 Open Access Article
Open Access ArticleRecent advances on the thermal destabilization of Mg-based hydrogen storage materials
Jianfeng Zhang ,
Zhinian Li*,
Yuanfang Wu,
Xiumei Guo,
Jianhua Ye,
Baolong Yuan,
Shumao Wang and
Lijun Jiang
,
Zhinian Li*,
Yuanfang Wu,
Xiumei Guo,
Jianhua Ye,
Baolong Yuan,
Shumao Wang and
Lijun Jiang
General Research Institute for Nonferrous Metals, 2 Xinjiekou Wai Street, Beijing 100088, China. E-mail: lzngrinm@163.com; Fax: +86-10-60662619; Tel: +86-10-60662633
First published on 2nd January 2019
Abstract
Magnesium hydride and its compounds have a high hydrogen storage capacity and are inexpensive, and thus have been considered as one of the most promising hydrogen storage materials for on-board applications. Nevertheless, Mg/MgH2 systems suffer from great drawbacks in terms of kinetics and thermodynamics for hydrogen uptake/release. Over the past decades, although significant progress has been achieved with respect to hydrogen sorption kinetics in Mg/MgH2 systems, their high thermal stability remains the main drawback, which hinders their practical applications. Accordingly, herein, we present a brief summary of the synthetic routes and a comprehensive overview of the advantages and disadvantages of the promising strategies to effectively tune the thermodynamics of Mg-based materials, such as alloying, nanostructuring, metastable phase formation, changing reaction pathway, and nano Mg-based composites. Among them nanostructuring and metastable phase formation, which have the superiority of changing the thermodynamics without affecting the hydrogen capacity, have attracted increasing interest in this field. To further optimize the hydrogen storage performance, we specially emphasize novel nanostructured materials, which have the advantage of combining alloy engineering, nanostructuring and the synergistic effect to change the thermodynamics of Mg/MgH2 to some extent. Furthermore, the remaining challenges and the directions of further research on MgH2, including the fundamental mechanism of the Mg–H bond instability, advanced synthetic routes, stabilizing nanostructures, and predicting novel composite materials, are proposed.
1. Introduction
Energy is the basic requirement for human productivity and social activities. Recently, the consumption of fossil energy has remarkably increased with the development of the economy. Especially, after the eighteenth century industrial revolution, the consumption of fossil fuels such as coal, oil, and natural gas has increased dramatically in society. However, the problems of environmental pollution and exhaustion of energy resources caused by the over-use of fossil fuels have made it necessary to search for alternative energy carriers. Hydrogen is an energy carrier, which holds tremendous promise as a new clean energy option. Upon its conversion into energy using a fuel cell, only water is produced without any harmful emissions. Therefore, many countries have focused on the development and utilization of hydrogen energy a key research area. The research on good performance and high capacity hydrogen storage materials plays a crucial role in the large-scale applications and the realization of a “hydrogen economy”. Hydrogen storage sectors can be classified into three broad categories, namely, a high-pressured gaseous state, a cryogenic liquid state, and a solid state. However, currently, the storage of hydrogen gas in gas cylinders under high pressure is not safe. Similarly to pressurized hydrogen gas, liquid hydrogen possesses many difficulties related to its very low density and is not economic since a large amount of energy is consumed to cool the gas temperature to 20.2 K. Apart from the gaseous and liquid states for hydrogen storage, solid-state hydrogen storage systems have been considered as the most reliable and safe practical solution for providing clean energy for different applications at a desired temperature with low cost and high hydrogen capacity,1 such as proton-exchange fuel cells membranes (PEM).2In the 1960s, scientists in Holland found that the SmCo5 magnetic alloy can absorb a large amount of hydrogen. Based on this, the LaNi5-type hydrogen storage alloy was developed with a reversible hydrogen storage capacity of 1.4 wt% with a formation enthalpy of −30.2 kJ mol−1 at ambient temperature.3 Around the same period, J. J. Reilly, a researcher at the Brookhaven National Laboratory in the United States, discovered the Mg2Ni hydrogen storage alloy, with a reversible hydrogen storage capacity of 3.6 wt%.4 In metal hydrides, the hydrogen atoms are chemically bonded with metallic elements such as intermetallic hydrides, complex hydrides, and elemental hydrides, where around 36 metallic elements can interrelate with hydrogen atoms. These metal hydrides, such as LaNi5, TiFe, and V-based solid solution, have suitable hydrogen sorption thermodynamics and fast kinetics, which mean that hydrogen sorption can take place at a fast rate and suitable temperatures and hydrogen pressure conditions. Unfortunately, they all have a very low gravimetric hydrogen storage density (Fig. 1)5 and are unsuitable for practical application as energy sources, particularly as on-board automotive hydrogen storage systems according to the US-Department of Energy (DOE) (Table 1).6
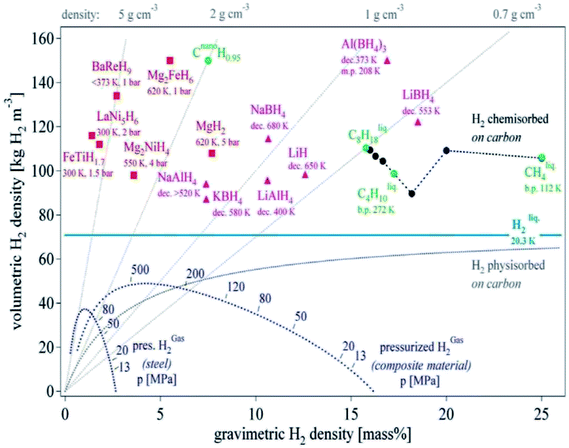 | ||
| Fig. 1 Gravimetric and volumetric hydrogen density of various hydrogen storage materials.5 | ||
| Parameters | Units | Year 2020 | Year 2025 | Ultimate | Related properties of materials |
|---|---|---|---|---|---|
| System gravimetric capacity | kW h kg system | 1.5 | 1.8 | 2.2 | Storage capacity |
| kg H2/kg system | 0.045 | 0.055 | 0.065 | ||
| System volumetric capacity | kW h L system | 1.0 | 1.3 | 1.7 | Storage capacity |
| kg H2/L system | 0.030 | 0.040 | 0.050 | ||
| Min/max delivery temperature | °C | 40/85 | 40/85 | 40/85 | Kinetics, thermodynamics |
| System fill time | Min | 3–5 | 3–5 | 3–5 | Kinetics |
| Operational cycle life (1/4 tank to full) | Cycles | 1500 | 1500 | 1500 | Cycle ability |
| Fuel cost | USD/kW h | 10 | 9 | 8 | Storage capacity |
| Net (USD/kg H2) | (333) | (300) | (266) | Composition |
From Fig. 1, Mg-based hydrides can be considered as an efficient on-board hydrogen storage candidate especially for vehicular application due to their high hydrogen content of 7.6 wt% and volumetric density of about twice that of liquid hydrogen (consistent with the goals of the DOE). http://www.youdao.com/w/eng/destabilized/javascript:void(0); The use of Mg for hydrogen storage purposes also has other potential advantages. Mg is the 8th most abundant element in the Earth's crust (2.3%) and the 3rd most abundant element dissolved in sea water. Thus, Mg-based storage materials have the potential of low cost and are considered to be the most promising hydrogen storage materials for light-duty vehicles. However, a number of issues need to be solved before their practical application, such as high desorption temperature, kinetic limitations of Mg/MgH2, and easy oxidation in oxygen and air. In the past few decades, significant progress has been made in improving the hydrogenation/dehydrogenation kinetics of Mg-based materials by adding a catalyst, multiphase composite, nanocrystalline and other measures. However, the tuning of their thermodynamic properties is still a great challenge. Herein, we present a summary on the recent advances and developments of Mg and Mg-based materials.
2. Magnesium hydride as hydrogen storage medium
The crystal structure of pure magnesium is close-packed hexagonal and it can react with hydrogen at the temperature of 573–673 K and 2.4–40 bar hydrogen pressure, which involves a number of different steps, such as physisorption, dissociation, chemisorption, diffusion of hydrogen into its subsurface sites and bulk lattice sites and finally the nucleation and growth of the hydride phase. Upon the application of hydrogen pressure to Mg, the hydrogen atoms initially occupy the tetrahedral interstitial sites within its lattice, forming a tetragonal α-phase with a hydrogen concentration of up to 0.4 wt%.7 At higher pressures, the nucleation of the β-phase is energetically favorable and the α-phase is converted into hexagonal structure β-MgH2. During this process, the hexagonal close-packed (hcp) lattice of Mg expands by approximately 30% (Table 2), and this phase further transitions to the metastable γ-MgH2 phase under higher compressive stress (70–80 bar).8–10 Recently, researchers discovered that the coexistence of different types MgH2 can produce a catalytic effect during hydrogen release/uptake, for example, γ-MgH2 will make β-MgH2 destabilize to release hydrogen at low temperature. Unfortunately, γ-MgH2 will irreversibly revert to β-MgH2 upon the first hydrogen cycle at 573 K.10| Component phase | Crystal structure | Unit cell parameters (Å) | ΔH (kJ mol−1 H2)/110 surface energy of plane12 (J m2) | ΔH (kJ mol−1 H2)/001 surface energy of plane12 (J m2) |
|---|---|---|---|---|
| Mg | Hexagonal | a = 3.2094, c = 5.2112, V = 46.48 Å3 | ||
| α-MgH2 | Tetragonal | a = 4.5010, c = 3.0100 | ||
| β-MgH2 | Hexagonal | a = 4.5180, c = 3.0205, V = 61.63 Å3 | 78.16/0.19 | 50.75/1.09 |
| γ-MgH2 | Orthorhombic | a = 4.5300, b = 5.4400, c = 4.9300, V = 121.49 Å3 | 44.66/1.23 | 54.41/0.97 |
Magnesium hydride differs to other metal hydrides in its M–H bonds and crystal structure, which is similar to ionic hydrides of alkali and alkaline earth metals. Vajeeston et al. revealed that all polymorphs of MgH2 have a dominant ionic character with Mg and H in nearly 2+ and 1− states, respectively, by means of charge-density, charge-transfer, electron-localization function and Mulliken population analyses.11 The strong chemical bonds formed between hydrogen and metals during chemisorption explain the high storage capacity of Mg and its stability at room temperature. Thereby, the reaction of hydrogen with Mg is exothermic with an enthalpy of reaction of −74.5 kJ (mol−1 H2) and an entropy variation of −135 J (K−1 mol−1 H2), which is much larger than the practical requirements for metal hydrides of 20–40 kJ mol−1 and results in a higher desorption temperature.13 Fig. 2 shows the variation in the plateau pressure as a function of temperature for MgH2. Accordingly, it can be concluded that magnesium cannot absorb hydrogen below a pressure of 10 bar if the temperature is higher than about 640 K. Similarly, magnesium hydride has to be heated to at least 550 K to desorb hydrogen under 1 bar of pressure. Thus, the challenge is to reduce the operation temperature while retaining as much as possible its high hydrogen storage capacity.14
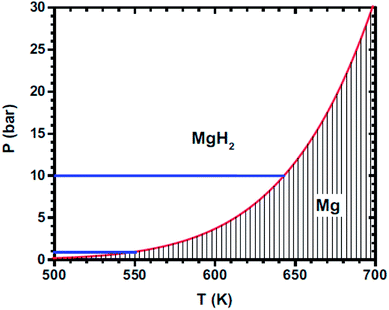 | ||
| Fig. 2 Temperature dependence of the dissociation pressure of MgH2.14 | ||
3. Preparation of MgH2
The reaction of Mg with hydrogen yields a stable hydride as follows (1):| Mg + H2 ↔ MgH2 | (1) |
Thus far, many techniques have been developed to synthesize MgH2 for hydrogen storage, such as mechanical milling,15 thin film technology,16 hydrogen plasma metal reaction,17 hydriding chemical vapor deposition,18 melt spinning,19 severe plastic deformation,20 chemical reduction,21 and electrochemical deposition.22 One of the most commonly used techniques is reactive mechanical milling (RMM), which has been widely used to tune metal microstructures to modify their hydrogenation properties.23 Its advantages as an efficient tool for the synthesis of hydrogen storage materials include the ease of hydrogenation of Mg-based alloys at low temperature, the generation of fresh and highly reactive surfaces, the ease of formation of multi-phase nanocomposites and creation of more defects and micro-strains in the crystal lattice due to mechanical action.24 As shown in Fig. 3, the desorption plateau of the milled sample is much higher and the hysteresis much smaller, indicating very fast desorption kinetics due to the novel nanostructures with a high surface area caused by ball milling (BM).25
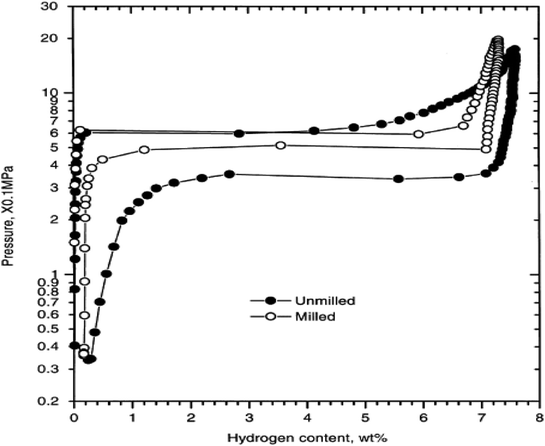 | ||
| Fig. 3 Pressure-composition-temperature (PCT) curves of the unmilled and ball-milled MgH2 at 623 K.25 | ||
Currently, the mechanochemical synthesis of metal hydrides using RMM has become one of the most frequently used methods. Generally, mill machines can be classified into four categories: planetary, rotational, vibratory and attritor mills.15 Considering the final products expected from BM, several milling parameters can be varied in the ball-milling synthesis, including the type of milling bowl and ball, milling speed, milling time, ball-to-powder weight ratio, milling atmosphere, pressure of the selected gas, temperature of milling, and process control agents to inhibit particle agglomeration. Varin et al.26 investigated the effect of ball milling time on the particle and grain/crystallite size and clearly showed that a particle size reduction occurs within a very similar time frame as the reduction in grain size (Fig. 4). Besides Jain et al.23 systematically investigated the role of ball milling in improving the surface and kinetic properties in the preparation of Mg-based alloys. They concluded that the pulverization and deformation processes during high energy ball milling play a major role in the hydriding reaction. Thus far, RMM under hydrogen gas allows the synthesis of binary and ternary metal hydrides (Mg–Fe–H, Mg–Co–H, Mg–Ni–H, Mg–Ti–H), Mg-based complex hydrides and alanates.27 However, the preparation of such small particles by ball milling on a large scale is a major challenge.
