of the Fe3O4 or Co@Fe3O4 nanozymes under the same conditions. The amount of hydroxyl radicals was quantitatively estimated by the ESR signal intensity of the hydroxyl radical spin adduct (BMPO/OH˙) using the peak-to-peak height of the second line of the ESR spectrum.Cell viability assay
The cytotoxicity of the Fe3O4 and Co@Fe3O4 nanozymes with the addition of 10 nM H2O2 was determined using the CCK-8 cell viability assay kit (Dojindo Molecular Technologies). Briefly, A-498 cells (Human renal cancer cell, ATCC, HTB-44) were plated in 96-well plates (BD Biosciences) with the density of 5 × 103 cells per well and cultured in 100 μL EMEM (Catalog No. 30-2003) for 1 day before the addition of Fe3O4,Co@Fe3O4 nanozymes, or only the buffer as a control. On each plate, blank wells (n = 6) with media were defined as 0% viability. Moreover, the wells with only PBS-treated cells (n = 6) were defined as 100% viability. The dilutions of the Fe3O4 and Co@Fe3O4 nanozymes were prepared using a buffer containing 10 nM H2O2. The cells were then exposed to the Fe3O4 or Co@Fe3O4 nanozymes at a series of concentrations (from 0 to 0.2 mg mL−1) for 24 hours. After stimulation, a 10 μL CCK-8 solution was added to each well. The plates were then incubated for 4 h at 37 °C. After this, the absorbance was determined at 450 nm using the Benchmark Plus microplate spectrophotometer (Bio-Rad Laboratories, Inc.). The results presented herein are the average of those obtained via three independent experiments.Localization of the Fe3O4 and Co@Fe3O4 nanozymes in cytoplasm
The cellular uptake and distribution of Fe3O4 or Co@Fe3O4 nanozymes in human renal tumor cells were investigated by a confocal laser scanning microscope. Briefly, the A-498 cells were plated on poly-L-lysine-treated coverslips (BD Biosciences) and cultured in a six-well plate (Corning) for 12 h before use. After stimulation for 48 h with the Alexa-488-labeled Fe3O4 or Co@Fe3O4 nanozymes (0.2 mg mL−1), the cells were washed with PBS, fixed in 4% cold formaldehyde in PBS for 5 min, and then permeabilized with 0.1% Triton X-100. After being washed with PBS, the cells were blocked in a 5% normal goat serum for 30 min at room temperature. To visualize the lysosomes, the cells were incubated with anti-Lamp1 mAb (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, clone H4A3; Invitrogen) at 37 °C for 1 h. The cells were then washed three times with PBS and incubated with goat anti-mouse IgG1 conjugated with Alexa-555 (1
200, clone H4A3; Invitrogen) at 37 °C for 1 h. The cells were then washed three times with PBS and incubated with goat anti-mouse IgG1 conjugated with Alexa-555 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500; Invitrogen) for 1 h at 37 °C. Finally, the nuclei of the cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI, 1 μg mL−1, Roche Applied Science) for 10 min at room temperature. The samples were examined using a confocal laser scanning microscope (Olympus FluoView FV-1000, Tokyo, Japan).
500; Invitrogen) for 1 h at 37 °C. Finally, the nuclei of the cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI, 1 μg mL−1, Roche Applied Science) for 10 min at room temperature. The samples were examined using a confocal laser scanning microscope (Olympus FluoView FV-1000, Tokyo, Japan).
