Open Access Article
Open Access ArticleInfluence of modification of Ti3C2 MXene with ceramic oxide and noble metal nanoparticles on its antimicrobial properties and ecotoxicity towards selected algae and higher plants†
A. Rozmysłowska-Wojciechowska*a,
E. Karwowskab,
S. Poźniaka,
T. Wojciechowskic,
L. Chlubnyd,
A. Olszynaa,
W. Ziemkowskac and
A. M. Jastrzębska *a
*a
aWarsaw University of Technology, Faculty of Materials Science and Engineering, Woloska 141, 02-507 Warsaw, Poland. E-mail: anita.rozmyslowska@gmail.com; szymonpozniak0@gmail.com; aolszyna@meil.pw.edu.pl; agsolgala@gmail.com; Fax: +48-22-234-57-19; Tel: +48-22-234-74-49
bWarsaw University of Technology, Faculty of Building Services, Hydro and Environmental Engineering, Nowowiejska 20, 00-653 Warsaw, Poland. E-mail: ewa.karwowska@pw.edu.pl
cWarsaw University of Technology, Faculty of Chemistry, Noakowskiego 3, 00-664 Warsaw, Poland. E-mail: twojciechowski@ch.pw.edu.pl; ziemk@ch.pw.edu.pl
dAGH University of Science and Technology, Faculty of Materials Science and Ceramics, Mickiewicza 30, 30-059 Krakow, Poland. E-mail: leszek@agh.edu.pl
First published on 30th January 2019
Abstract
The number of investigations regarding the application of 2D nanosheets of MXenes in different technological areas is growing rapidly. Different surface modifications of MXenes have been introduced to date in order to tailor their properties. As a result, surface-modified MXenes could be released in the environment from filtration membranes, adsorbents, or photocatalysts. On the other hand, assessment of their environmental impact is practically unexplored. In the present study, we examined how modification of the antimicrobial Ti3C2 MXene with ceramic oxide and noble metal nanoparticles affects its toxic behavior. The expanded 2D sheets of the Ti3C2 MXene phase were modified with Al2O3/Ag, SiO2/Ag, and SiO2/Pd nanoparticles using the sol–gel method and extensively characterized. The obtained 2D nanocomposite structures were characterized by antibacterial properties. The ecotoxicological assays considered green algae (Desmodesmus quadricauda) as well as two higher plants: sorghum (Sorghum saccharatum) and charlock (Sinapis alba). Our results revealed that obtained nanomaterials can cause both stimulating and inhibiting effects towards algae, and the ecotoxicity depended on the concentration and the type of modification. The study reveals the intriguing property of pristine Ti3C2 which highly stimulated green algae growth at low concentrations. It also shows that modification of pristine Ti3C2 MXene with different nanoparticles changes the ecotoxicological effects of the resulting nanocomposite 2D structures. We have also indicated nanocomposite structures that does not revealed the toxic effect on tested organisms i.e. the Ti3C2 MXene surface-modified with Al2O3/Ag was not phyto- and eco-toxic. This work helps with better understanding of the reactivity of surface-modified MXenes towards chosen organisms, giving more information concerning the potential impact of tested nanocomposites on the ecosystems.
1. Introduction
The number of investigations regarding the application of 2D nanosheets of MXenes in different technological areas is growing rapidly. The term 'MXene' reflects the stoichiometry of the material i.e. Mn+1Xn, in which M stands for early transition metal, X relates to carbon or nitrogen, while n = 1, 2 or 3. MXenes are obtained from their parent MAX phases, caused by expansion in concentrated HF which removes the A atom a metal from group 13 or 14 of the periodic table. MXenes are simply chemically expanded structures of MAX phases and are known as early transition metal carbides and nitrides.2 Their attractive properties have allowed several applications, including filtration membranes,3 adsorbents,4 photocatalysts,4 and antimicrobials.5 It should be noted, however, that the potential toxicity of MXenes towards the natural environment has been practically unexplored. So far, the only publication in this area is Nasrallah et al.,1 who reported the lack of significant toxicity of the pristine Ti3C2 MXene towards zebrafish embryos especially that Ti3C2 possess antimicrobial properties5,6 contrary to e.g. Ti2C MXene.7,8 Many studies have considered different modifications of MXenes' surfaces, which were carried out in order to tailor their specific chemical activities. The surface-modifications with nanoparticles that have been introduced for MXenes include: SnO2,9,10 TiO2,11–18 Nb2O5,13 Cu2O,19,20 Rh,21 Ru,22 bimetallic Ru/Ni23 or Co/Ni,24 Mn3O4,25 MnO2,26 Sb2O3,27 NiO2,28 the transition metals Fe, Co, and Ni,29 and Ni/Al hydroxide.30 Additionally, some interesting antimicrobial 2D heterostructures based on Ti3C2 MXene were also prepared.31 At the same time, some concerns have been raised about the effects if such nanocomposite 2D systems were released into the environment. The effective method of overcoming this problem is to use the surface-modification. It enables sophisticated managing of the biological activity of 2D materials. It was previously shown, that modification of the surface of 2D materials with ceramic/noble metal nanoparticles has large impact on material properties in terms of biocidal action.32,33 Also, changing type of noble metal in e.g. TiO2/M nanocomposites changes toxicity of the material.34 Additionally, changing TiO2 to Al2O3, SiO2 or ZnO2 in the MexOy/Ag can shift the toxicity from one particular bacteria strain towards another.In the present study, for the first time, we examine how modification of the antimicrobial Ti3C2 MXene with ceramic oxide and noble metal nanoparticles affects its ecotoxic behavior. In our ecotoxicological studies with nanocomposites: Ti3C2/3% Al2O3/2% Ag, Ti3C2/3% SiO2/2% Ag, and Ti3C2/3% SiO2/2% Pd we have chosen the green algae (Desmodesmus quadricauda) and two species of higher plants: sorghum (Sorghum saccharatum) and charlock (Sinapis alba). All of them are representatives of the primary producers – the first level of the matter circulation and a food chain in the ecosystems. This study will be suitable for a better understanding of the impact of surface-modified MXenes in the organisms belonging to the group playing the essential role in the production of organic matter in ecosystems and in consequence make it easier to estimate the potential impact of tested nanocomposites on the natural environment.
2. Experimental
2.1. Synthesis procedure of the Ti3C2 MXene modified with SiO2, Al2O3, Ag and Pd nanoparticles
The materials examined for ecotoxicity and phytotoxicity were: Ti3C2 MXene (used here as a reference sample) as well as nanocomposites of Ti3C2/3% Al2O3/2% Ag, Ti3C2/3% SiO2/2% Ag, and Ti3C2/3% SiO2/2% Pd.The layered Ti3AlC2 MAX phase was synthesized using the SHS technique with a local ignition system.35 Briefly, the SHS derived Ti3Al powder, graphite powder (Merck no. 1.04206.9050, 99.8% pure, grain size 99.5% < 50 μm) were reacted in stoichiometric proportions, placed in the reactor and ignited for 60 seconds until end of the SHS process. The cooled product was immersed in dry isopropanol and grinded with WC balls in a rotary-vibratory mill for 8 hours. The dried Ti3AlC2 powder was then immersed in 48% hydrofluoric acid for 24 h at room temperature. About 10 cm3 of hydrofluoric acid was used per 1 g of the starting material. The resulting suspension was washed four times with deionized water and four times with technical grade ethanol. The solid product, layered Ti2C, was dried overnight at room temperature.
The expanded 2D sheets of Ti3C2 MXene phase were modified with Al2O3, SiO2, Ag, and Pd nanoparticles using the sol–gel method. The 2D nanocomposite of Ti3C2/3% Al2O3/2% Ag was prepared as follows: to a stirred suspension of Ti3C2 MXene (1.00 g), 1 cm3 of iso-propanol (Avantor, Bytom, Poland), 0.120 g of Al(OiPr)3, 0.036 g of silver lactate (as a precursor of Ag2O), and 0.5 cm3 of distilled water were added. The reaction mixture was stirred in an open beaker until the volatiles evaporated (about 2 days).
2D nanocomposites of Ti3C2/3% SiO2/2% Ag and Ti3C2/3% SiO2/2% Pd were prepared as follows: to a stirred suspension of Ti3C2 MXene (1.00 g), 1 cm3 of iso-propanol, 0.094 g of Si(OEt)4, precursors of Ag2O or PdO nanoparticles (0.036 g of silver lactate or 0.053 g of palladium acetate, respectively), and 0.5 cm3 of distilled water were added. The reaction mixtures were stirred in open beakers until the volatiles evaporated (about 2 days). All reagents were obtained from Sigma-Aldrich (Poznań, Poland) and were used as delivered. The composition of the nanomaterials was determined assuming the purity of the Ti3C2 to be 100%.