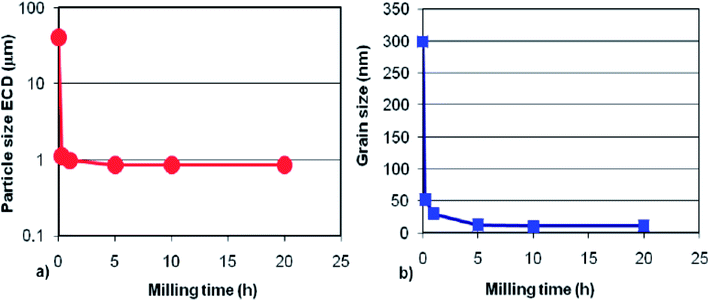 | ||
| Fig. 4 Simultaneous reduction in the (a) particle size and (b) grain size as a function of ball milling time of MgH2.26 | ||
4. Approaches to modify the thermodynamics of Mg/MgH2 systems
As mentioned before, the stable thermodynamics of MgH2 is the major obstacle in its practical application. According to the van't Hoff equation, we can conclude that changing the enthalpy and entropy of the hydrogenation reaction is an efficient way to decrease the desorption temperature of Mg/MgH2 systems. Considering that changing the entropy has little effect on metal hydrogen systems, decreasing the enthalpy change for the reaction is the basic guideline for decreasing the desorption temperature of Mg/MgH2 systems. In this section, we present the strategies for modifying the thermodynamics of MgH2, such as alloying, nanostructuring, metastable phase formation and changing the reaction path.4.1 Alloying
Generally, hydrogen storage alloys consist of a high temperature hydride forming element A and a non-hydride forming element B (Fig. 5).28 If Mg-based alloys are made with a hydride non-forming element and form less stable hydrides to destabilize the hydrogenated state, the hydriding reaction enthalpy can be remarkably decreased. Therefore, alloying is a traditional but effective strategy for altering the thermodynamics of Mg-based alloys.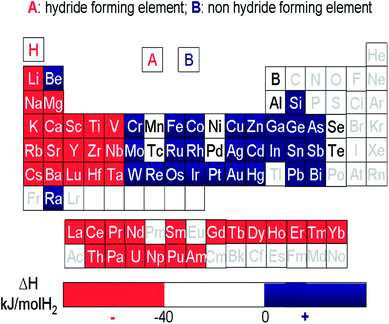 | ||
| Fig. 5 Hydride and non-hydride forming elements in the periodic table of elements.28 | ||
Similarly to the Mg–Ni system, Mg and Cu can form the Mg2Cu alloy. Upon the application of 30 MPa hydrogen pressure to Mg2Cu, it decomposes to MgH2 and MgCu2 according to the following disproportionation reaction (2):
| 2Mg2Cu + H2 → 3MgH2 + MgCu2 | (2) |
The hydride formation enthalpy and entropy of reaction (2) is −73.5 kJ (mol−1 H2) and −146 J (K−1 mol−1 H2), respectively, with a hydrogen capacity of 2.6 wt%,33 and the equilibrium temperature for 1 bar hydrogen pressure is reduced to about 513 K. Unfortunately, this reaction is irreversible. Advanced research has shown that Mg–Cu–H nanoparticle systems can further reduce the hydride formation enthalpy and entropy to −67.5 kJ (mol−1 H2) and −124.4 J (K−1 mol−1 H2), respectively.34 Thus from the above research, we can preliminarily conclude that nanostructuring of Mg-based alloys provides a potential strategy to further alter their thermodynamic properties without loss in their hydrogen capacity.
Normally, Mg and Fe are immiscible, but the ternary hydride Mg2FeH6 can be formed in the presence of hydrogen by mechanical milling. The hydride formation enthalpy and entropy are −77.9 kJ (mol−1 H2) and −140 J (K−1 mol−1 H2), respectively. Furthermore, it has a gravimetric storage capacity of 5.6 wt% and the highest known volumetric hydrogen density, amounting to 150 kg m−3, which is more than double that of liquid hydrogen at 20.2 K and moderate pressures up to 20 bar.35 The theoretical research conducted by Batalović36 demonstrated the influence of transition metal (Ni, Co, and Mn) doping on the hydrogen sorption properties of Mg2FeH6. They found that the largest decrease in desorption enthalpy was in the case of nickel doping, resulting in a 27.7 kJ (mol−1 H) energy change. Similarly to the Mg–Fe system, reactive ball-milling of Mg and Co under hydrogen gas led to the formation of Mg2CoH5 with the gravimetric storage capacity of 4.5 wt%, hydride formation enthalpy of −82 kJ mol−1 H2 and desorption temperature for 1 bar hydrogen pressure of about 513 K.37 It is important to highlight that the formation of Mg2FeH6 consists of two steps that always involve MgH2 as a precursor, which was also confirmed by the in situ hydrogen uptake curve (Fig. 6) monitored during the synthesis of Mg2TM (TM = Fe and Co) hydrides according to reaction (3);38 whereas, a unique absorption step was detected in the case of Ni.39
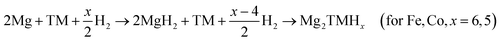 | (3) |
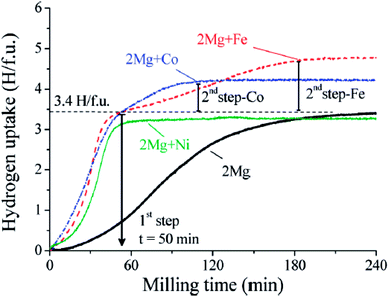 | ||
| Fig. 6 Absorbed hydrogen atoms by formula unit of the formed hydride during RBM of Mg and Mg+2TM mixtures (TM = Ni, Fe, and Co) (The number of absorbed H atoms per unit metal or alloy formula (H/f.u.)).38 | ||
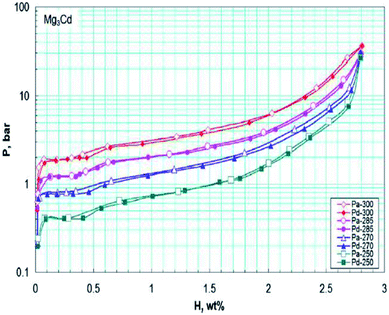 | ||
| Fig. 7 PCT diagrams of the studied Mg3Cd alloy in the temperature range of 250–300 °C. The open and filled symbols correspond to the absorption and desorption branches of the curves, respectively.42 | ||
According to the binary phase diagram of the Mg–In system, it has a high solubility of up to 10 at% indium in a wide temperature range.43 The Mg (In) solid solution absorbs hydrogen to form MgH2 and a disordered Mg–In compound. Table 3 summaries the lattice constants and hydrogen storage properties of Mg (In) solid solutions. The Mg0.95In0.05 solid solution can reversibly react with hydrogen at 573 K according to the following reaction (4):
| Mg95In5 + 0.9H2 ↔ 0.9MgH2 + 0.05MgIn | (4) |
| Solid solution | Lattice constants (nm) | |ΔH| (kJ (mol−1 H2)) | ΔS (J (K−1 mol−1 H2)) | Capacity (wt%) | |
|---|---|---|---|---|---|
| a | c | ||||
| Mg0.9In0.1 | 0.31929 | 0.52061 | 65.2 | 121.8 | 4.2 |
| Mg0.95In0.05 | 0.32027 | 0.52086 | 68.1 | 125.5 | 5.3 |
| Mg0.98In0.02 | 0.32077 | 0.52106 | 69.6 | 126.0 | 6.4 |
| Mg0.75Al0.25 | 0.31990 | 0.51960 | 76.8 | 138.6 | 5.0 |
| Mg0.9In0.05Al0.05 | 0.31980 | 0.51929 | 66.3 | 121.2 | 4.8 |
| Mg90In5Cd5 | 0.31925 | 0.51964 | 86 | 154.8 | 4.3 |
The hydrogen storage capacity of Mg0.95In0.05 is about 5.3 wt%, but the poor kinetics of its desorption/absorption reaction is a key problem.44 Thus, Zhu et al. conducted research on Mg-based solid solutions, including Mg (In, Al), Mg (In, Cd) and Mg (In, Y) ternary alloys. Compared with the binary Mg (Al) solid solution, the Mg (In, Al) ternary solid solution presented improved dehydriding reversibility and a substantial decrease in ΔH because Al was dissolved in the β phase.45
With respect to the Mg–In–Y ternary system, the reversible dissolving of Y in Mg was realized by the decomposition of the In3Y phase in dehydriding, but the reversible solubility of Y is rather low due to the formation of YH2. The Mg90In5Y5 solid solution exhibited a decreased reaction enthalpy of 62.9 kJ (mol−1 H2), which was reduced by 5 kJ (mol−1 H2) and 12 kJ (mol−1 H2) compared to that of the Mg95In5 binary solid solution and pure Mg, respectively.46 Similarly to the Mg–In–Y system, the hydriding reaction of the Mg90In5Cd5 alloy can be described as reaction (5):
| Mg90In5Cd5 + 70H2 ↔ 70MgH2 + 5MgIn + 5Mg3Cd | (5) |
The dehydriding reaction enthalpy ΔH and entropy ΔS for the Mg90In5Cd5 alloy are 86.0 kJ (mol−1 H2) and 154.8 J (K−1 mol−1 H2), respectively. The PCT results showed a reversible hydrogen storage capacity of 4.3 wt% and elevated equilibrium pressure for the MgH2 in the Mg90In5Cd5 alloy compared with pure MgH2 (Fig. 8). The Mg90In5Cd5 alloy also exhibited enhanced hydrogenation kinetics with a decreased hydrogenation activation energy of 61.0 kJ mol−1, but its dehydrogenation rate is slow due to the long-range diffusion of In and Cd in MgH2.47 Mg-based solid solutions can reduce the reaction enthalpy with a higher hydrogen capacity than the Mg–Ni alloy and even can compete with the Mg–Fe alloy in terms of hydrogen capacity, but their sluggish kinetics is still an issue.