Intracellular ROS assay
The fluorescent probe 2′,7′-dichlorofluorescin diacetate (H2DCFDA, Sigma-Aldrich, D6883) was used to measure the intracellular generation of ROS by the Fe3O4 or Co@Fe3O4 nanozymes. Briefly, the confluent A-498 cells on the coverslips (BD Biosciences) were incubated with Fe3O4 or Co@Fe3O4 nanozymes (0.2 mg mL−1) for 4 hours. After being washed with PBS, the cells were incubated with 10 μM H2DCFDA in a serum-free DMEM for 20 min at 37 °C in the dark. The fluorescence intensities of H2DCFDA were measured by a confocal laser scanning microscope (Olympus FluoView FV-1000, Tokyo, Japan).Apoptosis analysis
The apoptosis analysis of the treated tumor cells was conducted by PI and annexin V staining and flow cytometry (FACSCaliburTM, Becton Dickinson, Franklin Lakes, NJ, USA). Briefly, the Fe3O4 and Co@Fe3O4 (0.2 mg mL−1) nanozymes were incubated with the A-498 tumor cell lines for 24 h. After trypsinization, the treated A-498 tumor cells were incubated with annexin V and PI for 15 min to achieve nuclear staining. After this, the cells were fixed and incubated with streptavidin-fluorescein (5 μg mL−1) (Sigma, USA) for 15 min. Cell death was evaluated by the quantification of annexin-stained apoptotic cells and PI-stained necrotic cells using flow cytometry.Therapy studies
Herein, eighteen female BALB/c nude mice bearing A-498 tumors were randomly assigned to four groups (n = 6 mice per group). All the mice were intratumorally treated with a single dose of Fe3O4 and Co@Fe3O4 nanozymes (3 mg mL−1, 100 μL) with 10 nM H2O2 when the diameter of the tumors was about 100 mm3. For the controls, PBS was administered. The tumor size was measured 3 times a week. The tumor size was calculated as volume [mm3] = length × width2 × π/6. The measured values are presented as mean ± SD.Results
Characterization of the Co@Fe3O4 nanozymes
The Fe3O4 nanozymes and Co-doped Fe3O4 nanozymes (Co@Fe3O4) used in this study were synthesized by the solvothermal method. To study the composition of the as-prepared nanozymes, the EDX analysis was performed. As shown in Fig. S1,† the EDX spectrum of the Co@Fe3O4 nanozymes indicated that the Fe and Co elements were present in the nanoparticles. Based on the EDX mapping analysis, the content of Fe and Co in the Co@Fe3O4 nanozymes were determined as 33.48% and 16.23%, respectively (Table S1†). In conclusion, herein, the synthesized Co@Fe3O4 nanozymes contained Fe and Co with the ratio of approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; this confirmed that Co was successfully doped into the Fe3O4 nanozymes by the simple solvothermal method.
1; this confirmed that Co was successfully doped into the Fe3O4 nanozymes by the simple solvothermal method.
To characterize the structure of the Co@Fe3O4 nanozymes, TEM, SEM, DLS and X-ray diffraction (XRD) analysis were performed. The TEM images of the as-prepared Fe3O4 and Co@Fe3O4 nanozymes are shown in Fig. 1A and B, respectively. The SEM images of the Fe3O4 and Co@Fe3O4 nanozymes are presented in Fig. S2A and B,† respectively. The results indicate that the Fe3O4 and Co@Fe3O4 nanozymes present a typical spherical morphology. The average size of the Fe3O4 nanozymes was determined to be 89.8 ± 7.9 nm by the TEM images, whereas that of the Co@Fe3O4 nanozymes was determined to be 94.6 ± 8.6 nm. Moreover, the Fe3O4 and Co@Fe3O4 nanozymes exhibited the average size of 90.31 ± 0.62 nm and 95.82 ± 3.57 nm in solution (Fig. S2C and D†), respectively. The XRD patterns of the as-prepared nanozymes are shown in Fig. 1C and D, which indicate that both the Fe3O4 and Co@Fe3O4 nanozymes are well crystallized. Moreover, each characteristic diffraction peak of the Co@Fe3O4 nanozymes was similar to that of the Fe3O4 nanozymes and the standard PDF card of Fe3O4 (JCPDS card no. 19-0629); this indicated that Co-doping of the Fe3O4 nanozymes did not affect the phase pattern of Fe3O4.