2.2. Characterization of the morphology and structure of the obtained nanocomposite structures
The morphologies of the surface of Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites were analyzed using scanning electron microscopy (SEM). The samples were directly deposited onto sticky carbon tape and coated with a carbon layer using a BAL-TEC SCD 005 sputter coater. Samples were subjected to morphology analysis with a LEO 1530 (Zeiss, USA) microscope, at an accelerating voltage of 2.0 kV.The elemental composition was analyzed using an energy dispersive X-ray spectroscopy (EDS) unit coupled with a scanning electron microscope. The analysis gave information on the presence of specific elements in a chosen region of the sample.
The chemical compositions of the surfaces of Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites were characterized using X-ray photoelectron spectroscopy (XPS). X-ray photoelectron spectroscopic measurements were performed using a PHI 5000 VersaProbe (ULVAC-PHI) spectrometer with monochromatic Al Kα radiation (hν = 1486.6 eV) from an X-ray source operating at 100 μm spot size, 25 W, and 15 kV. The high-resolution (HR) XPS spectra were collected with the hemispherical analyzer at a pass energy of 117.4 and an energy step size of 0.1 eV. The X-ray beam was incident at the sample surface at an angle of 45° with respect to the surface normal, and the analyzer axis was located at 45° with respect to the surface. Casa XPS software (version 2.3.18) was used to evaluate the XPS data. Deconvolution of all HR XPS spectra were performed using a Shirley background and a Gaussian peak shape with 30% Lorentzian character.
2.3. Analysis of physical properties and porous structure
The physical properties and porous structure of Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites were analyzed using physical nitrogen sorption isotherms. The preparation of samples included degassing under vacuum at a temperature of 300 °C for 24 hours. Physical nitrogen sorption isotherms were measured experimentally in a liquid nitrogen bath (−195.8 °C) using Quadrasorb-SI equipment (Quantachrome Instruments, USA). The specific surface area, SBET, was determined using the Brunauer, Emmett, and Teller method (BET). The total pore volume, Vpores, and the surface area occupied by the pores, Spores, were determined using the Barrett, Joyner, and Halenda method (BJH). The distributions of the pore sizes were further used for estimating the average pore size, DBJH.2.4. Analysis of antimicrobial properties
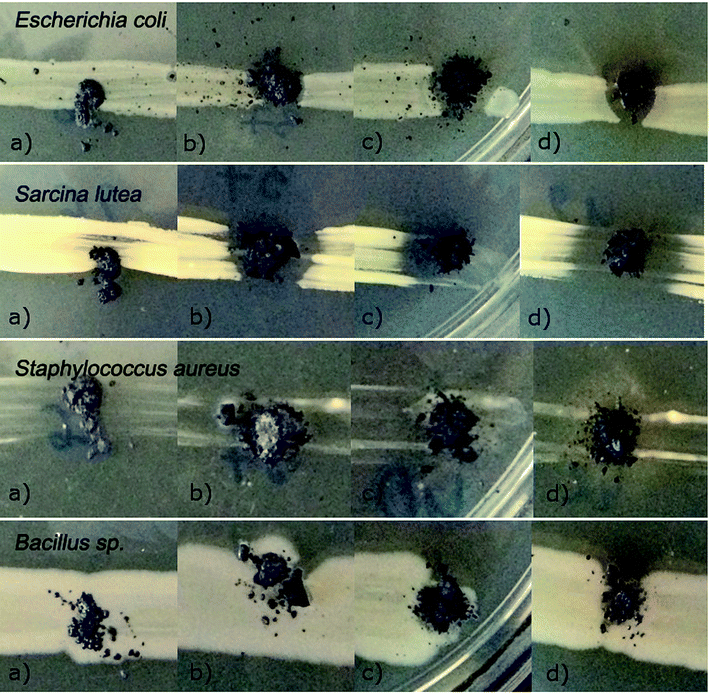
The bioactivities of Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites were analyzed qualitatively by an agar diffusion method using selected strains of both Gram-negative (Escherichia coli) and Gram-positive bacteria (Bacillus sp., Staphylococcus aureus, and Sarcina lutea), from the private collection of the Biology Department, Faculty of Building Services, Hydro and Environmental Engineering, Warsaw University of Technology.In the diffusion method, microorganisms were spread in a line on the surface of a solid nutritive culture medium (Nutrient LAB-AGAR™, Biocorp) in Petri dishes. The samples of nanocomposites were subsequently placed on the surface of the bacterial inoculation. The prepared bacterial cultures were then incubated for 48 h at 26 °C (Bacillus sp. and Sarcina lutea) or 37 °C (Escherichia coli and Staphylococcus aureus). After incubation, the cultures were photographed, and the growth inhibition zones around the nanocomposite samples were measured. The size of the inhibition zone 2 mm and below was estimated as moderate inhibition (+) and the inhibition exceeding 2 mm was stated as the effective one (++). Five parallel measurements were accomplished and the final result was given as the average.
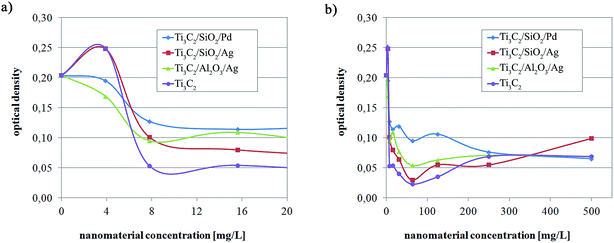
In order to estimate the antibacterial effect against a potentially pathogenic microorganisms, the additional quantitative analysis of the nanocomposites' bioactivity was accomplished with Staphylococcus aureus, using a dilution method. The test was carried out in nutrient broth medium (Biocorp), diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with the nanocomposite suspension, giving final nanocomponent concentrations from 0 mg l−1 (control sample) to 500 mg l−1. The cultures were incubated at 37 °C for 24 hours. After the incubation, the optical density of the culture was measured in a spectrophotometer at a wavelength of 610 nm.
1 with the nanocomposite suspension, giving final nanocomponent concentrations from 0 mg l−1 (control sample) to 500 mg l−1. The cultures were incubated at 37 °C for 24 hours. After the incubation, the optical density of the culture was measured in a spectrophotometer at a wavelength of 610 nm.
2.5. Analysis of ecotoxicity towards green algae
The ecotoxicity of Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites was tested using the green algae Desmodesmus quadricauda, which are organisms commonly used in ecotoxicological tests. The D. quadricauda cultures were grown at room temperature under continuous artificial lighting. The algal cultures were carried out in cameras recommended for standardized ALGAL TOXKIT test, in the Jankowski L5 growth medium, containing KNO3 – 0.1 g l−1, Ca(NO3)2 – 0.1 g l−1, KH2PO4 – 0.04 g l−1, MgSO4·7H2O – 0.03 g l−1, citric acid – 0.003 g l−1, ferric citrate – 0.003 g l−1, distilled water – 1000 ml, and supplied with 1 ml l−1 of a microelements solution (H3BO3 – 2.86 g l−1, MnCl2·7H2O – 1.81 g l−1, ZnSO4·7H2O – 0.222 g l−1, MoO3 – 0.176 g l−1, NH4VO3 – 0.23 g l−1, CuSO4·7H2O – 0.01 g l−1, and Co(NO3)2·H2O – 0.02 g l−1). The test was carried out at nanocomposite concentrations from 12.5 mg l−1 to 100 mg l−1. The nanocomposite suspension was prepared by sonication. Two parallel series of algal cultures were grown for each concentration of the nanopowders; the results were presented as the average values of both experimental series. The number of algal cells was determined by direct counting under a light microscope, at the beginning of the experiment and after 7 days. The algal culture without nanocomposites was used as a control sample.2.6. Analysis of phytotoxicity
In the present study, the phytotoxicity of Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites was evaluated using a standardized PHYTOTOXKIT set (Micro-BioTests, Sterling, VA), allowing to estimate the intensity of germination and root growth according to the ISO Standard 11269-1.32. The PHYTOTOXKIT also permits a preliminarily assessment of the condition of the plant seedlings during the experiments, based on the estimation of the growth of the sprout and its morphology at an early stage of plant growth. The test was carried out using the seeds of two species of plants, sorghum (Sorghum saccharatum) and charlock (Sinapis alba), in an artificial soil analogous to that recommended by the OECD for soil toxicity tests. The tests were conducted at two nanocomposite concentrations, 100 and 200 mg kg−1 of soil. The control was the same soil without any nanocomposite. After 3 days of cultivation at 26 °C, the germination intensity, the length of the sprout and roots, and the intensity of growth of the root system were determined. The results were documented photographically and interpreted using ImageJ software.3. Results and discussion
The morphologies of Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites were examined using a scanning electron microscope (SEM) and are presented in Fig. 1. The typical expanded structure of Ti3C2 MXene (Fig. 1a), i.e. the nano-sized sheets with the slit-shaped pores, can clearly be seen. The Ti3C2 structure was then surface-modified with composite nanoparticles of Al2O3/Ag (Fig. 1b), SiO2/Ag (Fig. 1c), and SiO2/Pd (Fig. 1d). The SEM observations also indicated that the sizes of the composite nanoparticles varied and were generally below 100 nm, which confirmed the formation of nanocomposite systems.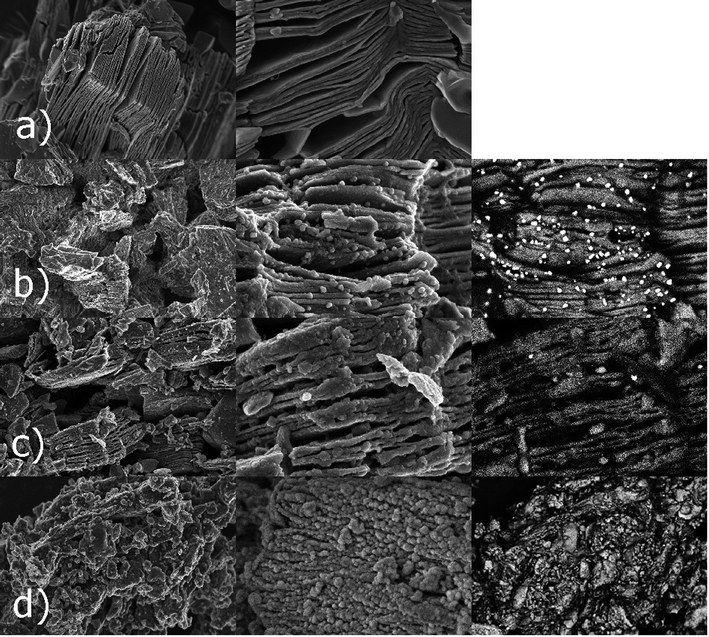 | ||
| Fig. 1 SEM images obtained for the Ti3C2 MXene (a), Ti3C2/Al2O3/Ag (b), Ti3C2/SiO2/Ag (c), and Ti3C2/SiO2/Pd (d) nanocomposites. | ||
EDS analyses of the Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites are presented in Fig. 2 and show signals associated with the presence of C, Ti, O, and F that are similar for all samples. Additionally, the presence of signals related to Ag confirmed the presence of silver in Ti3C2/Al2O3/Ag (Fig. 2b) and Ti3C2/SiO2/Ag (Fig. 2c). Also, for the Ti3C2/SiO2/Pd sample, the presence of palladium was confirmed by a Pd-related signal (Fig. 2d).