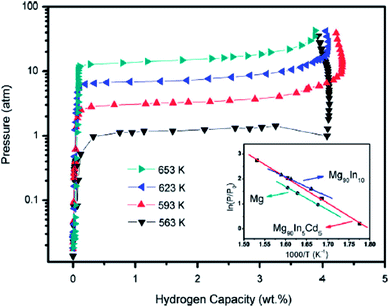 | ||
| Fig. 8 PCT and van't Hoff plot (the inset) for the hydrogen desorption of the Mg90In5Cd5 alloy.47 | ||
Generally, alloying is a feasible way to modify the thermodynamics of Mg-base hydrogen storage alloys. However, there still exists some disadvantages, such as the hydrogen capacity decreases sharply after alloying due to the introduction of heavy metals and most of these Mg-base alloys still suffer from poor reversibility because the bonds between Mg and the other element break during the process of hydrogenation. Besides, Mg–Fe, Mg–Co, and Mg (In, Cd) present a higher ΔH for the dehydrogenation reaction than Mg/MgH2; thus further research should be performed to address their remaining problems.
4.2 Nanostructuring
A major feature that discriminates various types of nanostructures is the dimensionality of their architecture, for example, zero-dimensional (0D; such as nanoparticles (NPs), quantum dots, and hollow spheres), one-dimensional (1D; such as nanowires, nanofibers, nanorods and nanobelts), two-dimensional (2D; such as thin films and nanosheets), and three-dimensional (3D; such as frameworks, nanoflowers, and dendritic structures), as demonstrated in Fig. 9.48 Nanomaterials exhibit numerous advantages for batteries, fuel cells, and hydrogen storage systems. For example, with the decreasing size of a material, its surface-to-volume ratio increases apparently. Moreover, the atoms on the surface may have some dangling bonds, which are extremely active and tend to form bonds with surrounding molecules to reduce the surface energy. Thus, reducing the particle and grain size of Mg-based materials is a promising way to further decrease their hydride formation enthalpy ΔH.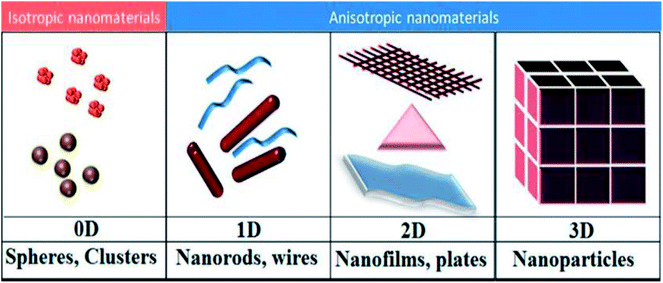 | ||
| Fig. 9 Schematic representation of the various dimensions (0D, 1D, 2D, and 3D) of nanomaterials.48 | ||
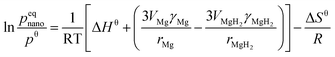 | (6) |
Bulk MgH2 is destabilised when the contribution related to size becomes sufficiently negative, i.e. the surface energy density of MgH2 is significantly larger than that of magnesium, and some of the heat of formation will be stored as excess surface energy, which will reduce the heat released upon hydride formation and hydrogen release. Wagemans et al. predicted a considerable reduction (>10%) in ΔH for radii smaller than 4 nm, while DFT calculations predicted a result below 2 nm. The calculations showed that MgH2 is more destabilized than Mg upon decreasing the cluster size below 19 Mg atoms (Fig. 10). For instance, an Mg9H18 cluster size of 0.9 nm corresponds to a desorption temperature of only 473 K with an enthalpy formation of −63 kJ mol−1 H2.50 Liu et al. successfully synthesized Mg nanoparticles by reducing di-n-butyl magnesium with lithium in the presence of naphthalene as an electron carrier. Further determination of the hydrogen thermodynamics of these materials revealed a significant evolution in both enthalpy and entropy with a decrease in particle sizes (Fig. 11). Hence, a ΔH of 63.5 ± 1.8 kJ (mol−1 H2) and ΔS of 118.4 ± 3.1 J (K−1 mol−1 H2) were measured for MgLi4.6Napth0.5 with an average particle size of 16 nm.51
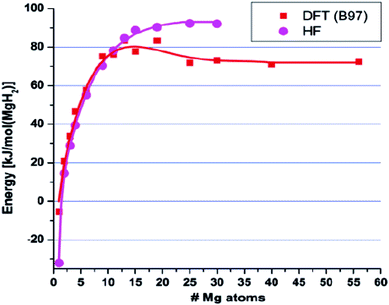 | ||
| Fig. 10 Calculated desorption energies for MgH2 clusters using both the HF method and DFT method (B97 functional). The energies were normalized per mole of H2 released.50 | ||
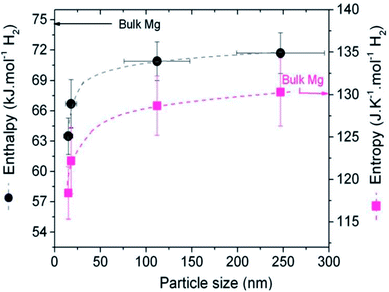 | ||
| Fig. 11 Evolution of ΔH and ΔS versus size of the magnesium nanoparticles. The dotted line is a guide to the eyes.51 | ||
Recently, there have been numerous methods reported for the preparation of MgH2 NPs, including ball milling, hybrid combustion, melt spinning, and chemical vapour deposition (CVD). The nanostructured uniform mixture of MgH2–0.1TiH2 powder is a reversible hydrogen storage material with a cyclic hydrogen storage capacity of 6 wt%, which demonstrated good cycling stability with no loss in capacity over 80 cycles. The ΔH for the dehydrogenation of MgH2–0.1TiH2 is 68 kJ (mol−1 H2), which is lower than that for MgH2 (75 kJ (mol−1 H2)). This can be attributed to two factors, its nanosize and the addition of TiH2.52 Besides, Asano et al.53 demonstrated clearly that embedding Mg nanometer-sized clusters in a matrix of immiscible metals is a powerful strategy to destabilize MgH2, resulting in a lower working temperature for hydrogen storage applications. Carbon-based materials also show huge potential in improving the hydrogen storage performance of MgH2. Several works have been devoted to combining MgH2 with various carbon materials, for example, nanocomposite (Mg–V)nano/G exhibits a reversible hydrogen capacity of 6.03 wt% with an enthalpy of formation of −68.8 ± 2.3 kJ (mol−1 H2)54 and the magnesium particles with a carbon additive were milled to 20–60 nm with enthalpy and entropy changes of 44.5 kJ (mol−1 H2) and 83.8 J (K−1 mol−1 H2), respectively according to Fig. 12.55 Graphite and carbon as a process control agent (PCA) can reduce the agglomeration of the milled particles, and thus allow better particle refinement by stabilizing the surface charges created at the fresh surfaces of the fractured particles. In summary, it has been clearly demonstrated that the use of carbon materials offers many opportunities in tailoring the properties of metal hydrides. Since their porosity and surface chemistry can be adjusted in a broad range, their use as a support shows great potential for further improvements in hydrogen storage applications.
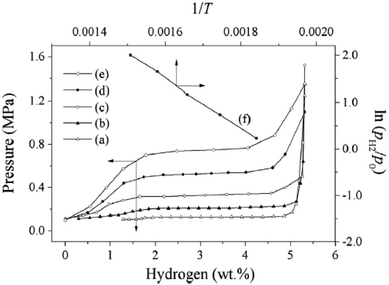 | ||
Fig. 12 Dehydrogenation isotherms of the material from Mg and C in a ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, milled for 3 h under 1 MPa H2 at 543 K (a), 573 K (b), 603 K (c), 633 K (d), and 663 K (e), and plot of ln(PH2/P0) versus 1/T (f).55 30, milled for 3 h under 1 MPa H2 at 543 K (a), 573 K (b), 603 K (c), 633 K (d), and 663 K (e), and plot of ln(PH2/P0) versus 1/T (f).55 | ||
The formation of a nanocrystalline structure and associated defects through mechanical milling is often perceived as leading to enhanced hydrogen kinetics. Similarly to the case of surfaces discussed above, grain boundaries between nanocrystals contain excess energy, resulting in surface energy, thus providing another potential tool for reducing the formation enthalpy of metal hydrides. Calizzi et al.56 prepared Mg–Ti nanostructured samples with a metastable Mg–Ti–H fcc phase and the mean crystallite size of Mg and β-MgH2 decreased with an increase in Ti content (Fig. 13). However, their results only showed an enhanced kinetic properties in the Mg–Ti system since the modification in thermodynamics due to interface energy effects or coherency strain may be too small to be detected. Further research showed that the hydriding enthalpy of Mg–Fe-based materials with a crystallite size of 10 nm decrease by 6 kJ (mol−1 H2) between 523 K and 673 K (ref. 57) and an La2Mg17–LaNi5 nanocrystalline composite material had a lower enthalpy of −53.23 kJ (mol−1 H2).58
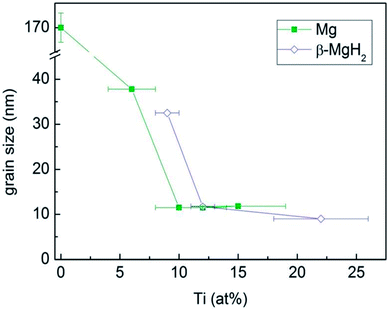 | ||
| Fig. 13 Mean crystallite size of the Mg and β-MgH2 phases in the Mg–Ti and Mg–Ti–H samples as a function of Ti content.56 | ||
Although the surface energy, lattice strain and defects, and excess enthalpy in the grain boundaries provide effective ways to lower the enthalpy of formation without loss in hydrogen storage capacity, they are usually unstable since the excess enthalpy will increase the free energy and drive recrystallization. Nanocrystalline MgH2 resulting from ball milling undergoes a significant growth in crystallite size or subsequent structural relaxation of defects upon the very first hydrogen cycle, which sluggishly decreases the advantages of nanocrystallization. Therefore, finding a proper way to protect NPs from aggregation or recrystallization is urgent.
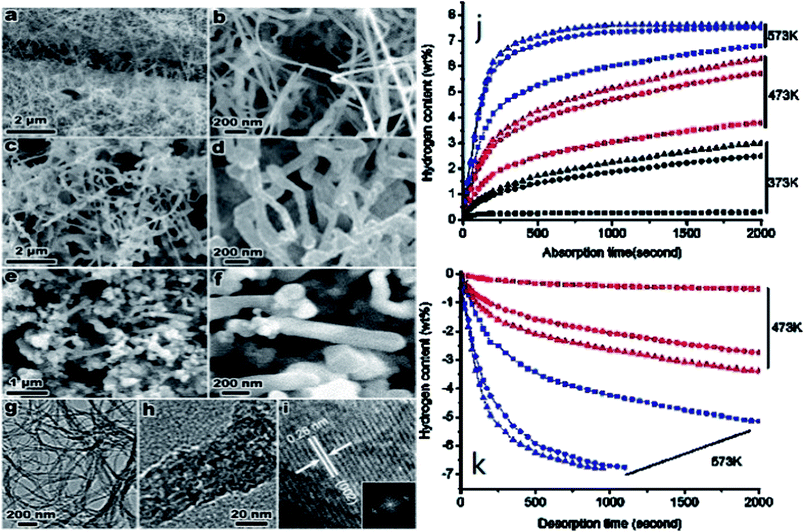 | ||
| Fig. 14 Scanning electron microscopy (SEM) images of the Mg nanowires: (a and b) 30–50 nm, (c and d) 80–100 nm, and (e and f) 150–170 nm. TEM (g and h), and HRTEM (i) images of (a and b) with the corresponding fast Fourier transform (FFT) pattern (inset). Hydrogen absorption (j) and desorption (k) of the Mg nanowires (sample 1, triangles; sample 2, circles; and sample 3, squares) at different temperatures.59 | ||
They further studied the size effects on the thermodynamic stability of MgH2 nanowires as well as the size dependency on the energetic stability of magnesium and MgH2 nanowires using first-principles density functional theory.60 The desorption enthalpy, ΔHdes, was calculated to be −20.64, 34.54, and 61.86 kJ (mol−1 H2) for the nanowires of A1_MgH2 (φ0.68 nm), A2_MgH2 (φ0.85 nm), and A3_MgH2 (φ1.24 nm), as shown in Fig. 15. Thus, we can conclude that a decrease in diameter leads to energetic instability in MgH2 and magnesium nanowires; however, MgH2 nanowires are more strongly destabilized than magnesium nanowires, which result in the thermodynamic destabilization of MgH2 nanowires as their diameter decreases. Accordingly, the nanowire structured Mg can be considered as a promising hydrogen storage material due to its thermodynamic destabilization.