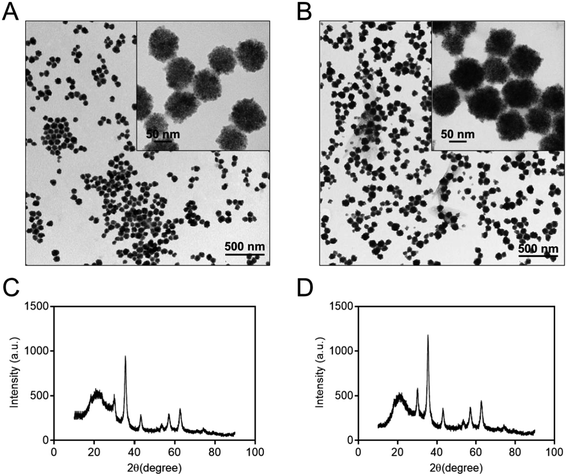 | ||
| Fig. 1 TEM images and XRD diffraction patterns of the Fe3O4 (A and C) and Co@Fe3O4 nanozymes (B and D), respectively. | ||
To characterize the oxidation state of cobalt in the Co@Fe3O4 nanozyme, we further performed XPS analysis of the as-prepared Co@Fe3O4 nanozyme. The high-resolution XPS spectrum of Co 2p is shown in Fig. 2A. The Co 2p XPS peak at 780.8 eV was assigned to Co (2p3/2), with a shake-up satellite peak at 785.9 eV. In addition, the Co 2p XPS peak at 797.2 eV was attributed to Co (2p1/2), with a satellite peak at 803.0 eV.28 These characteristic and satellites peaks confirm that Co2+ is present in the Co@Fe3O4 nanozyme. Moreover, as shown in Fig. 2B, the Fe 2p XPS spectrum exhibited characteristic peaks with the binding energy values at 711.0 and 724.0 eV, assigned to the Fe (2p3/2) and Fe (2p1/2) peaks,29 respectively. Since the atomic radius of iron (140 pm) is similar to that of the cobalt atom (135 pm), these results suggest that the cobalt atoms are probably located only at the lattice positions of the Fe3O4 crystal structure.
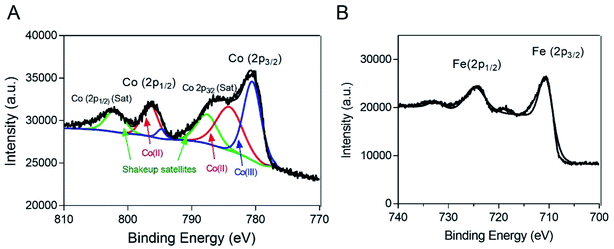 | ||
| Fig. 2 XPS spectra of the Co@Fe3O4 nanozyme. (A) The Co 2p XPS spectrum of the Co@Fe3O4 nanozyme. (B) The Fe 2p XPS spectrum of the Co@Fe3O4 nanozyme. | ||
Peroxidase-like activity and steady-state kinetic assay of the Co@Fe3O4 nanozymes
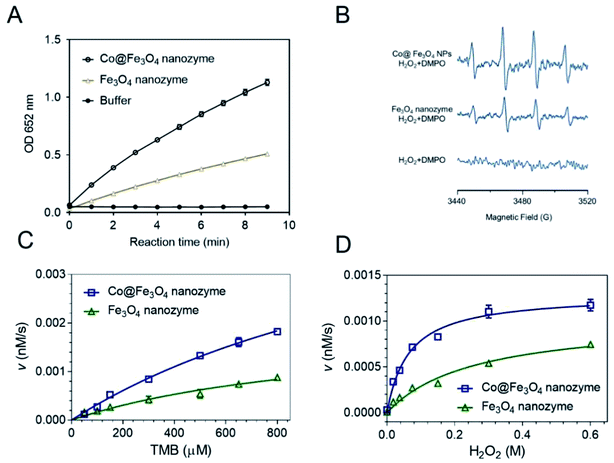
To directly compare the peroxidase-like activity of the Fe3O4 and Co@Fe3O4 nanozymes, we performed typical catalytic experiments using the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB) and H2O2 as previously reported.11 The results showed that both the Fe3O4 and Co@Fe3O4 nanozymes catalyzed the oxidation of TMB with H2O2 to produce blue color products with absorption at 652 nm (Fig. 3A). Moreover, the results demonstrated that the Co@Fe3O4 nanozymes exhibited a significant improvement in the peroxidase-like activity as compared to the Fe3O4 nanozymes; this indicated that a significant improvement in the nanozyme activity was achieved by Co doping of the Fe3O4 nanozymes.The mechanism of action of the Co@Fe3O4 nanozymes was investigated using the ESR method. As shown in Fig. 3B, similar to the previously reported Fe3O4 nanozymes, the Co@Fe3O4 nanozymes significantly enhanced the generation of hydroxyl radicals under acidic conditions. Importantly, the Co@Fe3O4 nanozymes generated more hydroxyl radicals than the Fe3O4 nanozymes under the same conditions; this further confirmed that Co doping significantly improved the peroxidase-like activity of the Fe3O4 nanozymes.