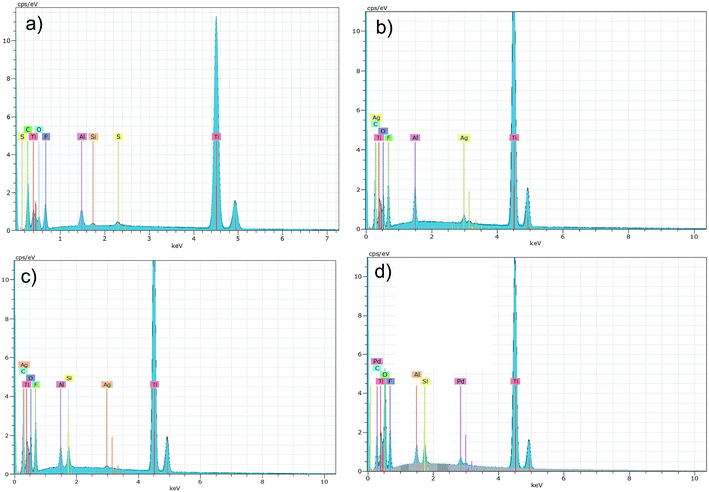 | ||
| Fig. 2 Results of EDS analysis obtained for the Ti3C2 MXene (a) as well as: Ti3C2/Al2O3/Ag (b), Ti3C2/SiO2/Ag (c), Ti3C2/SiO2/Pd (d) nanocomposites. | ||
The quantitative results on the chemical state of the elements present on the surface of the Ti3C2 MXene, as well as nanocomposites of Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd, are shown in Tables 1–4.
| Name | Peak BE | At% | SF | Peak BE (−0.9 eV) | Chemical bonds |
|---|---|---|---|---|---|
| Ti 2p3 | 460.3 | 3.6 | 5.22 | 459.4 | Ti–O (TiO2) |
| Ti 2p3 | 454.8 | 2.2 | 5.22 | 453.9 | Ti–C |
| Ti 2p3 | 455.8 | 5.3 | 5.22 | 454.9 | Ti–O |
| Ti 2p3 | 457.3 | 4.5 | 5.22 | 456.4 | Ti–O (amorphous TiO2) |
| C 1s | 285.0 | 33.6 | 1 | 284.1 | C–C |
| C 1s | 281.8 | 9.0 | 1 | 280.9 | Ti–C |
| C 1s | 286.6 | 6.4 | 1 | 285.7 | C–O |
| C 1s | 288.9 | 2.4 | 1 | 288.0 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (O O (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH) C–OH) |
| O 1s | 533.8 | 5.3 | 2.93 | 532.9 | C–O |
| O 1s | 531.9 | 6.2 | 2.93 | 531.0 | Ti–O |
| O 1s | 530.5 | 4.6 | 2.93 | 529.6 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| O 1s | 535.7 | 1.1 | 2.93 | 534.8 | — |
| F 1s | 685.4 | 9.5 | 4.43 | 684.5 | Al–F (AlF3) |
| F 1s | 687.3 | 2.9 | 4.43 | 686.4 | Al–F (AlF3) |
| F 1s | 689.4 | 0.7 | 4.43 | 688.5 | C–F |
| Al 2p | 76.3 | 1.7 | 0.537 | 75.4 | Al–F (AlF3) |
| Al 2p | 78.4 | 0.6 | 0.537 | 77.5 | Al–F (AlF3) |
| Al 2p | 73.8 | 0.7 | 0.537 | 72.9 | Al–O (Al2O3) |
| Name | Peak BE | At% | SF | Peak BE (−0.9 eV) | Chemical bonds |
|---|---|---|---|---|---|
| Ti 2p3 | 459.2 | 1.5 | 5.22 | 458.3 | Ti–O (TiO2) |
| Ti 2p3 | 455.4 | 1.9 | 5.22 | 454.5 | Ti–C |
| Ti 2p3 | 457.0 | 1.1 | 5.22 | 456.1 | Ti–O (amorphous TiO2) |
| C 1s | 285.9 | 29.0 | 1 | 285.0 | C–C |
| C 1s | 287.5 | 9.2 | 1 | 286.6 | C–O |
| C 1s | 290.2 | 4.4 | 1 | 289.3 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (O O (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH) C–OH) |
| C 1s | 282.0 | 2.5 | 1 | 281.1 | Ti–C |
| O 1s | 534.6 | 9.8 | 2.93 | 533.7 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| O 1s | 531.0 | 3.9 | 2.93 | 530.1 | Ti–O |
| O 1s | 533.0 | 8.0 | 2.93 | 532.1 | C–O |
| O 1s | 536.0 | 2.7 | 2.93 | 535.1 | — |
| F 1s | 687.8 | 4.2 | 4.43 | 686.9 | Al–F |
| F 1s | 685.9 | 2.6 | 4.43 | 685.0 | Ag–F |
| F 1s | 689.3 | 1.8 | 4.43 | 688.4 | C–F |
| Al 2p | 77.2 | 8.7 | 0.537 | 76.3 | Al–F |
| Al 2p | 75.9 | 8.1 | 0.537 | 75.0 | Al–O |
| Ag 3d5 | 368.2 | 0.3 | 10.66 | 367.3 | Ag–F |
| Ag 3d5 | 369.5 | 0.2 | 10.66 | 368.6 | Ag metallic |
| Ag 3d5 | 371.1 | 0.1 | 10.66 | 370.2 | Ag–O (Ag2O) |
| Name | Peak BE | At% | SF | Peak BE (−0.54 eV) | Chemical bonds |
|---|---|---|---|---|---|
| Ti 2p3 | 459.7 | 3.6 | 5.22 | 459.1 | Ti–O (TiO2) |
| Ti 2p3 | 455.8 | 3.4 | 5.22 | 455.2 | Ti–C |
| Ti 2p3 | 457.5 | 1.8 | 5.22 | 456.9 | Ti–O (amorphous TiO2) |
| Ti 2p3 | 461.5 | 0.7 | 5.22 | 461.0 | Ti–O (TiO2) |
| C 1s | 285.5 | 26.5 | 1 | 285.0 | C–C |
| C 1s | 282.2 | 4.2 | 1 | 281.6 | Ti–C |
| C 1s | 287.0 | 9.9 | 1 | 286.4 | C–O |
| C 1s | 289.5 | 4.4 | 1 | 288.9 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (O O (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH) C–OH) |
| O 1s | 533.7 | 17.4 | 2.93 | 533.1 | C–O |
| O 1s | 531.3 | 8.5 | 2.93 | 530.7 | Ti–O |
| O 1s | 535.3 | 9.6 | 2.93 | 534.8 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| F 1s | 687.2 | 1.0 | 4.43 | 686.7 | Si–F |
| F 1s | 689.2 | 0.9 | 4.43 | 688.7 | C–F |
| F 1s | 691.1 | 0.3 | 4.43 | 690.5 | Ag–F |
| F 1s | 685.4 | 1.2 | 4.43 | 684.9 | Al–F |
| Si 2p | 105.3 | 3.3 | 0.817 | 104.8 | Si–F |
| Si 2p | 107.2 | 0.9 | 0.817 | 106.6 | — |
| Si 2p | 103.8 | 2.4 | 0.817 | 103.2 | Si–O (SiO2) |
| Ag 3d5 | 368.3 | 0.0 | 10.66 | 367.8 | Ag metallic |
| Ag 3d3 | 374.3 | 0.0 | 7.38 | 373.8 | Ag–O (Ag2O) |
| Name | Peak BE | At% | SF | Peak BE (−0.56 eV) | Chemical bonds |
|---|---|---|---|---|---|
| Ti 2p3 | 459.7 | 6.3 | 5.22 | 459.1 | Ti–O (TiO2) |
| Ti 2p3 | 461.1 | 3.2 | 5.22 | 460.5 | Ti–O (amorphous TiO2) |
| C 1s | 285.6 | 30.9 | 1 | 285.0 | C–C |
| C 1s | 287.1 | 12.8 | 1 | 286.5 | C–O |
| C 1s | 289.3 | 5.2 | 1 | 288.7 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (O O (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH) C–OH) |
| C 1s | 290.3 | 1.7 | 1 | 289.8 | C–O/C–F |
| O 1s | 531.1 | 13.7 | 2.93 | 530.5 | Ti–O |
| O 1s | 532.8 | 11.4 | 2.93 | 532.2 | C–O |
| O 1s | 534.3 | 7.5 | 2.93 | 533.8 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| O 1s | 535.9 | 2.3 | 2.93 | 535.3 | — |
| F 1s | 685.3 | 1.2 | 4.43 | 684.8 | Al–F |
| F 1s | 687.2 | 0.8 | 4.43 | 686.7 | Si–F |
| F 1s | 689.2 | 0.5 | 4.43 | 688.7 | C–F |
| F 1s | 691.4 | 0.2 | 4.43 | 690.8 | Pd–F |
| Si 2p | 104.9 | 1.0 | 0.817 | 104.3 | Si–F |
| Si 2p | 103.3 | 0.6 | 0.817 | 102.8 | Si–O (SiO2) |
| Si 2p | 106.6 | 0.4 | 0.817 | 106.1 | — |
| Pd 3d5 | 335.7 | 0.2 | 9.48 | 335.2 | Pd metallic |
| Pd 3d5 | 337.4 | 0.1 | 9.48 | 336.9 | Pd–O (PdO) |
| Pd 3d5 | 338.8 | 0.1 | 9.48 | 338.3 | Pd–O (PdO2) |