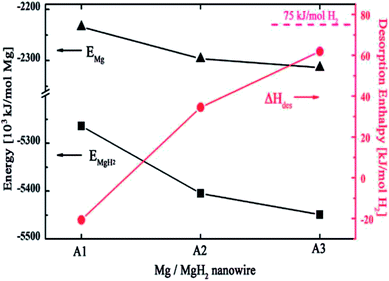 | ||
| Fig. 15 Desorption enthalpy for MgH2 nanowires (red line) and the size effect of the calculated energies for magnesium (triangle) and MgH2 (tetragon) nanowires.60 | ||
Thermodynamic destabilization of MgH2 via mechanical methods was first reported by Fujii.61 As shown in Fig. 16(a), the contraction of PdHx layers to Pd upon heating induced a large compressive stress in the lattice of MgH2. The intrinsic stress is well correlated to grain size, where an increase in grain size accommodates a reduction in stress. In the Pd/Mg/Pd layered films fabricated by sputtering, the Mg layer was composed of fine columnar structures along the c-axis with a diameter in the range of 10–30 nm (Fig. 16(b)). This produced a large thermodynamic shift in the stability of MgH2 and reduced its sorption temperature to just above room temperature (measured in vacuum), which can be attributed to the cooperative phenomenon due to the elastic interaction between the nanostructured Mg and Pd layers.62
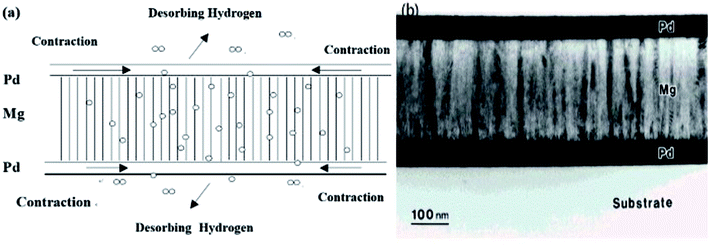 | ||
| Fig. 16 Schematic representation of the co-operative phenomenon (a) and TEM micrographs of the cross-section of Pd (50 nm)/Mg (200 nm)/Pd (50 nm) films before hydrogenation (b).61,62 | ||
Subsequently, the hydrogen storage characteristics of multi-layer Mg nanofilms have been investigated intensively. MmM5/Mg multi-layer thin films were deposited on a (0 0 1) Si substrate by direct current magnetron sputtering with a dual-target, and an advanced study proved that the apparent absorption of hydrogen in the Mg layer occurs at temperatures higher than 473 K and that the hydrogen absorbed can be fully released at 523 K.63 Nanostructured Mg thin films grow in the shape of closely-stacked columns extending throughout the film thickness, while containing polycrystalline grains and grain boundary defects, which is responsible for the significant reduction in sorption temperature from 670 to 475 K by capping the Mg films with a thin Pd layer.64 Other studies proved that thermodynamic properties and cycle stability of Mg–Al–Ti thin films or Mg–Cr–V films are significantly improved compared with pure MgH2.65,66
Baldi et al.67 revealed that the absorption plateau pressure can be altered when very thin layers of Mg are capped with a miscible element (Fig. 17). They assumed that the origins of this effect is due to ‘elastic constraints’, in accord with Fujii et al., meaning that the volume change in MgH2 is effectively restricted due to the small layer of alloying at the interface. The fundamental reasoning for this effect is the repulsive attraction between the H–H atoms within metals when volume expansion is restricted. Chung et al.68 argued that the observed destabilization has a chemical origin, whereby the interfacial mixing of the Mg/Pd regions requires additional energy for the formation of MgH2, which is exacerbated within thinner layers. In addition, Mooij et al. revealed that at very small thicknesses, the interface energies from adjacent layers become more important by synthesizing a wedge shaped Mg film with a thickness varying between 1–10 nm.69 Hughes et al.70 provided new insight into the destabilization mechanism by investigating the influence and comparative differences of Ti- and Y-based multilayers. It was remarkable that the Ti multilayers showed some destabilization with thinner layers down to 10 nm, which is proposed to be due to interfacial energy contributions. In comparison, Mg/Y-based multilayers can provide a new route towards MgH2 destabilization due to the accommodation of lattice mismatch between the strained FCC Y/Mg interfaces.
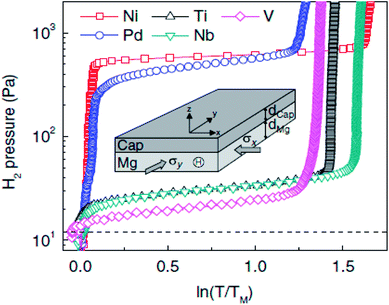 | ||
| Fig. 17 Effect of cap layer: PTI measured at 333 K for Ti(10 nm)Mg(20 nm)X(10 nm)Pd(10 nm) samples deposited on glass with X = Ni, Pd, Ti, Nb, and V.67 | ||
Recently, magnesium nanoparticles confined in carbon aerogels (CA) have attracted much attention.71–73 Liu et al.72 reported magnesium nanoparticles confined in carbon aerogels through the hydrogenation of infiltrated dibutyl-magnesium followed by hydrogen desorption at 623 K, which has a size in the range of 5.0 to 20.0 nm. The hydrogenation and dehydrogenation enthalpies of the confined Mg were determined to be 65.1 ± 1.56 kJ (mol−1 H2) and 68.8 ± 1.03 kJ (mol−1 H2), respectively. Jia et al.74 prepared the MgH2@CMK-3 nanoconfinement system and experimentally realized low temperature hydrogen release starting from 323 K. Comparing the calculated reaction energy (Er) of clusters of pure MgH2 and MgH2/C (Fig. 18), they concluded that the interfacial effect can effectively improve the low-temperature release properties of MgH2 clusters, even if the cluster is not ultra-small (Fig. 18c). Consequently, they proposed a new mechanism for destabilizing Mg–H bonding via the combination of the size effect and MgH2–carbon scaffold interfacial bonding, which is more superior than nanoparticles or 2D films.
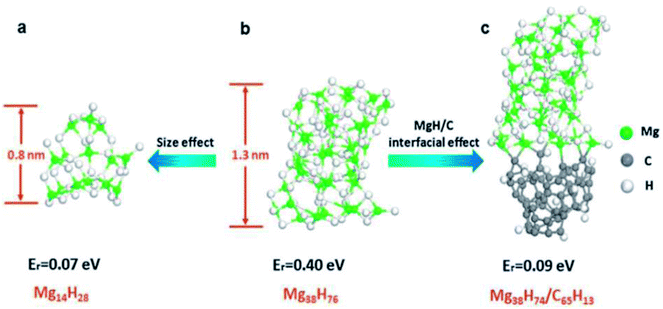 | ||
| Fig. 18 Reaction energy (Er) for hydrogen release from clusters of pure MgH2 and MgH2/C. (a) Mg14H28 cluster, (b) Mg38H76 cluster, and (c) Mg38H74 adsorbed on a cluster of amorphous carbon.74 | ||
Modifying the surface of NPs with a gas-selective outer layer of polymer or elemental metal has been well demonstrated to be a feasible route to obtain superior gas-selective permeability and structural stability. Jeon et al.75 reported the synthesis of an air-stable composite material consisting of metallic Mg nanocrystals (NCs) in a gas-barrier polymer matrix, which enabled them to absorb and release hydrogen without oxidation (Fig. 19(a)). The average Mg NCs diameter was 4.9–2.1 nm (Fig. 19(b)) and the composite material enabled both the storage of a high density of hydrogen (up to 6 wt% of Mg and 4 wt% for the composite) and rapid kinetics (loading in <30 min at 473 K) (Fig. 19(c)). Moreover, nanostructuring of Mg results in rapid storage kinetics without using expensive heavy metal catalysts. Besides, graphene nanosheets and gas-selective reduced graphene oxide (rGO) have received much attention due to their high hydrogen selectivity.76,77 Cho et al.78 reported the development of similar materials, which were comprised of nickel-doped Mg nanocrystals encapsulated by molecular-sieving reduced graphene oxide (rGO) layers, with simultaneously high hydrogen storage capacity (6.5 wt% for the total composite) and excellent kinetics, while maintaining robustness. Overall, this approach of synthesizing nanosized air-sensitive metal nanocrystals protected in a gas-selective polymer provides new opportunities for low-cost high-capacity hydrogen storage media, batteries and fuel cells.
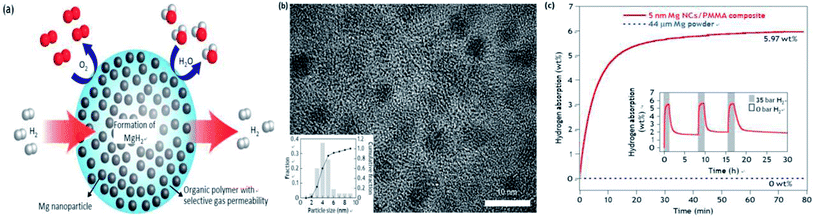 | ||
| Fig. 19 (a) Schematic of hydrogen storage composite material: high-capacity Mg NCs are encapsulated by a selectively gas-permeable polymer, (b) HRTEM of the nanocomposite and particle size distribution of Mg in the polymer matrix, and (c) hydrogen absorption kinetic analyses of bulk Mg and Mg/PMMA composites at 473 K and 35 bar hydrogen.75 | ||
Remarkably, Cui et al.79 provided another way to successfully load Mg into the nano-sized pores of an anodic aluminum oxide (AAO) template for realizing the nano-confinement of Mg via vapor transport deposition with a slight reduction in the hydrogen desorption enthalpy of MgH2 from (74.42 ± 0.12) to (73.21 ± 0.04) kJ (mol−1 H2). There was more evidence for the reduction in desorption temperature when MgH2 was embedded within an LiCl salt matrix.80 The particle size of 7 nm showed a reduction in desorption temperature of 6 K. Although this is smaller than expected, it shows the possibility of destabilization.
Over the past few decades, the thermodynamic destabilization mechanisms of nanostructured materials for hydrogen storage applications have been well demonstrated, including size effects, lattice strain, interface energies, elastic constraints, and combination of the size effect and interface energies. Remarkable advances have been achieved in destabilizing thermodynamics by taking advantage of the above mechanisms. However, there is an urgent need to address the agglomeration of particles in support-free nano-scaled hydrides or nanowires since it results in collapse of the nanostructure after repeated hydrogen uptake and release cycles, which is still a significant challenge. It is generally accepted that nanoconfinement leads to an obvious loss in the gravimetric hydrogen storage capacity compared with that of MgH2 and the low loading of Mg NPs is still a big problem. Besides, the synthesis of nanostructured materials is another challenge for practical applications.