To obtain the apparent kinetic parameters of the Co@Fe3O4 nanozymes, the Michaelis–Menten experiments were performed. Fig. 3C and D show the typical kinetics for TMB and H2O2, respectively. The apparent Michaelis–Menten constant (KM) and the maximum initial reaction rate (Vmax) of the Co@Fe3O4 and Fe3O4 nanozymes were calculated. Moreover, these kinetic parameters of the Co@Fe3O4 nanozymes were compared with those of the Fe3O4 and Co3O4 nanozymes and the natural enzyme HRP (Table 1). The Fe3O4 nanozymes typically exhibited low affinity to H2O2. The KM value to H2O2 for the Co@Fe3O4 nanozymes was much lower than that for the Fe3O4 and Co3O4 nanozymes; this indicated that there was a significant improvement in the affinity of the nanozymes towards substrates after Co doping. More importantly, the KM value to H2O2 for Co@Fe3O4 was nearly 50-fold and 100-fold lower than that of the HRP enzyme and the Fe3O4 nanozymes, respectively; this demonstrated that the Co@Fe3O4 nanozymes exhibited much higher affinity to H2O2 than HRP and the other nanozymes. The Vmax values to H2O2 for the Co@Fe3O4 nanozymes were also significantly improved.
Anti-tumor activities and mechanistic study of the Co@Fe3O4 nanozymes
Tumor cells typically possess higher levels of endogenous H2O2 and reactive oxygen species (ROS) than normal cells.9,20 The balance of the ROS determines the fate of the tumor cells. It has been previously shown that stimulation of ROS is a common strategy for cancer chemotherapy.30,31 Thus, we employed the Co@Fe3O4 nanozymes to trigger the burst of ROS to kill the tumor cells.Fe3O4 nanozymes, as the first well-studied nanozyme, have already been evaluated in tumor catalytic therapy for catalyzing the decomposition of hydrogen peroxide to generate ROS.19,20 However, because of the low affinity of these nanozymes to H2O2, the Fe3O4 nanozyme-based catalytic therapy typically requires additional high doses of H2O2 (approximately 10−3 to 10−4 M);19,20 this makes this nanozyme-based catalytic tumor therapy strategy unfeasible for practical application. In this study, we demonstrated that the Co@Fe3O4 nanozymes exhibited a 100-fold higher affinity to H2O2 than the Fe3O4 nanozymes. Therefore, we next evaluated the catalytic antitumor activity of the Co@Fe3O4 nanozymes with ultra-low doses of H2O2.
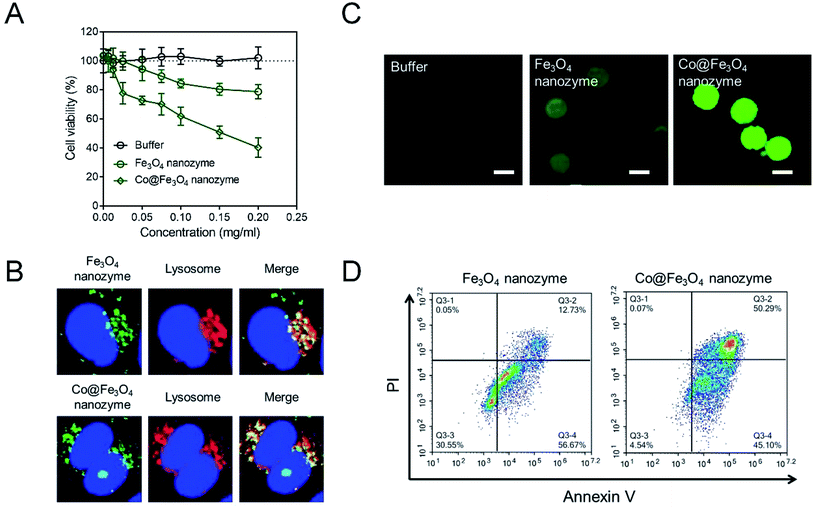
Considering that the typically used concentration of H2O2 is around 10−3 to 10−4 M, we have tried to use 10 nM (10−8 M) H2O2 to evaluate the antitumor activities of the Co@Fe3O4 nanozymes. As shown in Fig. 4A, the buffer group containing 10 nM H2O2 exhibited no significant toxicity to kidney cancer cells; this indicated that the tumor cells were able to survive at 10 nM H2O2. After incubation with 0.2 mg mL−1 Fe3O4 nanozymes and 10 nM H2O2 for 24 hours, only less than 20% tumor cells were killed. These results are consistent with the previously reported results. Only a high dose of H2O2 allows the Fe3O4 nanozymes to effectively kill tumor cells. In the case of the Co@Fe3O4 nanozymes, 0.02 mg mL−1 Co@Fe3O4 nanozymes with 10 nM H2O2 achieved similar antitumor activities as 0.2 mg mL−1 Fe3O4 nanozymes. Moreover, 0.2 mg mL−1 Co@Fe3O4 nanozymes and 10 nM H2O2 killed more than 60% of the tumor cells within 24 hours. Thus, the Co@Fe3O4 nanozymes effectively killed tumor cells with the addition of H2O2 at ultralow doses.