Our results indicated the presence of Ti–C species in the Ti3C2/Al2O3/Ag and Ti3C2/SiO2/Ag nanocomposites by a Ti 2p3 signal located at ca. 455 eV which presence is also the characteristic of Ti3C2 MXene (Table 1). The atomic percent of these bonds were, however, different in the investigated nanocomposites, which corresponded with the ceramic oxide/noble metal layer on the surface of Ti3C2 MXene, i.e. 1.9 at% in Ti3C2/Al2O3/Ag (Table 2) and 3.4 at% in Ti3C2/SiO2/Ag (Table 3). For Ti3C2/SiO2/Pd (Table 4) the signal coming from Ti–C bonds was not detected due to the large amount of SiO2/Pd nanoparticles covering the Ti3C2 surface with a thick layer (see also Fig. 1d).
XPS investigations also revealed the presence of crystalline TiO2 (by the Ti 2p3 signal located at ca. 458 eV) as well as a smaller amount of amorphous phase TiO2 (Ti 2p3 at ca. 457 eV). The amount of crystalline TiO2 phase increased between the three investigated nanocomposites, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd, i.e. 1.5, 3.6, and 6.3 at%, respectively (Tables 2–5). In contrast, the amount of amorphous TiO2 phase was relatively comparable between the Ti3C2/Al2O3/Ag and Ti3C2/SiO2/Ag nanocomposites, i.e. 1.1 and 1.8, respectively, and was a little higher for the Ti3C2/SiO2/Pd nanocomposite (3.2 at%).
| SBET | VBJH | rBJH | St-ext | St-micr | Vt-micr | |
|---|---|---|---|---|---|---|
| Ti3C2 MXene (reference sample) | 80.08 | 0.0868 | 15.33 | 54.40 | 25.68 | 0.0152 |
| Ti3C2/3% Al2O3/2% Ag | 14.81 | 0.02408 | 14.92 | 11.95 | 2.859 | 0.0007748 |
| Ti3C2/3% SiO2/2% Ag | 14.92 | 0.05043 | 19.83 | 14.92 | — | — |
| Ti3C2/3% SiO2/2% Pd | 56.71 | 0.05050 | 25.90 | 31.67 | 25.04 | 0.01596 |
Our investigations also indicated the presence of different chemical species containing fluorine. For the Ti3C2/Al2O3/Ag nanocomposite, Al–O (F 1s localized at 75.9 eV), Al–F (F 1s localized at 686.9 eV) and C–F (F 1s at 688.4 eV) bonds were detected. For nanocomposites containing silica (i.e. Ti3C2/SiO2/Ag and Ti3C2/SiO2/Pd) apart from Si–O connections, the additional Si–F species (Si 2p at 104 eV) were identified (Tables 3 and 4, respectively) and SiO2 (Si 2p signal localized at 103 eV). Also, for the nanocomposites modified with nanosilver, signals corresponding to mainly metallic Ag were detected, i.e. Ag 3d5 signal localized at 368.6 eV for Ti3C2/Al2O3/Ag (Table 2) and 367.8 eV for Ti3C2/SiO2/Ag (Table 3). For the Ti3C2/SiO2/Pd nanocomposite (Table 4), the Pd 3d5 signal appeared in the XPS spectrum at 335.2 eV and corresponded to mainly metallic Pd at a level of 0.2 at%. For all the investigated nanocomposites, some oxidized Ag and Pd were also detected, but in amounts of around 0.1 at%.
We also identified the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (O 1s signal at ca. 533 eV) as well as O
O groups (O 1s signal at ca. 533 eV) as well as O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH species (C 1s signal at ca. 289 eV) in all the nanocomposites (Tables 2–4). However, they can be related to the surface of Ti3C2 as well as to the surfaces of Al2O3 or SiO2 (Table 6).
C–OH species (C 1s signal at ca. 289 eV) in all the nanocomposites (Tables 2–4). However, they can be related to the surface of Ti3C2 as well as to the surfaces of Al2O3 or SiO2 (Table 6).
| Sample name | Influence on the particular bacteria species |
|---|---|
| Ti3C2 MXene (reference sample) | Escherichia coli − |
| Sarcina lutea − | |
| Staphylococcus aureus + | |
| Bacillus sp. − | |
| Ti3C2/3% Al2O3/2% Ag | Escherichia coli + |
| Sarcina lutea ++ | |
| Staphylococcus aureus + | |
| Bacillus sp. + | |
| Ti3C2/3% SiO2/2% Ag | Escherichia coli ++ |
| Sarcina lutea ++ | |
| Staphylococcus aureus + | |
| Bacillus sp. + | |
| Ti3C2/3% SiO2/2% Pd | Escherichia coli ++ |
| Sarcina lutea ++ | |
| Staphylococcus aureus + | |
| Bacillus sp. + |
It should be also noted that the XPS analysis of parent Ti3C2 MXene was previously shown in our previous,7 as well as,35 the same MXene material was used. In these studies we indicated the presence of surface chemistry typical for MXenes as well as the purity of used material. Our investigations of bioactive properties toward model Gram-negative Escherichia coli bacterial strain also showed that antibacterial properties of the Ti3C2 MXene.7
An analysis of physical properties and porous structures of Ti3C2 MXene and the Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites was carried out, based on the isotherms of physical nitrogen sorption. The obtained isotherms are shown in Fig. 3. The obtained results indicated that the Ti3C2/SiO2/Pd nanocomposite adsorbed almost twice as much nitrogen as the other modified samples. As can be seen in Fig. 1d, the Ti3C2 MXene is covered with SiO2/Pd nanoparticles which are much more dispersed in comparison to SiO2/Ag (Fig. 1c). This effect has been also observed for TiO2/Pd nanocomposite structures.36 Palladium usually promotes better dispersion. This effect typically results in higher surface are but is not strictly connected with toxicity because nanoparticles are attached to the surface of Ti3C2 MXene.