4.3 Metastable phases
Many groups have reported the preparation methods, crystal structures, thermodynamic properties and hydrogen desorption paths of γ-MgH2. Generally, high-pressure phase γ-MgH2 can be obtained by heating α-MgH2 (ref. 81) or BM α-MgH2.82 The deuterium atoms surround magnesium in a distorted octahedral configuration with bond distances of Mg-D = 1.915(3), 1.943(3) and 2.004(3) Å.82 The thermodynamic properties of γ- and β-MgH2 have also been determined by the first-principle calculations, where the supercell based on the γ-MgH2 (1 1 0) plane is the most unstable with a surface energy of 1.23 J m−2 and dehydriding enthalpy change of 44.66 kJ mol−1, while that of the β-MgH2 (1 1 0) plane has a surface energy of 0.19 J m−2 and enthalpy change of 78.16 kJ mol−1. This is because in γ-MgH2, the H octahedrons surrounding the Mg atoms are deformed and the octahedron chain takes a zigzag form instead of the straight chain in the β phase.12 Thus, we can conclude that conversion to the metastable γ-MgH2 phase should lead to improved hydrogen sorption properties and provide another approach to modify the thermodynamics of Mg-based materials. However, irreversible reconversion of the γ-phase into the β-phase83 or Mg84 occurs during heat treatment at 583 K in 1 bar hydrogen atmosphere.Numerous researchers have shown that ball milling is an efficient way to obtain a high proportion of γ-MgH2. Zhou et al.12 prepared magnesium hydride via reactive milling under a hydrogen atmosphere using carbon as a milling aid. The enthalpy change determined by experimental isotherms gradually decreased with more γ-MgH2 from a longer milling time. They also found that the unstable γ-MgH2 in the as-milled material disappeared during desorption/absorption cycle. Xiao et al.83 made use of a simple wet chemical route by ball milling MgH2 with LiCl as a PCA at room temperature followed by tetrahydrofuran (THF) treatment under an Ar atmosphere to obtain a magnesium hydride composite (MgH2-E), which was composed of 18 wt% orthorhombic γ-MgH2 with particle lengths/widths ranging from 50–400 nm. The β-/γ-MgH2 nanocomposite exhibited a dehydrogenation capacity of 6.6 wt% and started to release hydrogen at 533 K (Fig. 20), which can be attributed to both the existence of the MgH2 nanostructure and the presence of γ-MgH2. However, they failed to determine the hydrogen desorption path of γ-MgH2 due to the limited available data and experimental methods.
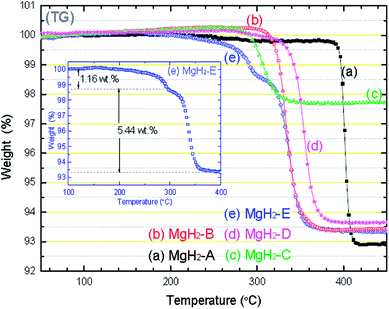 | ||
| Fig. 20 TG-DTA curves of (a) MgH2-A, (b) MgH2-B, (c) MgH2-C, (d) MgH2-D, and (e) MgH2-E samples. The heating rate was 2 °C min−1 from room temperature to 450 °C in each case. The inset shows the detail of the mass loss profile for MgH2-E.83 | ||
Shen et al.85 reported the electrochemical synthesis of nanosized Mg, leading to the formation of a mixed γ/β hydride phase upon hydrogen absorption with a high γ-MgH2 content (29.6%) with a crystallite size of 10.9 ± 0.7 nm. High-resolution transmission electron microscopy (HRTEM) also locally confirmed the formation of γ- and β-MgH2 (Fig. 21(b)), and this indicated that individual magnesium particles may contain both phases. As shown in Fig. 21(a), the decomposition of γ-MgH2 starts the same time as that of β-MgH2 with the simultaneous generation of hexagonal Mg. Thus, it is evident that both phases are intrinsically linked and γ-MgH2 may trigger the decomposition of β-MgH2. The presence of γ-MgH2 was found to cause a significant reduction in the apparent activation energy from 106.2 ± 4.0 to 69.1 ± 2.9 kJ mol−1 and a reduction in the reaction enthalpy from 74.8 ± 1.0 to 57.7 ± 5.3 kJ (mol−1 H2). It is notable that metastable γ-MgH2 has potential to lead to better kinetics and thermodynamic properties for the Mg/MgH2 system. This is consistent with the study conducted by Ponthieu,86 in which for Ti-containing nanocomposites, both γ- and β-MgD2 desorbed simultaneously above 475 K together with a concomitant increase in the Mg phase according to the reaction (7):
| x(γ-MgH2) + (1 − x)(β-MgH2) → Mg + H2 | (7) |
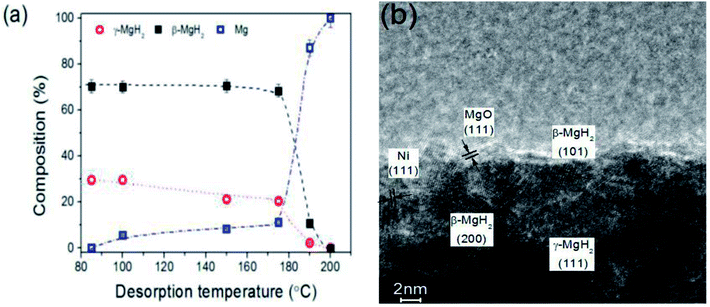 | ||
| Fig. 21 Thermal stability of γ-MgH2 during hydrogen desorption as function of desorption temperature (a) and HRTEM images of electro-synthesized Mg after hydrogen absorption at 100 °C (b).85 | ||
The results show that the presence of γ-MgH2 destabilizes the β-MgH2 phase and shows a synergetic effect during hydrogen desorption, reducing the β-MgH2 desorption temperature.87
Considering the achieved progress, although γ-MgH2 can be synthesized by heating the low-pressure phase, ball milling or hydrogenation of nanosized Mg and alter the thermodynamic properties of Mg/MgH2 without loss in its hydrogen storage capacity, the problems of bad cycle performance under high temperature and low proportion of γ-MgH2 still need to be addressed in further studies. However, it is promising that γ-MgH2 can exist stably with a decrease in desorption temperature and increase of proportion of γ-MgH2. Besides, Song et al.88 predicted a metastable phase of MgH2 by first principle calculations, where the metastable phase has a tetragonal symmetry (I41/amd, group 141) and meets all the mechanical stability criteria, and its formation enthalpy is −58.03 kJ mol−1 H2. Hence, the tetragonal structure is more desirable than α-MgH2 for practical hydrogen storage applications. However, this needs to be further confirmed by experimental data.
4.4 Changing reaction pathway
Fig. 22 shows a general enthalpy diagram illustrating the destabilization of a strongly bound hydride through the addition of a reactive additive.89 The alloying additive, B, reduces the dehydrogenation enthalpy through the formation of ABx and effectively destabilizes the hydride, AH2. This reaction occurs with a reduced enthalpy and therefore, increases the equilibrium pressure and decreases the dehydrogenation temperature.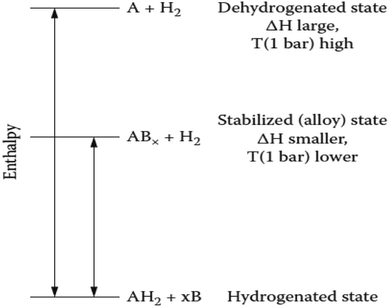 | ||
| Fig. 22 Generalized enthalpy diagram illustrating destabilization through alloy formation upon dehydrogenation.89 | ||
An intensively system investigated is the MgH2–Si system, in which, Mg2Si forms upon dehydrogenation according to reaction (8):
| 2MgH2 + Si → Mg2Si + 2H2 | (8) |
Thermodynamic calculations indicate the 1 bar equilibrium pressure is at approximately 293 K and 100 bars at approximately 423 K. Thus, it is concluded that the MgH2/Si system, with a hydrogen capacity of 5.0 wt%, is practical for hydrogen storage at reduced temperatures. Hence, alloying Si with MgH2 is supposed to significantly destabilize the Mg–H bond with the enthalpy reduced to 41 kJ (mol−1 H2) due to the formation of Mg2Si in eqn (8).90 However, the re-hydrogenation of Mg2Si was found to be severely limited by the reaction kinetics and the mass transport of Mg and Si into separate phases.91,92 Gennari et al.93 found that the presence of Ge decreases the hydride decomposition temperature in the range of 323 to 423 K, which was prepared by mechanical milling. Meanwhile, Walker et al.94 started with MgH2 and milled it with Ge under an Ar atmosphere to prepare Mg2Ge (eqn (9)).
| 2MgH2 + Ge → Mg2Ge + 2H2 | (9) |
Their results showed that Ge acts as a thermodynamic destabilization agent for MgH2, resulting in a dramatic decrease in the ΔH of dehydrogenation to 14 kJ (mol−1 H2). This dramatically reduced the temperature of dehydrogenation to 403 K, but unfortunately, the desorption product Mg2Ge could not absorb hydrogen to form MgH2 and Ge. In addition, the additives Si and Ge cannot form hydrides; thus, the hydrogen capacity was partially lost.
Aluminum also forms stable compounds or alloys with Mg, such as Mg17Al12 (γ phase) and Mg2Al3 (eqn (10)).
| 17MgH2 + 12Al ↔ 9MgH2 + 4Mg2Al3 + 8H2 ↔ Mg17Al12 + 17H2 | (10) |
However, the destabilization effect of this systems is rather small (only about 50 K decrease in decomposition start temperature), and the theoretical hydrogen content of MgH2–Al can reach 4.4 wt% with a small decrease in the desorption enthalpy ΔH of 6 kJ (mol−1 H2).95 The reversible hydrogenation was accomplished in three transformation steps (Fig. 23), where initially, only Mg reacts and forms MgH2, then the γ-Mg17Al12 phase is decomposed into the Mg-poor β-Mg2Al3 phase. Finally, the Mg-hydride phase is fully formed from the resulting Mg2Al3.96
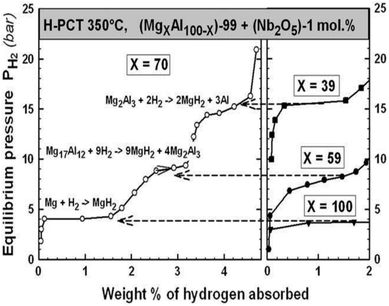 | ||
| Fig. 23 Summary of the PCT absorption curves of the (MgXAl100−X) − 99 + (Nb2O5) − 1 mol% samples. The 3 steps of transformation from the X = 70 samples are compared to the absorption curves of the X = 100 (Mg), X = 59 (Mg17Al12) and X = 39 (Mg2Al3) compounds.96 | ||
Significant progress has been achieved to alter the hydrogen storage properties of Mg-based materials, where their ΔH undergoes a great decrease through alloying, nanostructuring, forming metastable phases and changing the reaction path, as shown in Table 4. However, none of these strategies totally satisfy the requirements of practical applications; thus, so further studies on the thermodynamic properties of Mg/MgH2 need to be performed to realize the practical application goals by DOE (Table 1). The future looks bright for the combination of alloying, nanostructuring, and metastable phase.