As is well-known, the Fe3O4 nanozymes exhibit peroxidase-like activity only under acidic conditions.12 Since the Co@Fe3O4 nanozymes exhibit significant antitumor activity, we infer that the Co@Fe3O4 nanozymes localize in the lysosome (pH 4–5) after incubation with the tumor cells. To verify this hypothesis, we labeled the nanozymes with Alexa Fluor 488 to track their intracellular localization. As shown in Fig. 4B, we found that after incubation with tumor cells for 4 hours, most of the internalized Fe3O4 nanozymes co-localized with lysosomes. Similar to the Fe3O4 nanozymes, nearly all of the internalized Co@Fe3O4 nanozymes localized in the lysosomes, the highly acidic microenvironment of which would favor the peroxidase-like activities. Thus, the co-localization analysis of the nanozymes and lysosomes demonstrated that the nanozyme-based tumor catalytic therapy strategy is feasible.
In our hypothesis, the antitumor activities of the Co@Fe3O4 nanozymes are attributed to the catalytic generation of ROS by the decomposition of hydrogen peroxide, resulting in oxidative stress in the tumor cells. To verify this hypothesis, the intracellular ROS levels in the tumor cells were detected by employing 2′,7′-dichlorofluorescein diacetate (H2DCFDA), a typical ROS fluorescent dye. As shown in Fig. 4C, the tumor cells treated with only 10 nM H2O2 exhibited no significant ROS signal. After incubation with the Fe3O4 nanozymes and 10 nM H2O2, the green fluorescence intensity increased. In contrast, the tumor cells treated with the Co@Fe3O4 nanozymes and 10 nM H2O2 presented strong green fluorescence intensity, indicating that the Co@Fe3O4 nanozymes catalyzed the decomposition of H2O2 to generate an ROS burst to cause cell apoptosis. As shown in Fig. 4D, the tumor cells treated with the Co@Fe3O4 nanozymes and 10 nM H2O2 exhibited a significant apoptosis pattern. When the tumor cells were stimulated with the nanozymes at same concentration, the apoptosis induced by the Co@Fe3O4 nanozymes in the tumor cells was 4-fold higher than that of the Fe3O4 nanozymes.