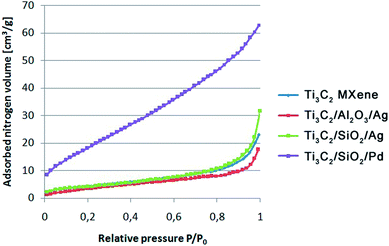 | ||
| Fig. 3 Nitrogen sorption isotherms obtained for the Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites. | ||
The shape of the obtained isotherms suggested the presence of not only slit pores, which are also characteristic for graphenes, but also cylindrical ones.37,38
The results of the physical properties of the analyzed samples are presented in Table 5. As expected, the BET specific surface area of the pure Ti3C2 was significantly larger than the specific surface area of modified samples. The total volume of pores (VBJH) was larger in the unmodified Ti3C2 than in the modified samples. It was due to the nanoparticles which filled the open slit-shaped pores of the parent MXene. The same effect was previously seen for covalent modifications of graphene oxide with nanoparticles.37,38
The pore size distributions obtained for Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd are presented in Fig. 4. The obtained BJH results indicate that, in all of our materials, there were mainly micropores (in the range of 3–6 nm) present. As can be also observed, mesopores (in the range of 5–50 nm) were present in small quantities in comparison to the general amount of micropores.
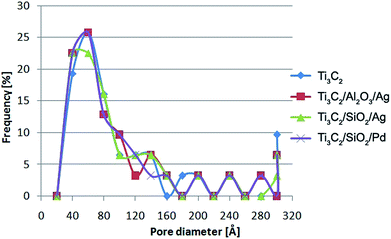 | ||
| Fig. 4 The distributions of pore diameter obtained using BJH method for the Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites. | ||
The investigations of the bioactive properties of the samples, performed using the classical culture method, revealed that the modification of Ti3C2 MXene with Al2O3 + Ag, SiO2 + Ag, and SiO2 + Pd resulted in significantly increased antibacterial properties (Fig. 5); the results of the experiment with Staphylococcus aureus, with an application of the dilution method revealed that all the tested samples were able to inhibit the growth of potentially pathogenic bacterial strains (Fig. 6). The considered mechanism of action for Ti3C2 MXene is related to the direct physical interactions between the edges of the nanosheets and the surface of bacterial cell wall or membrane. The Ti3C2 MXene nanosheets were previously found to damage physically the microorganisms' cells.39 As a result cells lose their integrity and the internal cytoplasm was released together with DNA.
Some differences in the optical density of the bacterial culture (reflecting the differences in the number of bacterial cells) were observed in low concentrations of nanocomposites. Ti3C2 MXene appeared as the most biocidal particle. However, in higher nanoparticles concentrations the antimicrobial properties of tested nanocompounds were similar despite Ti3C2/SiO2/Ag in concentration 500 mg l−1. It can be stated that the modification of Ti3C2 MXene probably does not influence significantly its antimicrobial properties. When taking into consideration systems where ceramic nanoparticles are present or noble metal nanoparticles, different effects should be considered. It should be noted, that when nanoparticles are present on the surface of Ti3C2, the sharp edges are also covered with nanoparticles (Fig. 1). Moreover, nanoparticles are strongly attached to the surface of Ti3C2 such as it was previously considered for modifications of graphene oxide.33,40 Accordingly, the antimicrobial effect relates to the presence of bioactive noble metal nanoparticles.41 Because they are incorporated into ceramic Al2O3 or SiO2 (i.e. immobilized in a porous Al2O3 or SiO2 matrix) – the only bioactive agent can be Ag+ or Pd+ ions. These ions are mostly active when direct contact occur or when the basic material (i.e. MXene). Additionally, the possibility of promoting of bioactive ions release should be considered for Ti3C2. It was previously shown, that the presence of graphene oxide in a core of a core–shell hybrid changes bioactivity of a system due to changes of zeta potential which can influence releasing of active ions.42
Nowadays, numerous nanoproducts are introduced into aquatic environments with wastewater streams.43 The estimation of nanomaterial toxicity to algae is essential because algae serve as the basis of numerous aquatic food chains. Some toxicity tests of nanoparticles' influence on the microalgae Pseudokirchneriella subcapitata have been carried out.44 The impact of ZnO, TiO2, and CuO nanoparticles on the prolongation of the lag phase of growth of Pseudokirchneriella subcapitata was observed. The formation of characteristic aggregates entrapping algal cells was revealed for nano-TiO2.45 The experiment carried out by Karwowska et al.46 on Al2O3 nanopowders' influence on green algae Scenedesmus quadricauda revealed that after 7 days the algal growth was inhibited by 5–54% in nanopowder concentrations of 500 mg l−1, while in concentrations of 1000 mg l−1 it reached 39–69%, depending on the properties of the individual nanopowder. Marine phytoplankton, including Thalassiosira pseudonana, Skeletonema marinoi, Dunaliella tertiolecta, and Isochrysis galbana, were also affected by metal oxide nanoparticles.47 It has been confirmed that a nanoproduct's toxicity depends on its concentration, physico-chemical properties, and the environmental conditions.48
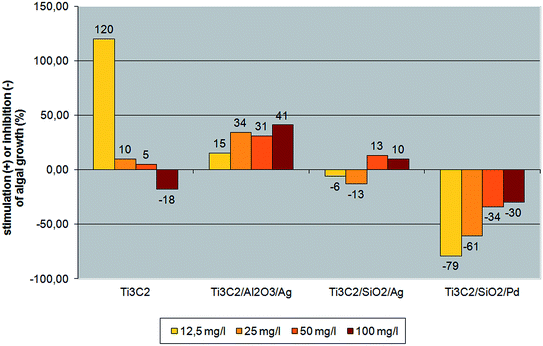
The results obtained in the present experiment showed that the impact of individual nanocomposites on tested algae differed, depending both on the concentration and the type of nanocomponents (Fig. 7). Pristine Ti3C2 MXene at the lowest concentration significantly stimulated algal growth. With increasing nanocomponent concentration, the stimulation effect diminished: for a concentration of 100 mg l−1, an inhibition of the bacterial effect was observed. The effect of growth intensification is also known for Monoraphidium minutum in presence of cobalt.49 Also arsenic was reported to stimulate the growth of microalgae Chlorella sp.50,51 This effect is related to the effect of dissolution of inorganic ions which support microalgae growth. The six times growth intensification of Scenedesmus was observed when vanadium was present. The vanadium ions were therefore almost entirely consumed by algae cells.52
It should be mentioned that algae are very sensitive for even small changes in chemical composition of the tested material as well as its concentration.53 Indeed, four different analysed compositions (i.e. Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag and Ti3C2/SiO2/Pd) showed different effects on algae and those effects became more viable when concentration was changed. There were considered two variants of material composition i.e.: (i) the same basic composition (Ti3C2 and Ag) and changing the type of ceramic used i.e. Al2O3 or SiO2, and (ii) the same basic composition (Ti3C2 and SiO2) and changing the type of noble metal used i.e. Ag or Pd. For both cases, different bio-effects were observed for green algae i.e. (1) the presence of Al2O3 at the material surface results in lower toxicity than presence of SiO2; (2) presence of Pd at the material surface results in much higher toxicity than presence of Ag. Different metallic and ceramic oxide nanoparticles were reported to exhibit the inhibitory activity of algae growth due to Reactive Oxygen Species (ROS) generation,54,55 the physical interactions,56 releasing of metal ions from the surface of the nanomaterial55,57 as well as their sizes.54 The probable mechanism of action that should be considered for the analyses nanocomposite materials is the presence of different ceramic oxide and noble metal components is the surface-dissolution of as well as physical interaction with algae cells.46,53,58 Ti3C2/Al2O3/Ag nanoparticles stimulated algal growth, with an increasing effect being caused by higher nanoparticle concentrations. The results obtained for Ti3C2/SiO2/Ag indicated both slight inhibition/stimulation. Ti3C2/SiO2/Pd nanocomposite revealed the strongest ecotoxic impact on Desmodesmus quadricauda, with the inhibition effectiveness increasing with a decreasing concentration of the nanopowder. It could be caused by an aggregating effect in the algal culture medium for the higher nanocomposite concentrations. It is evident that further study, covering the analysis of the mechanism of interaction between pristine Ti3C2 MXene and its derivatives, is necessary to explain this phenomenon.