| Strategy | Material | Synthetic method | |ΔH| (kJ (mol−1 H2)) | H2 capacity (wt%) | Ref. |
|---|---|---|---|---|---|
| Alloying | Mg2NiH4 | Mechanical alloying | 64 | 3.6 | 29 |
| Mg–Cu–H | Hydrogen-plasma-metal reaction | 67.5 | 2.6 | 34 | |
| (Mg0.68Ca0.32)Ni2 | Induce furnace melting | 37 | 1.4 | 32 | |
| Mg0.9In0.1 | Ball milling | 65 | 4.2 | 44 | |
| Nanostructuring | MgLi4.6Napth0.5 | Electrochemical reduction | 63.5 | 3.4 | 51 |
| MgH2–0.1TiH2 | Ball milling | 68 | 6 | 52 | |
| MgH2–C | Ball milling | 44.5 | 5.2 | 55 | |
| La2Mg17–LaNi5 | Mechanical milling | 53.23 | 4.9 | 58 | |
| Mg nanowires | Vapor-transport approach | 65.3 | 7.6 | 59 | |
| Mg–carbon aerogels | Solution impregnation | 65.1 | 2.35 | 72 | |
| MgH2@CMK-3 | Solution impregnation | 55.4 | 6 | 74 | |
| Metastable phase | γ-MgH2 (1.99 wt%) | Ball milling | 45.03 | 5 | 12 |
| γ-MgH2 (29.6 wt%) | Electrochemical synthesis | 57.7 | 3.5 | 85 | |
| T-MgH2 | First principles calculations | 58.04 | — | 88 | |
| Changing reaction pathway | MgH2–Si | Ball milling | 41 | 5 | 90 |
| MgH2–Ge | Ball milling | 14 | 3 | 94 | |
| MgH2–Al | Mechanical alloying | 68 | 4.4 | 95 |
5. Novel Mg-based composite materials
Altering the thermodynamic properties of Mg/MgH2 via a single measure cannot satisfy the requirements for its practical application. Therefore, the introduction of appropriate metal hydrides/dopants was proposed to further modify its thermodynamic properties. The widely studied Mg-based composites include Mg–Ni–RE,97–99 Mg–Ni–Ti,100 MgH2–LiBH4,101 LiNH2–MgH2,102 LiBH4–Mg2NiH4,103 and MgH2–NaAlH4.1045.1 Amorphization
Amorphization as a novel method can alter the hydrogen storage properties of Mg-based materials with more specific octahedral and/or tetrahedral sites for hydrogen occupation and extra potential vacancies for hydrogenation. Mg–RE–Ni amorphous alloys prepared by melt-spinning are typical examples with a hydrogen density as high as 5.5 wt% at 573 K, and the microstructure of the as-spun alloys consist of an amorphous matrix containing MgH2, Mg2NiH4 and REHx.97 Lin et al.98 found that the Mg–Ce–Ni metallic glasses (MGs) can reversibly absorb and desorb about 0.2–0.4 wt% H at room temperature without activation, and the hydrogenation capacity of the glassy Mg-based alloy is twice that of the corresponding crystalline alloy due to its free volume and disordered atomic structure.Recent studies showed that desirable properties can realized by combining crystalline and amorphous materials, which are better than that of the amorphous counterpart. Due to the combination of nanocrystalline magnesium matrix and the amorphous nickel inter-grain region, Mg–Ni nano/amorphous composite materials are superior over their amorphous counterpart in hydrogen storage capacity and kinetics.99 Besides, it was also found that Mg–Ni–La nano/amorphous composite materials with the starting temperature of hydrogen desorption from 473 K to 298 K have better properties than their Mg 50%–Ni 50% composite material. This indicates that the thermodynamic stability of Mg–Ni nano/amorphous composite materials can be reduced by additives such as La.99 Another research reported that the nanostructured 50% Mg1.5Mn0.5Ni/50% LaNi3.75Mn0.75Al0.25Co0.25 composite material can release H2 at room temperature as a result of the combined engineering of many factors, including alloying, surface properties, microstructure, grain size and others.105
5.2 Novel reactant mixtures
The U.S. Department of Energy Metal Hydride Center of Excellence (MHCoE) conducted substantial research into new reversible hydrogen storage materials for light-duty vehicles, which is composed of three main fields: mechanisms and modeling, materials development, and system design and materials engineering.106 Alapati et al.102 theoretically predicted the LiNH2 + MgH2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system due to its very attractive theoretical desorption enthalpy of ΔH = 32 kJ (mol−1 H2), with a hydrogen capacity of 8.2 wt% by the following reaction:
1) system due to its very attractive theoretical desorption enthalpy of ΔH = 32 kJ (mol−1 H2), with a hydrogen capacity of 8.2 wt% by the following reaction:| MgH2 + LiNH2 → LiMgN + 2H2 | (11) |
The high predicted weight percentage of hydrogen is due to its full dehydrogenation to LiMgN, bypassing the undesirable imide intermediate, as shown in Fig. 24. Based on this, substantial work has been done to demonstrate the feasibility of reaction (11) experimentally.107–109 The total weight loss was 8.1 wt% of the initial weight after the sample was held at 493 K for 20 min, with a ΔH of 33.5 kJ mol−1 H2, which is very close to the theoretically predicted reaction enthalpy.110 However, its application has been greatly hindered by its rather high activation energy barriers.
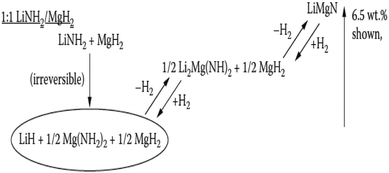 | ||
Fig. 24 Mechanism for hydrogen absorption/desorption of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LiNH2/MgH2.102 1 LiNH2/MgH2.102 | ||
Based on these results, Dolotko et al.111 demonstrated thermochemical transformations in 2MNH2–3MgH2 systems (M = Li or Na). A total of 6.45 wt% of hydrogen was released by the 2LiNH2–3MgH2 system beginning at 459 K, and a total of 5.1 wt% H2 was released by the 2NaNH2–3MgH2 system starting at 403 K according to the following solid state transformation:
| 2MNH2 + 3MgH2 → Mg3N2 + 2MH + 4H2↑ | (12) |
Other groups further studied LiNH2–nMgH2 (n = 0.5, 0.7, 0.9, 1.0, 1.5 and 2.0) nanocomposites to shed light on the combined effects of molar ratio and ball milling energy on the phase transformations and mechanical dehydrogenation.112,113 For all molar ratios, n, the hydride nanocomposites are fully reversible at 448 K under a relatively mild pressure of 50 bar H2. To further decrease the de-/re-hydrogenation temperature of the Li–Mg–N–H system, Xia et al.114 reported a novel multi-reaction methodology for the synthesis of nanosized Li2Mg (NH)2 space confined in thin-film hollow carbon spheres (THCSs) with a uniform dispersion, resulting in a stable cycling capacity and reversible hydrogen sorption at a temperature of 378 K. Besides, Zhang et al.115 reported a brief review of the state-of-the art advances in improving the performances of the lightweight complex hydride Li–Mg–N–H system. Due to nano effects, the space-confinement and nanoconfinement seem to be more effective for improving the hydrogen storage performance, and it is significant to develop hydrogen storage materials by studying the nanoconfined effects on the Li–Mg–N–H systems.
Besides the Li–Mg–N–H system, Vajo et al.103 studied the LiBH4/Mg2NiH4 system due to its unique features, including full reversibility, reaction through a direct low temperature kinetic pathway, formation of a unique ternary boride phase (MgNi2.5B2), and low reaction enthalpy coupled with low entropy. The reaction begins at temperatures as low as 523 K, which is possibly due to the catalytic nature of Ni in the NiH42− anion and is superior than MgH2–LiBH4.101 Thus far, this system appears to be the only reversible destabilized system that reacts through a direct reaction pathway. However, its capacity (2.6 wt%) for the direct low temperature step shown above is too low for practical use.
5.3 Synergistic effect
Recent research has shown that the synergistic effect, which exists in the γ/β hydride phase or multi-layer Mg nanofilms induced by elastic constraints or interface energies, can effectively alter the desorption temperature of Mg/MgH2, but this effect has not been observed directly in powders because the metal lattice is often free to expand. However, nano Mg-based composite materials prepared by ball milling have proven that the synergistic effect produced by the high density interface between different phases can further enhance the adsorption/dehydrogenation process, as shown in Fig. 25. The desorbing phase may be surrounded by an alternative phase and the tensile/compressive stress placed on the adjacent phase then acts as a driving force for destabilization. Significant investigations have been undertaken to improve the hydrogen storage properties of Mg toward the requirements of practical applications, including Mg–Mg2Ni,116 Mg–Ni–RE,117 Mg–TiCrV,118–120 Mg–LaNix,121,122 and Mg–TiB2/graphene nanosheets (GNSs).123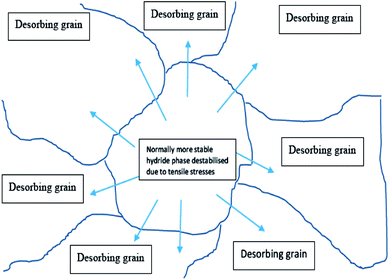 | ||
| Fig. 25 Schematic representation of the potential synergistic effects observed in multiphase powders.70 | ||
Zaluska et al.116 reported the synergistic effects of desorption for mixtures of Mg2NiH4/MgH2 phases prepared by ball-milling. The DSC results of the mixtures showed that they could operate at temperatures of 493–513 K. Li et al.117 prepared an Mg-20 wt% Ni–Y composite by reactive mechanical alloying (RMA), and the MgH2 and Mg2NiH4 phases co-existed in the milled composite. They found that the dehydrogenation temperature of the Mg-20 wt% Ni–Y composite was depressed by about 100 K compared to that of the milled pure MgH2 due to the co-contribution of the synergetic effect of hydrogen sorption of MgH2 and Mg2NiH4 and many crystal defects created by the RMA. This proves that mechanically-treated hydrides offer a new opportunity for Mg-based materials, exploiting the high capacity of magnesium hydride while operating at a much lower temperature than conventional MgH2.
Different from the synergistic effect resulting from elastic instability, Lim et al.,124 for the first time, prepared Mg nanocrystals embedded in a metal–organic framework (MOF). The Mg@SNU-9′b sample was loaded with 6.52 wt% Mg NPs with a diagonal hexagon length ranging from 44–88 nm (Fig. 26(b)). They demonstrated that Mg–NCs@MOF is a hybrid-hydrogen-storage material that has both physical adsorption and chemisorption properties, and exhibits synergistic effects to increase the isosteric heat of H2 physisorption and to decrease the temperatures for the chemisorption/desorption of H2. Although the hydrogen storage capacities of Mg NCs@MOF were rather low at high temperature (0.2 wt% and 0.24 wt% at 325 K and 415 K, respectively) as shown in Fig. 26(d and e), the study of hybrid-hydrogen-storage material provides another new strategy for H2 storage and transport for the future.
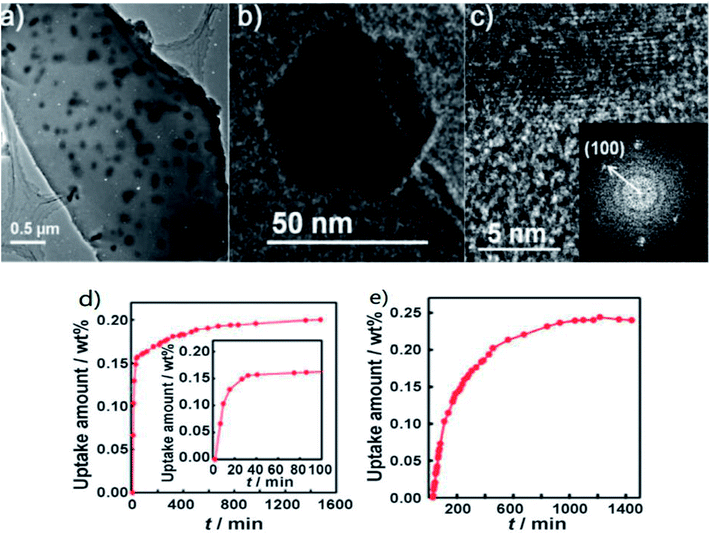 | ||
| Fig. 26 High-resolution transmission electron microscopy (HRTEM) images of (a) Mg@SNU-9′b, (b) hexagonal-shaped Mg nanocrystal and (c) its edge image showing the lattice fringe (100) d-spacing of Mg0 (2.7782 Å, JCPDS 04-0770). Inset: selected-area electron diffraction (SAED) pattern. H2 absorption kinetics of Mg@SNU-9′c at (d) 325 K and 80 bar and (e) 415 K and 40 bar.124 | ||
Substantial Mg-based composite materials have been found by combining theoretical predictions and experimental approaches. Furthermore, the mechanism of altering the hydrogen storage properties of Mg/MgH2 can be attributed to the advantages of combining many strategies including alloying, surface properties, nanostructuring, synergistic effect and others, which holds potential as an on-board storage material for light-duty vehicles with a high hydrogen capacity and low desorption temperature.