To further evaluate the antitumor activity of the Co@Fe3O4 nanozymes in vivo, we employed the human renal cancer cell A-498 xenograft in nude mice as a tumor model. The Fe3O4 nanozymes and Co@Fe3O4 nanozymes were intratumorally injected at the dose of 0.3 mg in 100 μL PBS and 10 nM H2O2 when the tumor volume reached 100 mm3. After this, the tumor volumes were determined 3 times a week. As shown in Fig. 5, the Co@Fe3O4 nanozyme-treated mice exhibited significant tumor inhibition after Co@Fe3O4 administration, whereas the Fe3O4 nanozyme-treated mice exhibited only slight tumor inhibition when compared with the PBS-treated mice. Thus, the Co@Fe3O4 nanozymes exhibited excellent in vivo renal tumor catalytic therapy activity, whereas the Fe3O4 nanozymes only partially inhibited the renal tumor growth due to their relative low peroxidase activity and low binding affinity to H2O2;11 this was consistent with previous studies.9
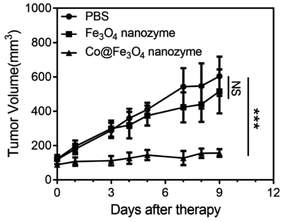 | ||
| Fig. 5 Antitumor activities of the Fe3O4 and Co@Fe3O4 nanozymes in vivo. n = 6, ***p < 0.001, NS, no significance, unpaired Student's t test on day 9. | ||
Overall, these results provide strong evidence that the Co@Fe3O4 nanozymes possess the ability to regulate intracellular ROS upon the addition of H2O2 at ultralow concentrations. Once located in the acidic microenvironment of lysosomes, these nanozymes induce cell death by boosting the level of ROS. The Co@Fe3O4 nanozymes exhibited significant antitumor activities against human renal tumor both in vitro and in vivo.
Discussion and conclusion
ROS-induced apoptosis is a popular strategy for cancer therapy.32–34 The tumor therapy strategies utilizing nanozymes mainly act by stimulating the production of ROS.9 The Fe3O4 nanozymes can simulate peroxidase and thereby efficiently catalyze the decomposition of H2O2 to generate ROS to inhibit tumors in vivo. However, the low binding affinity of the Fe3O4 nanozyme to H2O2 and its relatively low catalytic activity limit the development of the Fe3O4 nanozyme-based tumor catalytic therapy.Transition metal doping has been demonstrated to be an effective and easy way to improve the peroxidase-like activity of Fe3O4 nanozymes.23 Among the transition metals, cobalt, a non-noble metal, has been proven to be a promising dopant to enhance the enzymatic activity of the Fe3O4 nanozyme.25 Importantly, Chen et al. have systematically studied the effects of doping Fe/Co at different ratios on the enzymatic activity of the Fe3O4 nanozyme. They have demonstrated that when the ratio of Fe/Co is around 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the peroxidase-like activity of the Co-doped Fe3O4 nanozyme is the best enzymatic activity.24 In this study, by employing a simple solvothermal method, we fabricated the Co@Fe3O4 nanozyme with the ratio of Fe/Co around 2
1, the peroxidase-like activity of the Co-doped Fe3O4 nanozyme is the best enzymatic activity.24 In this study, by employing a simple solvothermal method, we fabricated the Co@Fe3O4 nanozyme with the ratio of Fe/Co around 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Compared with the case of other strategies, including metal doping, biomimetic coating, and C-dot modification methods, that significantly improved the peroxidase-like activity of the Fe3O4 nanozyme, our Co@Fe3O4 nanozyme exhibited the best binding affinity to H2O2 (Table S2†).
1. Compared with the case of other strategies, including metal doping, biomimetic coating, and C-dot modification methods, that significantly improved the peroxidase-like activity of the Fe3O4 nanozyme, our Co@Fe3O4 nanozyme exhibited the best binding affinity to H2O2 (Table S2†).
The XPS and EDX analysis of the Co@Fe3O4 nanozyme demonstrated that the cobalt atoms were probably located only at the lattice positions of the Fe3O4 crystal structure. Although the Co atom possesses a similar size as the Fe atom, the Co atoms doped into the Fe3O4 crystal may still slightly change the surface physical environment,35 resulting in an improved binding affinity of the nanozyme to H2O2. In addition, the Co dopant may produce more catalytically active sites and substrate-binding sites on the surface of the Co@Fe3O4 nanozyme when compared with the case of the Fe3O4 nanozyme.36 Moreover, the higher redox potential of Co3+/Co2+ (1.30 V) as compared to that of Fe3+/Fe2+ (0.771 V) in the Fe3O4 nanozyme may be another reason for the improvement in the peroxidase-like activities of Co@Fe3O4.37,38
In conclusion, using a simple solvothermal method, we successfully synthesized Co-doped Fe3O4 (Co@Fe3O4) nanozymes that contained Fe and Co at the ratio of approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The well-crystallized Co@Fe3O4 nanozymes exhibited excellent peroxidase-like activity. More importantly, Co doping makes the Co@Fe3O4 nanozymes exhibit a 50-fold and 100-fold higher affinity to H2O2 than that of the HRP and Fe3O4 nanozymes, respectively. The improvement of the H2O2 affinity renders the Co@Fe3O4 nanozymes with excellent antitumor activity upon the addition of H2O2 at ultralow concentrations. When the Co@Fe3O4 nanozymes with enhanced peroxidase-like activities are specifically located in the acidic microenvironment of the lysosomes, they induce apoptosis of human renal tumor cells (A-498) by catalyzing the decomposition of H2O2 to generate an ROS burst. Importantly, the Co@Fe3O4 nanozymes exhibited excellent antitumor activities both in vitro and in vivo for kidney tumor catalytic therapy.