The experiments performed in the present study, carried out in the presence of two comparatively high concentrations of nanocomposites – 100 mg l−1 and 200 mg l−1, revealed that SiO2-containing nanocomponents could influence the growth of the sprout and root of Sorghum saccharatum compared with the control sample. The inhibition effect was even stronger at a concentration of 200 mg kg−1 of the soil. The effect was not observed for the Al2O3-modified nanocomposite and the pristine Ti3C2 MXene (Fig. 8 and 9).
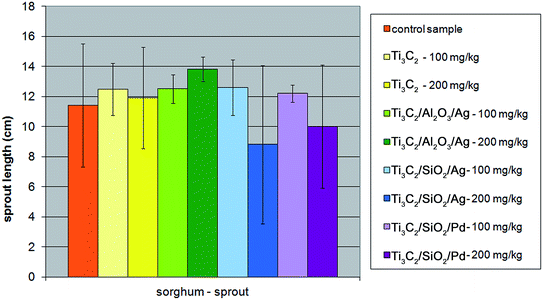 | ||
| Fig. 8 The influence of tested nanopowders at concentrations of 100 mg kg−1 and 200 mg kg−1 on the growth of the sprout of Sorghum saccharatum. | ||
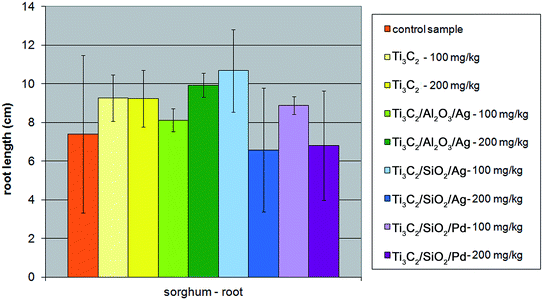 | ||
| Fig. 9 The influence of tested nanopowders at concentrations of 100 mg kg−1 and 200 mg kg−1 on the growth of the root of Sorghum saccharatum. | ||
In the case of charlock (Sinapis alba), all the modifications of the Ti3C2 MXene nanocomposite resulted in a decrease in phytotoxic properties towards the plant's sprout, although the root growth of the plant was not inhibited only in the presence of the Ti3C2/Al2O3/Ag nanocompound (Fig. 10 and 11). The strongest inhibition of the seed germination (up to 40%) was observed for the Ti3C2 MXene, while it did not exceed 20% in the presence of the other tested nanocomposites.
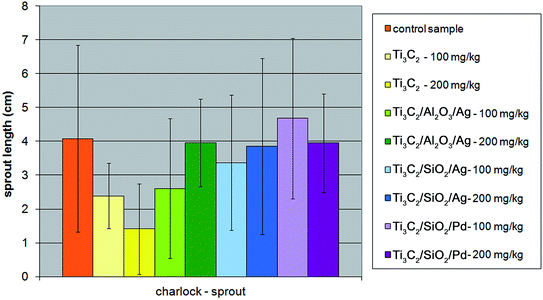 | ||
| Fig. 10 The influence of tested nanopowders at concentrations of 100 mg kg−1 and 200 mg kg−1 on the growth of the sprout of Sinapis alba. | ||
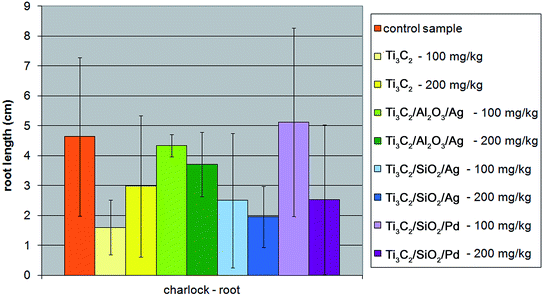 | ||
| Fig. 11 The influence of tested nanopowders at concentrations of 100 mg kg−1 and 200 mg kg−1 on the growth of the root of Sinapis alba. | ||
There is evidence that several inorganic nano-oxides may interact with plant cells. The mechanisms for plant root adsorption and incorporation into the cell wall or cell membrane have already been studied, although there is still a lack of knowledge concerning the nanoparticles' internalization.59 It has been confirmed that nanoparticles can influence both the cell structures and physiological mechanisms, bonding to different intracellular structures, including the Golgi apparatus and endoplasmic reticulum. One of the recognized mechanisms is the ability to form reactive oxygen species.60 Accordingly, we can now understand better the reactivity of surface-modified MXenes towards chosen organisms, giving more information concerning the potential impact of tested nanocomposite structures on the ecosystems. The further investigations between Ti3C2 MXenes and plant cells will be undoubtedly a subject of future research for scientific community working with MXenes.
4. Summary
The number of investigations regarding the application of 2D nanosheets of MXenes in different technological areas is growing rapidly. Different surface modifications of MXenes have been introduced to date in order to tailor their properties. As a result, surface-modified MXenes could be released in the environment from filtration membranes, adsorbents, or photocatalysts. On the other hand, assessment of their environmental impact is practically unexplored. So far, the only publication in this area is Nasrallah et al.,1 who reported the lack of significant toxicity of pristine Ti3C2 MXene towards zebrafish embryos. In the present study, we made some preliminary tests allowing to evaluate how modification of the antimicrobial Ti3C2 MXene with ceramic oxide and noble metal nanoparticles can influence its toxicity. The ecotoxicological assessment covered the experiments with a application of the green algae Desmodesmus quadricauda as well as higher plants – sorghum (Sorghum saccharatum) and charlock (Sinapis alba).It was observed that in the growth tests on solid media all the modifications of the pristine Ti3C2 MXene influenced positively its antibacterial properties against both Gram-positive and Gram-negative bacteria, despite a Staphylococcus aureus strain, in which effect was similar for all the nanocompounds. The last effect was also confirmed using the dilution method.
Our examinations of the ecotoxicity and phytotoxicity of the tested nanocomposites have shown that their ecotoxicity against algae depended not only on their concentration, but also on the type of modification. Both stimulating and inhibiting effects were observed for individual nanocomposites. We have observed that pristine Ti3C2 which highly stimulated green algae growth at low concentrations. The strongest anti-algal effect, especially in case of low concentrations, was observed for Ti3C2/SiO2/Pd nanocomposite.
The modifications of the pristine Ti3C2 MXene influenced its phytotoxic properties. The addition of SiO2/Ag or SiO2/Pd made it more phytotoxic, although the effect was not noted for Ti3C2/Al2O3/Ag. Germination inhibition was lower in modified nanocomposites compared with the pristine Ti3C2 MXene.
It is evident that the modification of the pristine Ti3C2 MXene with different nanoparticles can change its ecotoxicity. Some of modified nanocompounds can be more eco- or phyto-toxic to organisms playing the essential role of the primary producers in natural ecosystems. However, we have indicated nanocomposite structures characterized by significantly decreased ecotoxic properties: Ti3C2 MXene modified with Al2O3/Ag. The increase of antibacterial activity resulting from the modification of the pristine Ti3C2 MXene make them useful as the biocidal agents. On the other hand, the application of MXenes in different technologies should give rise to special attention to their toxicity, due to their potential ecotoxic properties. The results of our work confirm that modification of MXenes can result in the change of its potential toxic properties. Ti3C2 MXene modified with Al2O3/Ag was neither phyto- nor eco-toxic but still characterized by antimicrobial properties, which can be important from the point of view of its potential application in practice.
Authors' contributions
A. Rozmysłowska-Wojciechowska collected the obtained results, prepared the final version of the manuscript; carried out analysis of morphology, structure, physical properties and porous structure of the obtained materials; S. Poźniak was involved in the analysis of physical properties and porous structure of the obtained materials as well as preparation of figures, tables and interpretation of the results; E. Karwowska carried out and interpreted microbiological and ecotoxicity tests; T. Wojciechowski synthesized the expanded Ti3C2 MXene material and modified it with ceramic and noble metal nanoparticles; L. Chlubny synthesized the MAX phase using SHS method; A. Olszyna revised the characterization part of the manuscript. W. Ziemkowska supervised MXene preparation and modification; A. M. Jastrzębska supervised the whole research and coordinated the preparation of the manuscript. All the authors read and approved the manuscript.Conflicts of interest
The authors declare that there is no conflict of interest regarding publication of this manuscript.Acknowledgements
The scientific work was financed from the budget for science in the years 2016–2019, project no. JP2015027774 (Ministry of Science and Higher Education).Notes and references
- G. K. Nasrallah, M. Al-Asmakh, K. Rasool and K. A. Mahmoud, Ecotoxicological assessment of Ti3C2Tx (MXene) using a zebrafish embryo model, Environ. Sci.: Nano, 2018, 5, 1002–1011 RSC.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, Two-dimensional transition metal carbides, ACS Nano, 2012, 6, 1322–1331 CrossRef CAS PubMed.