6. Conclusion and outlook
Hydrogen energy is the one ideal means of energy storage for transportation and conversion of energy, which holds the advantages of high gravimetric energy density and carbon-free energy carrier.125 There is a generally accepted opinion that hydrogen storage materials and technologies are the key issues for the realization of a hydrogen energy economy. Among the hydrogen storage materials, magnesium-based alloys show great application prospect due to their high hydrogen storage capacity, low cost, high abundance and non-toxicity. However, they are severely limited by their high decomposition temperatures and/or poor reversibility due to unfavorable de-/hydriding thermodynamics and/or kinetics. Despite the significant progress achieved with respect to the kinetics of hydrogen absorption/desorption in magnesium, its stable thermodynamic property is still the main drawback, which hinders its practical applications.126,127 In this review, we summarized the effective measures to alter the hydrogen storage properties of MgH2, such as alloying, nanostructuring, metastable phase formation, changing reaction paths and synergistic effects. However, all of these strategies have limitations. Alloying and changing the reaction path are the traditional and effective ways to decrease the formation enthalpy; however, at the expense of hydrogen capacity and some reactions are irreversible. Nanostructuring is a good way to change the hydrogen storage properties, but the synthesis and poor stability of nanostructures remain challenging. γ-MgH2 with a low formation enthalpy gradually disappears during the absorption/desorption cycle process. Considering the advantages and disadvantages of these strategies, consequently, a new strategy, nano Mg-based composite materials, was proposed, which combined the advantages of alloy engineering, nanostructuring, and synergistic effect created by the RMM, resulting in instability in the thermodynamic properties of Mg/MgH2. However, there still a long way before the Mg/MgH2 system meets the criteria stipulated by the DOE for light-duty vehicles.Based on the substantial progress, further research on Mg-based material should focus on the following aspects:
(1) Novel preparation techniques require more attention. The synthetic approach is the first and most important step for the effective modification of the properties of Mg. Nowadays, mechanical milling, physical/chemical deposition, wet chemical routes, equal channel angular pressing and electrochemical approaches have been revisited for the synthesis of nanosized Mg. However, these approaches have failed in the advanced control over Mg atomic organization, crystalline-to-amorphous transitions, formation of metastable γ-MgH2 phase and stabilization of specific nanostructures. Thus, new synthetic routes need to be developed to control the assembly of Mg atoms, content of different phases, and the location and level of doping of additives/catalysts.
(2) Finding a better way to stabilize nanostructuring. Promisingly, nanostructuring can effectively alleviate kinetic barriers and reduce thermodynamic stability. However, many challenges still exist in terms of serious aggregation and structure collapse during dehydriding/hydriding. The use of scaffolds hinders particle growth and agglomeration; however, it results in a significant capacity decrease. Therefore, new forms of stabilization should be explored to maximize the storage capacity, while providing effective stabilization for nanosized Mg without scaffolds, such as the introduction of modified graphene nanoribbons, introduction of a second phase, storing additional hydrogen and core–shell methods, should be pursued.
(3) Predicting novel Mg-based composite materials by combining theoretical methods and experimental approaches, which can efficiently combine the advantages of alloy engineering, nanostructuring, and synergistic effects. More attention should be paid to systematic theoretical research including crystallographic/structural quantities, enthalpy/entropy/surface energy, defect formation energies and computational spectroscopy, which can be used as guidelines for the preparation of new Mg-based hydrogen materials.
Ideal storage materials with low reaction temperatures, a reaction heat in the range of |ΔH| = 20–30 kJ (mol−1 H2) and an on-board reversible hydrogen storage density of more than 6 wt% H2 have not been found. However, it is expected that significant breakthrough will realize the DOE's targets with more accurate fundamental guidance and more advanced technical approaches connecting targeted properties with material microstructures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from the Beijing Science and Technology Program (Z171100000917012) under the Beijing Municipal Science and Technology Commission.References
- B. Sakintuna, F. Lamari-Darkrim and M. Hirscher, Int. J. Hydrogen Energy, 2007, 32, 1121–1140 CrossRef CAS.
- C. Zhou, Z. Fang, C. Ren, J. Li and J. Lu, J. Phys. Chem. C, 2015, 117, 12973–12980 CrossRef.
- H. Zijlstra and F. F. Westendorp, Solid State Commun., 1969, 7, 857–859 CrossRef CAS.
- J. J. Reilly and R. H. Wiswall, Inorg. Chem., 1968, 7, 2254–2256 CrossRef CAS.
- A. Züttel, Mater. Today, 2003, 6(9), 24–33 CrossRef.
- ENERGY.GOV online, https://energy.gov/eere/fuelcells, accessed on June 10, 2017.
- A. San-Martin and F. D. Manchester, Bull. Alloy Phase Diagrams, 1987, 8, 431–437 CrossRef CAS.
- V. Bérubé, G. Radtke, M. Dresselhaus and G. Chen, Int. J. Energy Res., 2007, 31, 637–663 CrossRef.
- L. Schlapbach and A. Zuttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed.
- J. P. Bastide, B. Bonnetot, J. M. Létoffé and P. Claudy, Mater. Res. Bull., 1980, 15, 1779–1787 CrossRef CAS.
- P. Vajeeston, P. Ravindran, B. C. Hauback, H. Fjellvåg, A. Kjekshus, S. Furuseth and M. Hanfland, Phys. Rev. B, 2006, 73, 224102 CrossRef.
- S. Zhou, Q. Zhang, H. Chen, X. Zang, X. Zhou, R. Wang, X. Jiang, B. Yang and R. Jiang, Int. J. Hydrogen Energy, 2015, 40, 11484–11490 CrossRef CAS.
- J. F. Stampfer Jr, C. E. Holley Jr and J. F. Suttle, J. Am. Chem. Soc., 2002, 82, 3504–3508 CrossRef.
- J. C. Crivello, B. Dam, R. V. Denys, M. Dornheim, D. M. Grant, J. Huot, T. R. Jensen, P. de Jongh, M. Latroche, C. Milanese, D. Milčius, G. S. Walker, C. J. Webb, C. Zlotea and V. A. Yartys, Appl. Phys. A, 2016, 122(2), 1–20 Search PubMed.
- C. Suryanarayana, Prog. Mater. Sci., 2004, 46, 1–184 CrossRef.
- A. Baldi and B. Dam, J. Mater. Chem., 2011, 21, 4021–4026 RSC.
- C. E. Buckley, H. K. Birnbaum, J. S. Lin, S. Spooner, D. Bellmann, P. Staron, T. J. Udovic and E. Hollar, J. Appl. Crystallogr., 2001, 34, 119–129 CrossRef CAS.
- H. Shao, G. Xin, J. Zheng, X. Li and E. Akiba, Nano Energy, 2012, 1, 590–601 CrossRef CAS.
- Y. Zhang, S. Liu, T. Yang, G. Zhang, X. Li and D. Zhao, Rare Met., 2014, 34, 1–9 Search PubMed.
- V. M. Skripnyuk, E. Rabkin, Y. Estrin and R. Lapovok, Acta Mater., 2004, 52, 405–414 CrossRef CAS.
- G. Cao, Nanostructures and Nanomaterials, World Scientific, New Jersey, 2004 Search PubMed.
- I. Haas and A. Gedanken, Chem. Commun., 2008, 15, 1795–1797 RSC.
- I. P. Jain, C. Lal and A. Jain, Int. J. Hydrogen Energy, 2010, 35(10), 5133–5144 CrossRef CAS.
- J. Huot, E. Akiba and T. Takada, J. Alloys Compd., 1995, 231, 815–819 CrossRef CAS.
- J. Huot, G. Liang, S. Boily, A. V. Neste and R. Schulz, J. Alloys Compd., 1999, 293–295(51), 495–500 CrossRef CAS.
- C. Suryanarayana, E. Ivanov and V. V. Boldyrev, Mater. Sci. Eng., A, 2001, 304, 151–158 CrossRef.
- R. A. Varin, T. Czujko and Z. S. Wronski, Nanomaterials for Solid State Hydrogen Storage, Springer US, New York, 2009 Search PubMed.
- M. Dornheim, Tailoring Reaction Enthalpies of Hydrides[M], Wiley-VCH Verlag GmbH & Co. KGaA, 2010 Search PubMed.
- J. J. Reilly and R. H. Wiswall, Inorg. Chem., 1968, 7, 2254–2256 CrossRef CAS.
- M. Pozzo and D. Alfè, Phys. Rev. B, 2008, 77, 104103 CrossRef.
- X. Hou, H. Kou, T. Zhang, R. Hu, J. Li and X. Xue, Mater. Sci. Forum, 2013, 744, 44–52 Search PubMed.
- N. Terashita, K. Kobayashi, T. Sasai and E. Akiba, J. Alloys Compd., 2001, 327, 275–280 CrossRef CAS.
- J. J. Reilly and R. H. Wiswall, Inorg. Chem., 1968, 7, 2254–2256 CrossRef CAS.
- H. Shao, G. Xin, X. Li and E. Akiba, J. Nanomater., 2013, 6, 2527–2531 Search PubMed.
- M. Klell, Storage of Hydrogen in the Pure Form M, Wiley-VCH Verlag GmbH & Co. KGaA, 2010 Search PubMed.
- K. Batalović, J. Radaković, J. Belošević-čavor and V. Koteski, Phys. Chem. Chem. Phys., 2014, 16, 12356–12361 RSC.
- Y. Wang, T. Aizawa and C. Nishimura, Mater. Trans., 2006, 47, 1052–1057 CrossRef CAS.
- M. Polanski, T. K. Nielsen, Y. Cerenius, J. Bystrzycki and T. R. Jensen, Int. J. Hydrogen Energy, 2010, 35, 3578–3582 CrossRef CAS.
- J. Zhang, F. Cuevas, W. Zaïdi, J. P. Bonnet, L. Aymard, J. L. Bobet and M. Latroche, J. Phys. Chem. C, 2016, 115, 4971–4979 CrossRef.
- D. L. Douglass, Hydrides for Energy Storage, 1978, pp. 151–184 Search PubMed.
- G. Liang and R. Schulz, J. Mater. Sci., 2004, 39, 1557–1562 CrossRef CAS.
- V. M. Skripnyuk and E. Rabkin, Int. J. Hydrogen Energy, 2012, 37, 10724–10732 CrossRef CAS.
- B. Thaddeus, Binary Phase Alloy Diagrams, Materials Park Ohio, 1990, pp. 2705–2708 Search PubMed.
- H. Zhong, H. Wang, J. Liu, D. Sun and M. Zhu, Scr. Mater., 2011, 65, 285–287 CrossRef CAS.
- H. Wang, H. Zhong, L. Ouyang, J. Liu, D. Sun, Q. Zhang and M. Zhu, J. Phys. Chem. C, 2014, 118, 12087–12096 CrossRef CAS.
- F. Luo, H. Wang, L. Ouyang, M. Zeng, J. Liu and M. Zhu, Int. J. Hydrogen Energy, 2013, 38, 10912–10918 CrossRef CAS.
- Y. Lu, H. Wang, L. Ouyang, J. Liu and M. Zhu, J. Alloys Compd., 2015, 645, S103–S106 CrossRef CAS.
- P. R. Sajanlal, T. S. Sreeprasad, A. K. Samal and T. Pradeep, Nano Rev., 2011, 2, 1–62 Search PubMed.
- K.-F. Aguey-Zinsou and J.-R. Ares-Fernandez, Energy Environ. Sci., 2010, 3, 526–543 RSC.
- R. W. Wagemans, J. H. van Lenthe, P. E. de Jongh, A. J. van Dillen and K. P. de Jong, J. Am. Chem. Soc., 2005, 127, 16675–16680 CrossRef CAS PubMed.
- W. Liu and K. F. Agueyzinsou, J. Mater. Chem. A, 2014, 2, 9718–9726 RSC.
- J. Lu, Y. J. Choi, Z. Fang and H. Y. Sohn, J. Am. Chem. Soc., 2009, 131, 15843–15852 CrossRef CAS PubMed.
- K. Asano, R. J. Westerwaal, A. Anastasopol, L. P. A. Mooij, C. Boelsma, P. Ngene, H. Schreuders, S. W. H. Eijt and B. Dam, J. Phys. Chem. C, 2015, 119, 12157–12164 CrossRef CAS.
- S. Bouaricha, J. P. Dodelet, D. Guay, J. Huot and R. Schulz, J. Mater. Res., 2001, 16, 2893–2905 CrossRef CAS.
- S. Zhou, X. Zhang, T. Li, N. Wang, H. Chen, T. Zhang, H. Yu, H. Niu and D. Liu, Int. J. Hydrogen Energy, 2014, 39, 13628–13633 CrossRef CAS.
- M. Calizzi, F. Venturi, M. Ponthieu, F. Cuevas, V. Morandi, T. Perkisas, S. Bals and L. Pasquini, Phys. Chem. Chem. Phys., 2016, 18, 141–148 RSC.
- J. A. Puszkiel, P. A. Larochette and F. C. Gennari, J. Power Sources, 2009, 186, 185–193 CrossRef CAS.
- K. J. Gross, P. Spatz, A. Zuettel and L. Schlapbach, J. Alloys Compd., 1996, 27, 206–213 CrossRef.
- W. Li, C. Li, H. Ma and J. Chen, J. Am. Chem. Soc., 2007, 129, 16675–16680 Search PubMed.