1. The well-crystallized Co@Fe3O4 nanozymes exhibited excellent peroxidase-like activity. More importantly, Co doping makes the Co@Fe3O4 nanozymes exhibit a 50-fold and 100-fold higher affinity to H2O2 than that of the HRP and Fe3O4 nanozymes, respectively. The improvement of the H2O2 affinity renders the Co@Fe3O4 nanozymes with excellent antitumor activity upon the addition of H2O2 at ultralow concentrations. When the Co@Fe3O4 nanozymes with enhanced peroxidase-like activities are specifically located in the acidic microenvironment of the lysosomes, they induce apoptosis of human renal tumor cells (A-498) by catalyzing the decomposition of H2O2 to generate an ROS burst. Importantly, the Co@Fe3O4 nanozymes exhibited excellent antitumor activities both in vitro and in vivo for kidney tumor catalytic therapy.
Conflicts of interest
There are no conflicts to declare.References
- H. Wei and E. K. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- Y. H. Lin, J. S. Ren and X. G. Qu, Acc. Chem. Res., 2014, 47, 1097–1105 CrossRef CAS PubMed.
- R. Ragg, M. N. Tahir and W. Tremel, Eur. J. Inorg. Chem., 2016, 1906–1915, DOI:10.1002/ejic.201501237.
- X. Y. Wang, Y. H. Hu and H. Wei, Inorg. Chem. Front., 2016, 3, 41–60 RSC.
- H. Y. Shin, T. J. Park and M. I. Kim, J. Nanomater., 2015, 756278 Search PubMed.
- Z. Zhang, X. Zhang, B. Liu and J. Liu, J. Am. Chem. Soc., 2017, 139, 5412–5419 CrossRef CAS PubMed.
- B. W. Liu and J. W. Liu, Nano Res., 2017, 10, 1125–1148 CrossRef CAS.
- K. F. Xiangqin Meng, Prog. Biochem. Biophys., 2018, 45, 218–236 Search PubMed.
- K. Fan, J. Xi, L. Fan, P. Wang, C. Zhu, Y. Tang, X. Xu, M. Liang, B. Jiang, X. Yan and L. Gao, Nat. Commun., 2018, 9, 1440 CrossRef PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- K. Fan, H. Wang, J. Xi, Q. Liu, X. Meng, D. Duan, L. Gao and X. Yan, Chem. Commun., 2017, 53, 424–427 RSC.
- L. Gao, K. Fan and X. Yan, Theranostics, 2017, 7, 3207–3227 CrossRef CAS PubMed.
- J. Xie, G. Liu, H. S. Eden, H. Ai and X. Chen, Acc. Chem. Res., 2011, 44, 883–892 CrossRef CAS PubMed.
- K. Ulbrich, K. Hola, V. Subr, A. Bakandritsos, J. Tucek and R. Zboril, Chem. Rev., 2016, 116, 5338–5431 CrossRef CAS PubMed.
- L. L. Dugan, L. L. Tian, K. L. Quick, J. I. Hardt, M. Karimi, C. Brown, S. Loftin, H. Flores, S. M. Moerlein, J. Polich, S. D. Tabbal, J. W. Mink and J. S. Perlmutter, Ann. Neurol., 2014, 76, 393–402 CrossRef CAS PubMed.
- M. Huo, L. Wang, Y. Chen and J. Shi, Nat. Commun., 2017, 8, 357 CrossRef PubMed.
- J. Yao, Y. Cheng, M. Zhou, S. Zhao, S. Lin, X. Wang, J. Wu, S. Li and H. Wei, Chem. Sci., 2018, 9, 2927–2933 RSC.
- Y. Zhang, F. Wang, C. Liu, Z. Wang, L. Kang, Y. Huang, K. Dong, J. Ren and X. Qu, ACS Nano, 2018, 112(1), 651–661 CrossRef PubMed.
- D. Zhang, Y. X. Zhao, Y. J. Gao, F. P. Gao, Y. S. Fan, X. J. Li, Z. Y. Duan and H. Wang, J. Mater. Chem. B, 2013, 1, 5100–5107 RSC.
- S. Fu, S. Wang, X. Zhang, A. Qi, Z. Liu, X. Yu, C. Chen and L. Li, Colloids Surf., B, 2017, 154, 239–245 CrossRef CAS PubMed.
- L. Su, J. Feng, X. Zhou, C. Ren, H. Li and X. Chen, Anal. Chem., 2012, 84, 5753–5758 CrossRef CAS PubMed.
- L. Su, W. Qin, H. Zhang, Z. U. Rahman, C. Ren, S. Ma and X. Chen, Biosens. Bioelectron., 2015, 63, 384–391 CrossRef CAS PubMed.
- N. Chaibakhsh and Z. Moradi-Shoeili, Mater. Sci. Eng., C, 2019, 99, 1424–1447 CrossRef CAS PubMed.
- Y. Chen, H. Cao, W. Shi, H. Liu and Y. Huang, Chem. Commun., 2013, 49, 5013–5015 RSC.
- F. Vetr, Z. Moradi-Shoeili and S. Özkar, Appl. Organomet. Chem., 2018, 32, e4465 CrossRef.
- L. Z. Gao, J. M. Wu, S. Lyle, K. Zehr, L. L. Cao and D. Gao, J. Phys. Chem. C, 2008, 112, 17357–17361 CrossRef CAS.
- W. B. Shi, Q. L. Wang, Y. J. Long, Z. L. Cheng, S. H. Chen, H. Z. Zheng and Y. M. Huang, Chem. Commun., 2011, 47, 6695–6697 RSC.
- X. Niu, Y. Xu, Y. Dong, L. Qi, S. Qi, H. Chen and X. Chen, J. Alloys Compd., 2014, 587, 74–81 CrossRef CAS.
- W. Wu, Q. He, H. Chen, J. Tang and L. Nie, Nanotechnology, 2007, 18, 145609 CrossRef.
- L. Y. Tong, C. C. Chuang, S. Y. Wu and L. Zuo, Cancer Lett., 2015, 367, 18–25 CrossRef CAS PubMed.
- G. Y. Liou and P. Storz, Free Radical Res., 2010, 44, 479–496 CrossRef CAS PubMed.
- D. Trachootham, J. Alexandre and P. Huang, Nat. Rev. Drug Discovery, 2009, 8, 579–591 CrossRef CAS PubMed.
- T. I. Lakshmi Raj, A. U. Gurkar, A. Mandinova, S. L. Schreiber and S. W. Lee, Nature, 2011, 475, 231–234 CrossRef PubMed.
- J. S. Zijian Zhou, L. Nie and X. Chen, Chem. Soc. Rev., 2016, 45, 6597–6626 RSC.
- R. Gargallo-Caballero, L. Martin-Garcia, A. Quesada, C. Granados-Miralles, M. Foerster, L. Aballe, R. Bliem, G. S. Parkinson, P. Blaha, J. F. Marco and J. de la Figuera, J. Chem. Phys., 2016, 144, 094704 CrossRef PubMed.
- H. Sun, Y. Zhou, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2018, 57, 9224–9237 CrossRef CAS PubMed.
- J. Dong, L. Song, J. J. Yin, W. He, Y. Wu, N. Gu and Y. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 1959–1970 CrossRef CAS PubMed.
- B. Jiang, L. Yan, J. Zhang, M. Zhou, G. Shi, X. Tian, K. Fan, C. Hao and X. Yan, ACS Appl. Mater. Interfaces, 2019, 11(10), 9747–9755 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05487h |
| This journal is © The Royal Society of Chemistry 2019 |