- A. Shahzad, K. Rasool, W. Miran, M. Nawaz, J. Jang, K. A. Mahmoud and D. S. Lee, Two-Dimensional Ti3C2Tx MXene Nanosheets for Efficient Copper Removal from Water, ACS Sustainable Chem. Eng., 2017, 5, 11481–11488 CrossRef CAS.
- O. Mashtalir, K. M. Cook, V. N. Mochalin, M. Crowe, M. W. Barsoum and Y. Gogotsi, Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media, J. Mater. Chem. A, 2014, 2, 14334–14338 RSC.
- K. Rasool, M. Helal, A. Ali, C. E. Ren, Y. Gogotsi and K. A. Mahmoud, Antibacterial Activity of Ti3C2Tx MXene, ACS Nano, 2016, 10, 3674–3684 CrossRef CAS PubMed.
- A. A. Shamsabadi, M. Sharifian Gh., B. Anasori and M. Soroush, Antimicrobial Mode-of-Action of Colloidal Ti3C2Tx MXene, ACS Sustainable Chem. Eng., 2018, 6, 16586–16596 CrossRef.
- A. M. Jastrzębska, E. Karwowska, T. Wojciechowski, W. Ziemkowska, A. Rozmysłowska, L. Chlubny and A. Olszyna, The Atomic Structure of Ti2C and Ti3C2 MXenes is Responsible for Their Antibacterial Activity Toward E. coli Bacteria, J. Mater. Eng. Perform., 2018, 1–6, DOI:10.1007/s11665-018-3223-z.
- A. Jastrzębska, E. Karwowska, D. Basiak, A. Zawada, W. Ziemkowska, T. Wojciechowski, D. Jakubowska and A. Olszyna, Biological Activity and Bio-Sorption Properties of the Ti2C Studied by Means of Zeta Potential and SEM, Int. J. Electrochem. Sci., 2017, 12, 2159–2172 CrossRef.
- Z. Wei, Z. Peigen, T. Wubian, W. Ying, Z. Yamei, C. Jian and S. ZhengMing, Microwave-assisted synthesis of SnO2-Ti3C2 nanocomposite for enhanced supercapacitive performance, Mater. Lett., 2017, 209, 122–125 CrossRef.
- F. Wang, Z. Wang, J. Zhu, H. Yang, X. Chen, L. Wang and C. Yang, Facile synthesis SnO2 nanoparticle-modified Ti3C2 MXene nanocomposites for enhanced lithium storage application, J. Mater. Sci., 2017, 52, 3556–3565 CrossRef CAS.
- C. Peng, H. Wang, H. Yu and F. Peng, TiO2-x/Ti3C2: synergy of active facets, interfacial charge transfer and Ti3+ doping for enhance photocatalytic activity, Mater. Res. Bull., 2017, 89, 16–25 CrossRef CAS.
- M. Xue, Z. Wang, F. Yuan, X. Zhang, W. Wei, H. Tang and C. Li, Preparation of TiO2/Ti3C2Tx hybrid nanocomposites and their tribological properties as base oil lubricant additives, RSC Adv., 2017, 7, 4312–4319 RSC.
- C. J. Zhang, S. J. Kim, M. Ghidiu, M. Zhao, M. W. Barsoum, V. Nicolosi and Y. Gogotsi, Layered Orthorhombic Nb2O5@Nb4C3Tx and TiO2@Ti3C2Tx Hierarchical Composites for High Performance Li-ion Batteries, Adv. Funct. Mater., 2016, 26, 4143–4151 CrossRef CAS.
- H. Tang, S. Zhuang, Z. Bao, C. Lao and Y. Mei, Two-Step Oxidation of Mxene in the Synthesis of Layer-Stacked Anatase Titania with Enhanced Lithium-Storage Performance, ChemElectroChem, 2016, 3, 871–876 CrossRef CAS.
- C. Peng, X. Yang, Y. Li, H. Yu, H. Wang and F. Peng, Hybrids of Two-Dimensional Ti3C2 and TiO2 Exposing {001} Facets toward Enhanced Photocatalytic Activity, ACS Appl. Mater. Interfaces, 2016, 8, 6051–6060 CrossRef CAS PubMed.
- F. Wang, C. Yang, M. Duan, Y. Tang and J. Zhu, TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances, Biosens. Bioelectron., 2015, 74, 1022–1028 CrossRef CAS PubMed.
- Y. Gao, L. Wang, A. Zhou, Z. Li, J. Chen, H. Bala, Q. Hu and X. Cao, Hydrothermal synthesis of A. TiO2/Ti3C2 nanocomposites with enhanced photocatalytic activity, Mater. Lett., 2015, 150, 62–64 CrossRef CAS.
- H. Ghassemi, W. Harlow, O. Mashtalir, M. Beidaghi, M. R. Lukatskaya, Y. Gogotsi and M. L. Taheri, In situ environmental transmission electron microscopy study of oxidation of two-dimensional Ti3C2 and formation of carbon-supported TiO2, J. Mater. Chem. A, 2014, 2, 14339–14343 RSC.
- H. Zhang, H. Dong, X. Zhang, Y. Xu and J. Fransaer, Cu2O Hybridized Titanium Carbide with Open Conductive Frameworks for Lithium-ion Batteries, Electrochim. Acta, 2016, 202, 24–31 CrossRef CAS.
- Y. Gao, L. Wang, Z. Li, A. Zhou, Q. Hu and X. Cao, Preparation of MXene-Cu2O nanocomposite and effect on thermal decomposition of ammonium perchlorate, Solid State Sci., 2014, 35, 62–65 CrossRef CAS.
- M. Ming, Y. Ren, M. Hu, Y. Zhang, T. Sun, Y. Ma, X. Li, W. Jiang, D. Gao, J. Bi and G. Fan, Promoted effect of alkalization on the catalytic performance of Rh/alk-Ti3C2X2 (XO, F) for the hydrodechlorination of chlorophenols in base-free aqueous medium, Appl. Catal., B, 2017, 210, 462–469 CrossRef CAS.
- X. Li, G. Fan and C. Zeng, Synthesis of ruthenium nanoparticles deposited on graphene-like transition metal carbide as an effective catalyst for the hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2014, 39, 14927–14934 CrossRef CAS.
- X. Li, C. Zeng and G. Fan, Ultrafast hydrogen generation from the hydrolysis of ammonia borane catalyzed by highly efficient bimetallic RuNi nanoparticles stabilized on Ti3C2X2 (X = OH and/or F), Int. J. Hydrogen Energy, 2014, 40, 3883–3891 CrossRef.
- Z. Guo, T. Liu, Q. Wang and G. Gao, Construction of cost-effective bimetallic nanoparticles on titanium carbides as a superb catalyst for promoting hydrolysis of ammonia borane, RSC Adv., 2018, 8, 843–847 RSC.
- Q. Xue, Z. Pei, Y. Huang, M. Zhu, Z. Tang, H. Li, Y. Huang, N. Li, H. Zhang and C. Zhi, Mn3O4 nanoparticles on layer-structured Ti3C2 MXene towards the oxygen reduction reaction and zinc–air batteries, J. Mater. Chem. A, 2017, 5, 20818–20823 RSC.
- R. B. Rakhi, B. Ahmed, D. Anjum and H. N. Alshareef, Direct Chemical Synthesis of MnO2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications, ACS Appl. Mater. Interfaces, 2016, 8, 18806–18814 CrossRef CAS PubMed.
- X. Guo, X. Xie, S. Choi, Y. Zhao, H. Liu, C. Wang, S. Chang and G. Wang, Sb2O3/MXene(Ti3C2Tx) hybrid anode materials with enhanced performance for sodium-ion batteries, J. Mater. Chem. A, 2017, 5, 12445–12452 RSC.
- Q. X. Xia, J. Fu, J. M. Yun, R. S. Mane and K. H. Kim, High volumetric energy density annealed-MXene-nickel oxide/MXene asymmetric supercapacitor, RSC Adv., 2017, 7, 11000–11011 RSC.
- C. Ling, L. Shi, Y. Ouyang, Q. Chen and J. Wang, Transition Metal-Promoted V2CO2 (MXenes): A New and Highly Active Catalyst for Hydrogen Evolution Reaction, Adv. Sci., 2016, 3, 1600180–1600186 CrossRef PubMed.
- Y. Wang, H. Dou, J. Wang, B. Ding, Y. Xu, Z. Chang and X. Hao, Three-dimensional porous MXene/layered double hydroxide composite for high performance supercapacitors, J. Power Sources, 2016, 327, 221–228 CrossRef CAS.
- F. Alimohammadi, M. sharifian Gh., N. H. Attanayake, A. C. Thenuwara, Y. Gogotsi, B. Anasori and D. R. Strongin, Antimicrobial Properties of 2D MnO2 and MoS2 Nanomaterials Vertically Alined on Graphene Materials and Ti3C2 MXene, Langmuir, 2018, 34, 7192–7200 CrossRef CAS PubMed.
- A. M. Jastrzębska, A. Derecka, E. Karwowska, A. Pląsek, T. Wojciechowski, W. Ziemkowska and A. Olszyna, Comparative Assessment of Biocidal Activity of Different RGO/Ceramic Oxide-Ag Nanocomposites, J. Nano Res., 2017, 47, 89–95 Search PubMed.
- A. M. Jastrzębska, J. Karcz, E. Karwowska, A. Fiedorczuk and A. Olszyna, Synthesis and Bioactivity of Reduced Graphene Oxide/Alumina-Noble Metal Nanocomposite Flakes, Int. J. Appl. Ceram. Technol., 2016, 5, 856–870 CrossRef.
- A. M. Jastrzębska, E. Karwowska, P. Kurtycz, E. Miaśkiewicz-Pęska, D. Basiak, A. Olszyna, M. Załęska-Radziwiłł and N. Doskocz, The impact of zeta potential and physicochemical properties of TiO2-based nanocomposites on their biological activity, Int. J. Appl. Ceram. Technol., 2015, 6, 1157–1173 CrossRef.
- A. M. Jastrzębska, A. Szuplewska, T. Wojciechowski, M. Chudy, W. Ziemkowska, L. Chlubny, A. Rozmysłowska and A. Olszyna, In vitro studies on cytotoxicity of delaminated Ti3C2 MXene, J. Hazard. Mater., 2017, 339, 1–8, DOI:10.1016/j.jhazmat.2017.06.004.
- W. Ziemkowska, D. Basiak, P. Kurtycz, A. Jastrzębska, A. Olszyna and A. Kunicki, Nano-titanium oxide doped with gold, silver and palladium – synthesis and structural characterization, Chem. Pap., 2014, 68, 959–968 CAS.
- A. M. Jastrzębska, J. Karcz, R. Letmanowski, D. Zabost, E. Ciecierska, J. Zdunek, E. Karwowska, M. Siekierski, A. Olszyna and A. Kunicki, Synthesis of the RGO/Al2O3 core-shell nanocomposite flakes and characterization of their unique electrostatic properties using zeta potential measurements, Appl. Surf. Sci., 2016, 326, 577–594, DOI:10.1016/j.apsusc.2015.10.125.
- A. M. Jastrzębska, J. Jureczko, A. R. Kunicki and A. R. Olszyna, New reduced graphene oxide/alumina (RGO/Al2O3) nanocomposite: innovative method of synthesis and characterization, Int. J. Appl. Ceram. Technol., 2015, 12, 522–528 CrossRef.
- A. A. Shamsabadi, M. Sharifian Gh., B. Anasori and M. Soroush, Antimicrobial Mode-of-Action of Colloidal Ti3C2Tx MXene, ACS Sustainable Chem. Eng., 2018, 6, 16586–16596 CrossRef.
- A. M. Jastrzębska, J. Karcz, E. Karwowska, A. Fiedorczuk and A. Olszyna, Synthesis and Bioactivity of RGO/TiO2-Noble Metal Nanocomposite Flakes, J. Nano Res., 2017, 47, 33–48 Search PubMed.
- A. M. Jastrzębska, J. Karcz, E. Karwowska, A. Fiedorczuk and A. Olszyna, Biological properties of the RGO/Al2O3 nanocomposite flakes modified with Ag, Au and Pd for water purification, J. Alloys Compd., 2017, 724, 869–878 CrossRef.
- A. M. Jastrzębska, J. Karcz, R. Letmanowski, D. Zabost, E. Ciecierska, M. Siekierski and A. Olszyna, Synthesis of RGO/TiO2 nanocomposite flakes and characterization of their unique electrostatic properties using zeta potential measurements, J. Alloys Compd., 2016, 679, 470–484 CrossRef.
- W.-T. Liu, Nanoparticles and their biological and environmental applications, J. Biosci. Bioeng., 2006, 102, 1–7 CrossRef CAS PubMed.
- A. Kahru, A. Ivask, I. Blinova and H. C. Dubourguier, Bioavailability and ecotoxicology of nanoparticles, Toxicol. Lett., 2008, 180S, S20 CrossRef.
- V. Aruoja, H.-C. Dubourguier, K. Kasemets and A. Kahru, Toxicity of ZnO, TiO2 and CuO nanoparticles to microalgae Pseudokirchneriella subcapitata, Toxicol. Lett., 2008, 180S, S220 CrossRef.
- E. Karwowska, M. Mrozowicz, A. Zawada, T. Ząbkowski, W. Ziemkowska, A. R Kunicki and A. Olszyna, Impact of Al2O3 nanopowders characterized by various physicochemical properties on growth of green alga Scenedesmus quadricauda, Adv. Appl. Ceram., 2012, 111, 142–148 CrossRef CAS.
- R. J. Miller, H. S. Lenihan, E. B. Muller, N. Tseng, S. K. Hanna and A. A. Keller, Impacts of metal oxide nanoparticles on marinephytoplankton, Environ. Sci. Technol., 2010, 44, 7329–7334 CrossRef CAS PubMed.
- D. H. Lin and B. S. Xing, Root uptake and phytotoxicity of ZnO nanoparticles, Environ. Sci. Technol., 2008, 42, 5580–5585 CrossRef CAS PubMed.
- C. C. Chusuei, C. H. Wu, S. Mallavarapu, F. Y. Hou, C. M. Hsu, J. G. Winiarz, R. S. Aronstam and Y. W. Huang, Cytotoxicity in the age of nano: the role of fourth period transition metal oxide nanoparticle physicochemical properties, Chem.-Biol. Interact., 2013, 206, 319–326 CrossRef CAS PubMed.
- J. H. Lee, J. E. Ju, B. I. Kim, P. J. Pak, E. K. Choi, H. S. Lee and N. Chung, Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells, Environ. Toxicol. Chem., 2014, 33, 2759–2766 CrossRef CAS PubMed.
- V. Forest, L. Leclerc, J. F. Hochepie, A. Trouvé, G. Sarry and J. Pourchez, Impact of Cerium Oxide Nanoparticles Shape on their In Vitro Cellular Toxicity, Toxicol. In Vitro, 2017, 38, 136–141 CrossRef CAS PubMed.
- M. P. Delorme, Y. Muro, T. Arai, D. A. Banas, S. R. Frame, K. L. Reed and D. B. Warheit, Ninety-day inhalation toxicity study with a vapor grown carbon nanofiber in rats, Toxicol. Sci., 2012, 128, 449–460 CrossRef CAS PubMed.
- K. Miazek, W. Iwanek, C. Remacle, A. Richel and D. Goffin, Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial product Biosynthesis: A Review, Int. J. Mol. Sci., 2015, 16, 23929–23969 CrossRef CAS PubMed.
- H. J. Wang, A. C. Growcock, T. H. Tang, J. O'Hara, Y. W. Huang and R. S. Aronstam, Zinc oxide nanoparticle disruption of store-operated calcium entry in a muscarinic receptor signaling pathway, Toxicol. In Vitro, 2010, 24, 1953–1961 CrossRef CAS PubMed.
- T. H. Tang, C. T. Chang, H. J. Wang, J. D. Erickson, R. A. Reichard, A. G. Martin, E. K. Shannon, A. L. Martin, Y. W. Huang and R. S. Aronstam, Oxidative stress disruption of receptor-mediated calcium signaling mechanisms, J. Biomed. Sci., 2013, 20, 48 CrossRef CAS PubMed.
- X. Lai, Y. Wei, H. Zhao, S. Chen, X. Bu, F. Lu, D. Qu, L. Yao, J. Zheng and J. Zhang, The effect of Fe2O3 and ZnO nanoparticles on cytotoxicity and glucose metabolism in lung epithelial cells, J. Appl. Toxicol., 2015, 35, 651–664 CrossRef CAS PubMed.
- V. S. Periasamy, J. Athinarayanan, M. Alhazmi, K. A. Alatiah and A. A. Alshatwi, Fe3O4 nanoparticle redox system modulation via cell-cycle progression and gene expression in human mesenchymal stem cells, Environ. Toxicol., 2016, 31, 901–912 CrossRef CAS PubMed.
- Y.-W. Huang, M. Cambre and H.-J. Lee, The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms, Int. J. Mol. Sci., 2017, 18, 2702, DOI:10.3390/ijms18122702.
- R. D. Handy, R. Owen and E. Valsami-Jones, The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, an future needs, Ecotoxicology, 2008, 17, 315–325 CrossRef CAS PubMed.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A. J. Miao, A. Quigg, P. H. Santschi and L. Sigg, Environmental behavior and ecotoxicology of engineered nanoparticles to algae, plants and fungi, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07633b |
| This journal is © The Royal Society of Chemistry 2019 |