- L. Li, B. Peng, W. Ji and J. Chen, J. Phys. Chem. C, 2009, 113, 3007–3013 CrossRef CAS.
- H. Fujii, K. Higuchi, K. Yamamoto, H. Kajioka, S. Orimo and K. Toiyama, Mater. Trans., 2002, 43, 2721–2727 CrossRef CAS.
- K. Higuchi, K. Yamamoto, H. Kajioka, K. Toiyama, M. Honda, S. Orimo and H. Fujii, J. Alloys Compd., 2002, 330, 526–530 CrossRef.
- H. Wang, L. Ouyang, C. Peng, M. Zeng, C. Chung and M. Zhu, J. Alloys Compd., 2004, 370, L4–L6 CrossRef CAS.
- S. Singh, S. W. H. Eijt, M. W. Zandbergen, W. J. Legerstee and V. L. Svetchnikov, J. Alloys Compd., 2007, 441, 344–351 CrossRef CAS.
- W. P. Kalisvaart, C. T. Harrower, J. Haagsma, B. Zahiri, E. J. Luber, C. Ophus, E. Poirier, H. Fritzsche and D. Mitlin, Int. J. Hydrogen Energy, 2010, 35, 2091–2103 CrossRef CAS.
- B. Zahiri, M. Danaie, X. Tan, S. Amirkhiz, G. A. Botton and D. Mitlin, J. Phys. Chem. C, 2011, 116, 3188–3199 CrossRef.
- A. Baldi, M. Gonzalez-Silveira, V. Palmisano, B. Dam and R. Griessen, Phys. Rev. Lett., 2009, 102, 226102 CrossRef CAS PubMed.
- C. J. Chung, S. C. Lee, J. R. Groves, E. N. Brower, R. Sinclair and B. M. Clemens, Phys. Rev. Lett., 2012, 108, 106102 CrossRef PubMed.
- L. P. A. Mooij, A. Baldi, C. Boelsma, K. Shen, M. Wagemaker, Y. Pivak, H. Schreuders, R. Griessen and B. Dam, Adv. Energy Mater., 2011, 1, 754–758 CrossRef CAS.
- J. R. H. Luke, Hydrogen sorption properties of magnesium-based thin films, University of Birmingham, 2016 Search PubMed.
- S. Zhang, A. F. Gross, S. L. Van Atta, M. Lopez, P. Liu, C. C. Ahn, J. J. Vajo and C. M. Jensen, Nanotechnology, 2009, 20, 204027 CrossRef PubMed.
- Y. Liu, J. Zou, X. Zeng, X. Wu, H. Tian, W. Ding, J. Wang and A. Walter, Int. J. Hydrogen Energy, 2013, 38, 5302–5308 CrossRef CAS.
- Y. S. Au, M. K. Obbink, S. Srinivasan, P. C. M. M. Magusin, K. P. De Jong and P. E. de Jongh, Adv. Funct. Mater., 2014, 24, 3604–3611 CrossRef CAS.
- Y. Jia, C. Sun, L. Cheng, W. M. Abdul, J. Cui, J. Zou, M. Zhu and X. Yao, Phys. Chem. Chem. Phys., 2013, 15, 5814–5820 RSC.
- K. J. Jeon, H. R. Moon, A. M. Ruminski, B. Jiang, C. Kisielowski, R. Bardhan and J. J. Urban, Nat. Mater., 2011, 10, 286–290 CrossRef CAS PubMed.
- H. Li, Z. Song, X. Zhang, Y. Huang, S. Li, Y. Mao, H. Ploehn, Y. Bao and M. Yu, Science, 2013, 342, 95–98 CrossRef CAS PubMed.
- H. Jung, S. Yang, T. Kim, J. Kang and R. Chong, Carbon, 2013, 63, 165–174 CrossRef CAS.
- E. S. Cho, A. M. Ruminski, Y. Liu, P. T. Shea, S. Kang, E. W. Zaia, J. Y. Park, Y. D. Chuang, J. M. Yuk, X. Zhou, T. W. Heo, J. Guo, B. C. Wood and J. J. Urban, Adv. Funct. Mater., 2017, 27, 1704316 CrossRef.
- J. Cui, H. Wang, D. Sun, Q. Zhang and M. Zhu, Rare Met., 2016, 35, 401–407 CrossRef CAS.
- M. Paskevicius, D. A. Sheppard and C. E. Buckley, J. Am. Chem. Soc., 2010, 132, 5077–5083 CrossRef CAS PubMed.
- M. Bortz, B. Bertheville, G. Böttger and K. Yvon, J. Alloys Compd., 1999, 287(3), 4–6 CrossRef.
- J. Huot, G. Liang, S. Boily, A. V. Neste and R. Schulz, J. Alloys Compd., 1999, 293–295, 495–500 CrossRef CAS.
- X. Xiao, Z. Liu, S. Saremiyarahmadi and D. H. Gregory, Phys. Chem. Chem. Phys., 2016, 18, 10492–10498 RSC.
- C. Ren, Z. Fang, C. Zhou, J. Lu, Y. Ren, X. Zhang and X. Luo, Int. J. Hydrogen Energy, 2014, 39, 5868–5873 CrossRef CAS.
- C. Shen and K. F. Aguey-Zinsou, J. Mater. Chem. A, 2017, 5, 8644–8652 RSC.
- M. Ponthieu, F. Cuevas, J. F. Fernández, L. Laversenne, F. Porcher and M. Latroche, J. Phys. Chem. C, 2013, 117, 18851–18862 CrossRef CAS.
- F. C. Gennari, F. J. Castro and G. Urretavizcaya, J. Alloys Compd., 2001, 321, 46–53 CrossRef CAS.
- Y. Song and Z. Guo, Appl. Phys. Lett., 2006, 89, 151 Search PubMed.
- J. J. Vajo, T. T. Salguero, A. F. Gross, S. L. Skeith and G. L. Olson, J. Alloys Compd., 2007, 446(1), 409–414 CrossRef.
- J. J. Vajo, F. Mertens, C. C. Ahn, R. C. Bowman and B. Fultz, Cheminform, 2004, 35, 13977–13983 CrossRef.
- A. L. Chaudhary, D. A. Sheppard, M. Paskevicius, C. J. Webb, E. M. Gray and C. E. Buckley, J. Phys. Chem. C, 2013, 118, 1240–1247 CrossRef.
- S. T. Kelly, S. L. Van Atta, J. J. Vajo, G. L. Olson and B. M. Clemens, Nanotechnology, 2009, 20, 204017 CrossRef PubMed.
- F. C. Gennari, F. J. Castro, G. Urretavizcaya and G. Meyer, J. Alloys Compd., 2002, 334, 277–284 CrossRef CAS.
- G. S. Walker, M. Abbas, D. M. Grant and C. Udeh, Chem. Commun., 2011, 47, 8001–8003 RSC.
- S. Bouaricha, J. P. Dodelet, D. Guay, J. Huot, S. Boily and R. Schulz, J. Alloys Compd., 2000, 297, 282–293 CrossRef CAS.
- J. C. Crivello, T. Nobuki and T. Kuji, Int. J. Hydrogen Energy, 2009, 34, 1937–1943 CrossRef CAS.
- S. Kalinichenka, L. Röntzsch, T. Riedl, T. Weißgärber and B. Kieback, Int. J. Hydrogen Energy, 2011, 36, 10808–10815 CrossRef CAS.
- H. Lin, W. Wang and M. Zhu, J. Non-Cryst. Solids, 2012, 358, 1387–1390 CrossRef CAS.
- A. Ming, Mater. Sci. Eng., B, 2005, 117, 37–44 CrossRef.
- N. Zhou and D. Ju, Int. J. Hydrogen Energy, 2014, 39, 19630–19636 CrossRef CAS.
- S. Kumar, U. Jain, A. Jain, H. Miyaoka, T. Ichikawa, Y. Kojima and G. K. Dey, Int. J. Hydrogen Energy, 2017, 47, 3963–3970 CrossRef.
- S. V. Alapati, J. J. Karl and D. S. Sholl, Phys. Chem. Chem. Phys., 2007, 38, 1438–1452 RSC.
- J. J. Vajo, W. Li and P. Liu, Chem. Commun., 2010, 46, 6687–6689 RSC.
- H. Liu, X. Wang, Y. Liu, Z. Dong, H. Ge, S. Li and M. Yan, J. Phys. Chem. C, 2014, 118, 37–45 CrossRef CAS.
- M. Jurczyk, M. Nowak, L. Smardz and A. Szajek, Mg-BASED NANOCOMPOSITES FOR ROOM TEMPERATURE HYDROGEN STORAGE, Tms 1 Meeting & Exhibition, 2011 Search PubMed.
- L. E. Klebanoff and J. O. Keller, Int. J. Hydrogen Energy, 2013, 38, 4533–4576 CrossRef CAS.
- Y. Liu, K. Zhong, M. Gao, J. Wang, H. Pan and Q. Wang, Chem. Mater., 2008, 20, 3521–3527 CrossRef CAS.
- J. J. Hu, E. Rohm and M. Fichtner, Acta Mater., 2011, 59, 5821–5831 CrossRef CAS.
- J. Lu, Y. J. Choi, Z. Z. Fang and Y. Hong, J. Power Sources, 2010, 195, 1992–1997 CrossRef CAS.
- J. Lu, Z. Z. Fang, Y. J. Choi and H. Y. Sohn, MRS Online Proc. Libr., 2007, 111, 12129–12134 CAS.
- O. Dolotko, N. Paulson and V. K. Pecharsky, Int. J. Hydrogen Energy, 2010, 35, 4562–4568 CrossRef CAS.
- R. Parviz and R. A. Varin, Int. J. Hydrogen Energy, 2013, 38, 8313–8327 CrossRef CAS.
- R. A. Varin, R. Parviz, M. Polanski and Z. S. Wronski, Int. J. Hydrogen Energy, 2014, 39, 10585–10599 CrossRef CAS.
- G. Xia, X. Chen, C. Zhou, C. Zhang, D. Li, Q. Gu, Z. Guo, H. Liu, Z. Liu and X. Yu, J. Mater. Chem. A, 2015, 3, 12646–12652 RSC.
- B. Zhang and Y. Wu, Prog. Nat. Sci.: Mater. Int., 2017, 27(1), 21–33 CrossRef CAS.
- A. Zaluska, L. Zaluski and J. O. Ström-Olsen, J. Alloys Compd., 1999, 289, 197–206 CrossRef CAS.
- Z. Li, X. Liu, L. Jiang and S. Wang, Int. J. Hydrogen Energy, 2007, 32, 1869–1874 CrossRef CAS.
- L. Laversenne, J. Andrieux, D. Plante, L. Lyard and S. Miraglia, Int. J. Hydrogen Energy, 2013, 38, 11937–11945 CrossRef CAS.
- X. Liu, Z. Huang, L. Jiang and S. Wang, Int. J. Hydrogen Energy, 2007, 32, 965–968 CrossRef CAS.
- C. Zhou, Z. Z. Fang, C. Ren, J. Li and J. Lu, J. Phys. Chem. C, 2013, 117, 12973–12980 CrossRef CAS.
- F. Li, L. Jiang, J. Du, S. Wang, X. Liu and F. Zhan, Int. J. Hydrogen Energy, 2006, 31, 581–585 CrossRef CAS.
- G. Liang, S. Boily, J. Huot, A. V. Neste and R. Schulz, J. Alloys Compd., 1998, 268, 302–307 CrossRef CAS.
- G. Liu, Y. Wang, L. Jiao and H. Yuan, Int. J. Hydrogen Energy, 2014, 39, 3822–3829 CrossRef CAS.
- D. W. Lim, J. W. Yoon, K. Y. Ryu and M. P. Suh, Angew. Chem., 2012, 51, 9814–9817 CrossRef CAS.
- B. P. D. Ulf, E. P. D. Baldur and G. Taylor, Cogeneration and Competitive Power Journal, 2003, 18(3), 29–70 Search PubMed.
- X. B. Yu, Z. W. Tang, D. L. Sun, L. Z. Ouyang and M. Zhu, Prog. Mater. Sci., 2017, 88, 1–48 CrossRef.
- I. Z. Hlova, Mechanochemical synthesis of hydrogen-storage materials based on aluminum, magnesium and their compounds, Iowa State University, 2015 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |
