Open Access Article
Open Access ArticleTheoretical investigation on the electronic structure of one dimensional infinite monatomic gold wire: insights into conducting properties
Jin-Xia Liang a,
Yanxian Wua,
Hongfang Denga,
Changliang Longb and
Chun Zhu*b
a,
Yanxian Wua,
Hongfang Denga,
Changliang Longb and
Chun Zhu*b
aGuizhou Provincial Key Laboratory of Computational Nano-Material Science, Guizhou Synergetic Innovation Center of Scientific Big Data for Advanced Manufacturing Technology, Guizhou Education University, Guiyang, 550018, China
bSchool of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025, China. E-mail: czhu2014@163.com
First published on 11th January 2019
Abstract
Mixed-valence metal–organic nanostructures show unusual electronic properties. In our pervious investigation, we have designed and predicted a unique one-dimensional infinite monatomic gold wire (1D-IMGW) with excellent conductivity and the interesting characteristic of mixed valency (Auc3+ and Au0i). For further exploring its conduction properties and stability in conducting state, here we select one electron as a probe to explore the electron transport channel and investigate its electronic structure in conducting state. Density functional theory (DFT) calculations show the 1D-IMGW maintains its original structure in conducting state illustrating its excellent stability. Moreover, while adding an electron, 1D-IMGW is transformed from a semiconductor to a conductor with the energy band mixed with Auc (5d) and Aui (6s) through the Fermi level. Thus 1D-IMGW will conduct along its gold atom chain demonstrating good application prospect in nanodevices.
1. Introduction
One-dimensional metallic nanowires have received significant attention due to their particular physicochemical properties1–21 as well as their potential applications in nanodevices. Previous experimental and theoretical studies have been conducted to illuminate the formation of atomic wires and their unique properties,22–32 in particular, various novel gold nanostructures with unusual stoichiometries and structures have been synthesized because of the unique interactions between Au–Au, such as metallophilic interaction originating from electronic correlation being reinforced by relativistic effects,23–29,33–42 and short d10–d10 contacts36 arising from the bonding stabilization. However, the finite length limit the applications of Au monatomic chain. With the aim to obtain one-dimensional infinite monatomic gold wire with highly conductive and stability, we adopt the strategy to construct one-dimensional infinite monatomic metal wire by alternately connecting the organic metal macrocyclic compound and the single metal atom.As is well-known, corroles,43–50 are constructed by four conjugated pyrrole rings with three pyrrole-type nitrogen atoms and one pyridine-type nitrogen atom. Comparing with porphyrins,51 corroles' cavities are smaller due to the lack of one meso-carbon atom, which makes corroles superior tri-anionic ligands52,53 in stabilizing high oxidation-state transition metals and trivalent metals [M(III), M(IV) or M(V), M = Au, Mn, Fe, Co, Ni, Cu, Cr, Rh, Ga].54–63 The trivalent gold is generally stabled by a trianionic ligand to form stable complex. Furthermore, a stable square-planar Au(III)–corrole63 has been synthesized with excellent long-wavelength phosphorescence at ambient temperature.
Based on this stable isolated subunit, we have designed and investigated a novel one-dimensional (1D) infinite monatomic gold wire (1D-IMGW)22 with each of central Au at corrole rings connected by the single Au atoms, which has stabilized by tri-anionic corrole ligands. The 1D-IMGW exhibits excellent stability by the strong interaction between the dz2 orbitals of the Auc atoms in the centers of the corrole rings and the 6s orbitals of the Aui atoms in the middle of adjacent corrole rings. Moreover, the 1D-IMGW demonstrated the unique characteristic of mixed valency (Au3+ and Au0) and strong absorption across the entire visible range. We also further predicted the exceptional conductivity along the monatomic Au chains perpendicular to the corrole rings. However, the mechanism of charge transport on the materials of 1D-IMGW remains unclear. To explore it, here we have charged one electron on the 1D-IMGW and studied the electronic properties of this monatomic Au wire. In this work, the stable structure of charged 1D-IMGW ([1D-IMGW]−) has been optimized using density functional theory (DFT), and the band structure, the density of states (DOS), projection density of states (PDOS), Bader charges analysis and the partial charge densities have been calculated. These results presented here, help us to understand the micro mechanism of charge transport of 1D-IMGW that the electron transport along the energy band mixed with Auc (5d) and Aui (6s) through the Fermi level of [1D-IMGW]−.
2. Computational details
As used in our previous work,22 we performed ab initio calculations on the structural and electronic properties of the 1D-IMGW with one electron (the same amount of homogeneous positive background charge is added to ensure the charge neutrality by VASP program)64,65 under periodic boundary conditions using the plane-wave technique implemented in the Vienna ab initio simulation package (VASP).66,67 The generalized gradient approximation (GGA) with the PBE functional,68 including spin–orbit coupling (SOC), was employed to describe the exchange–correlation functional in all calculations. Here we've summed up over the data of quantisation axis (sx, sy, sz) for s, p, d orbitals of every atoms and given the total spin–orbit coupling effects for s, p and d orbitals for Au atoms in 1D gold wire. The projector-augmented wave (PAW) method69 was used to describe the electron–ion interactions, and the cutoff energy was set to 400 eV. All atomic positions were optimized by the conjugated gradient method with a converging tolerance of 0.02 eV Å−1 for the force on all atoms. The 1D periodic boundary conditions were considered along the growth direction of the nanowire. Two vacuum distances in the b and c directions over 15 Å were set to eliminate interactions between nanowires in adjacent cells. A Monkhorst–Pack grid 11 × 1 × 1 was used to sample the 1D periodic Brillouin zone, and 31 × 1 × 1 k-point meshes were used in the structure densities of states (DOS) integral for the periodic systems. The Brillouin zone was sampled by 31 k-points with line-mode used to calculate their electronic band structures.3. Results and discussion
3.1 The electronic structure of the [1D-IMGW]−
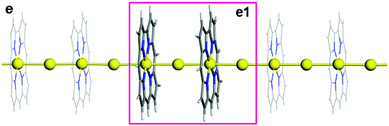
Our designed and predicted a unique one-dimensional (1D) infinite monatomic gold wire (1D-IMGW),22 in which monatomic Au wire is stabilized by the organic macrocyclic corrole compounds, and there are strong σ chemical bonding between the 5dz2 orbitals of the Auc (Au atom at the centre of the corrole rings) and the 6s orbitals of the Aui (Au atom between corrole rings) atoms. The 1D-IMGW displays excellent conductivity and strong absorption across the entire visible range. We here further investigate the mechanism of electron transport of 1D-IMGW. The optimized stable structure of charged 1D-IMGW ([1D-IMGW]−) is presented in Fig. 1. The partial bond lengths and the Bader charges analysis of Auc and Aui atoms are collected in Table 1. As seen from Fig. 1 and Table 1, the bond lengths of Au–N range from 1.967 Å to 1.987 Å, and from 1.971 Å to 1.993 Å, and the Au–Au bond lengths are 2.757 Å and 2.785 Å, and 2.766 Å and 2.777 Å in 1D-IMGW and [1D-IMGW]−, respectively. These bond lengths have slightly altered before and after charging on 1D-IMGW, indicating that the skeleton structure of 1D-IMGW has not distorted after charging and is relatively stable.| System | Bond lengths | Au charges | ||
|---|---|---|---|---|
| DAu–N | DAu–Au | Auc | Aui | |
| 1D-IMGW | 1.967–1.987 | 2.757–2.785 | 0.96, 0.98 | −0.15, −0.16 |
| [1D-IMGW]− | 1.971–1.993 | 2.766–2.777 | 0.94, 0.97 | −0.35, −0.35 |
As is our known, the mixed-valence character is usually determined by the spin density obtained under the broken symmetry approach70,71 in molecular systems. However, for the stable periodic system of [1D-IMGW]−, we adopt the Bader charges analysis on Au atoms to investigate the possible oxidation states of Au atoms. As seen from Table 1, the Bader charges of the two Auc atoms in [1D-IMGW]− are 0.94 |e| and 0.97 |e|, which are also very close to the Bader charges of 0.96 |e| and 0.98 |e| in 1D-IMGW, showing that the oxidation states of Auc atoms in [1D-IMGW]− are still triplet valence.22 The Bader charges of Aui atoms are −0.35 |e| in [1D-IMGW]−, higher than that (−0.15 |e| and −0.16 |e|) in 1D-IMGW, indicating that the added electron located mainly on the 6s of Aui atoms in [1D-IMGW]−. Obviously, these Bader charges demonstrate that the structure of [1D-IMGW]− remain the characteristic of mix valences with Auc3+ and Aui−, respectively.
3.2 The electron properties of [1D-IMGW]−
Here we used electron probe to further understand the electron transport mechanism of 1D-IMGW. Based on the optimized structure of charged 1D-IMGW ([1D-IMGW]−), the calculated electronic band structure, density of states (DOS) and the projection densities of states (PDOS) of [1D-IMGW]− are shown in Fig. 2 and 3. As seen from Fig. 2, the calculated electronic band structure of [1D-IMGW]− shows a metallic property, which is different from the direct semiconductor of neutral 1D-IMGW, because the partial electronic bands of [1D-IMGW]− have already crossed the Fermi level. We can also see that the distribution of the energy densities of states nearby the Fermi level is relatively higher in [1D-IMGW]− than that in 1D-IMGW,22 showing that the electron conduction is very excellent on 1D-IMGW materials.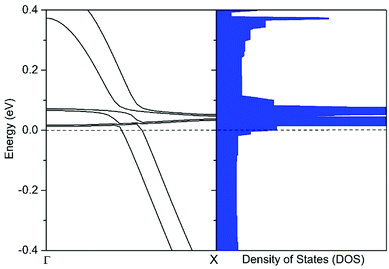 | ||
| Fig. 2 The band structure (left) and density of states (right) of the [1D-IMGW]− calculated by PBE. The Fermi level is set at zero. | ||
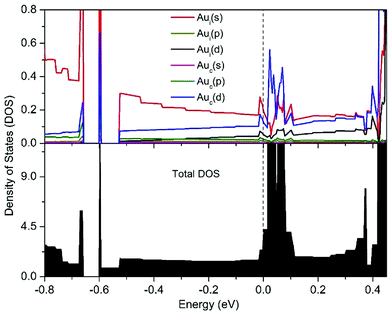 | ||
| Fig. 3 Calculated projection densities of states (PDOS) (up) and total densities of states (down) of the [1D-IMGW]−. | ||
To investigate the component of total DOS nearby the Fermi level of [1D-IMGW]−, the projection densities of states (PDOS) by calculating the total spin–orbit coupling effects for s, p and d orbitals for Au atoms in 1D gold wire, is presented in Fig. 3. Comparing the total DOS and the PDOS in Fig. 3, we can see the orbital-projected densities of states of Auc and Aui nearby the Fermi level are mainly 5d and 6s orbitals, respectively, indicating that there is a strong orbital interaction between the 5d orbitals of the Auc atoms and the 6s orbitals of the Aui atoms about the Fermi level. Therefore, as predicted by our previous research,22 1D-IMGW has remarkable conductivity and its electron conducting channel is along the monoatomic Au chains through the 5dz2 orbitals of the Auc atoms and the 6s orbitals of the Aui atoms.
3.3 The partial charge densities of [1D-IMGW]−
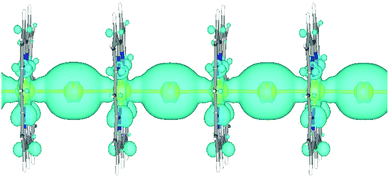
To further show the visualized strong interaction between the 5d orbitals of the Auc atoms and the 6s orbitals of the Aui atoms at Fermi level in [1D-IMGW]−, The partial charge densities of the Fermi level of [1D-IMGW]− are provided in Fig. 4. As seen from Fig. 4, the major contributions of the partial charge densities of the Fermi level arise from the strong σ bond formed by the 5dz2 orbitals of the Auc atoms and the 6s orbitals of the Aui atoms in the [1D-IMGW]−. Moreover, the extending σ bond in [1D-IMGW]− is very similar to that in 1D-1MGW and the charge densities on Aui of [1D-IMGW]− is much greater than that of 1D-IMGW, leading to the high stability for 1D-IMGW with electron transporting. Obviously, the conduction mechanism of 1D-IMGW is the electron transporting along the monoatomic Au chains perpendicular to the corrole rings through the electronic energy bands consisting of 5dz2 orbitals of the Auc atoms and the 6s orbitals of the Aui atoms.4. Conclusions
Based on our previous study of the newly-constructed 1D-infinite monatomic Au wire (1D-IMGW) with excellent conductivity, extensive density functional calculations have been performed to further investigate the electronic properties of 1D-IMGW after adding one electron ([1D-IMGW]−), which will bring us insight into the electron transport mechanism on 1D-IMGW. The optimized structure of the [1D-IMGW]− still remain linear, however, its electronic energy band structure displays a conductor property. Moreover, this theoretical work provide a method to study the electron transport mechanism of one-dimensional monoatomic Au chains and the calculated PDOS and partial charge densities indicate that the 1D-IMGW has extremely strong conductivity where electrons conduct along the monoatomic Au chains perpendicular to the corrole rings by their 5dz2 and 6s orbitals of Auc and Aui, respectively. Despite charging on the 1D-IMGW, the Auc and Aui still show mixing oxidation valence states of Au3+ and Au−.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge simulating discussions with Professors Zexing Cao, Jun Li and Yi Zhao. This work was supported by the National Natural Science Foundation of China (No. 21663008, 21763006, and 21503046), Natural Science Foundation of Guizhou Province of China (No. [2017]1029), Science and Technology Project of Guizhou Province (No. 2017[5788]), Natural Science Foundation of Guizhou Province of China (No. QKJ[2015]2122), Natural Science foundation of Department of Education of Guizhou Province (No. QJTD[2015]55), the construction project for Guizhou Provincial Key Disciplines (No. ZDXK[2015]10) and the undergraduate research program of GZEU (No. 2018DXS112). This project is partially supported by the Open Fund of Shaanxi Key Laboratory of Catalysis to J. X. L. (No. SXKLC-2017-01). We also thank the computational resources assistance by the State Key Laboratory of Physical Chemistry of Solid Surfaces (Xiamen University), the Shenzhen Supercomputing Center.Notes and references
- H. J. Seo, W. Jeong, S. Lee and G. D. Moon, Nanoscale, 2018, 10, 5424 RSC.
- L. Gao, L. Chen, H. Wei and H. Xu, Nanoscale, 2018, 10, 11923 RSC.
- R. MacKenzie, C. Fraschina, B. Dielacher, T. Sannomiya, A. B. Dahlin and J. Vörös, Nanoscale, 2013, 5, 4966 RSC.
- L. Shi, R. Wang, H. Zhai, Y. Liu, L. Gao and J. Sun, Phys. Chem. Chem. Phys., 2015, 17, 4231 RSC.
- C. J. Murphy and N. R. Jana, Adv. Mater., 2002, 14, 80 CrossRef CAS.
- T. M. Whitney, P. C. Searson, J. S. Jiang and C. L. Chien, Science, 1993, 261, 1316 CrossRef CAS PubMed.
- Z. L. Wang, Adv. Mater., 2000, 12, 1295 CrossRef CAS.
- M. Wu, J. D. Burton, E. Y. Tsymbal, X. C. Zeng and P. Jena, J. Am. Chem. Soc., 2012, 134, 14423 CrossRef CAS PubMed.
- T. Nishinaka, A. Takano, Y. Doi, M. Hashimoto, A. Nakamura, Y. Matsushita, J. Kumaki and E. Yashima, J. Am. Chem. Soc., 2005, 127, 8120 CrossRef CAS PubMed.
- H.-F. Wang, X.-Q. Gong, Y.-L. Guo, Y. Guo, G. Lu and P. Hu, J. Phys. Chem. C, 2009, 113, 6124 CrossRef CAS.
- F. Wang, Y. Dai, J. Zhao and Q. Li, Phys. Chem. Chem. Phys., 2014, 16, 24716 RSC.
- E. B. Guidez and C. M. Aikens, Nanoscale, 2012, 4, 4190 RSC.
- K. A. Dick and P. Caroff, Nanoscale, 2014, 6, 3006 RSC.
- L. Liu, J. Yuan, L. Cheng and J. Yang, Nanoscale, 2017, 9, 856 RSC.
- S. Ding, Y. Tian, J. Jiu and K. Suganuma, RSC Adv., 2018, 8, 2109 RSC.
- M. Hu, J. Gao, Y. Dong, S. Yang and R. K. Y. Li, RSC Adv., 2012, 2, 2055 RSC.
- G.-C. He, H. Lu, X.-Z. Dong, Y.-L. Zhang, J. Liu, C.-Q. Xie and Z.-S. Zhao, RSC Adv., 2018, 8, 24893 RSC.
- Y. C. Kim and S. Y. Kim, RSC Adv., 2018, 8, 19532 RSC.
- S. J. Percival, N. E. Vartanian and B. Zhang, RSC Adv., 2014, 4, 10491 RSC.
- X. Wang, X. Yin, H.-L. Yu and Y.-T. Liu, ChemistrySelect, 2018, 3, 6156 CrossRef CAS.
- H. Joshi, D. P. Rai, P. K. Patra, K. C. Bhamu and R. K. Thapa, Nanosci. Nanoeng., 2016, 4, 59 CAS.
- C. Zhu, J.-X. Liang and G. Wei, Phys. Chem. Chem. Phys., 2016, 18, 12338 RSC.
- H. Ohnishi, Y. Kondo and K. Takayanagi, Nature, 1998, 395, 780 CrossRef CAS.
- G. Rubio-Bollinger, S. R. Bahn, N. Agraït, K. W. Jacobsen and S. Vieira, Phys. Rev. Lett., 2001, 87, 026101 CrossRef.
- V. Rodrigues and D. Ugarte, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 073405 CrossRef.
- S. B. Legoas, D. S. Galvão, V. Rodrigues and D. Ugarte, Phys. Rev. Lett., 2002, 88, 076105 CrossRef PubMed.
- S. R. Bahn, N. Lopez, J. K. Nørskov and K. W. Jacobsen, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 081405 CrossRef.
- F. D. Novaes, A. J. R. da Silva, E. Z. da Silva and A. Fazzio, Phys. Rev. Lett., 2003, 90, 036101 CrossRef PubMed.
- N. V. Skorodumova and S. I. Simak, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 121404 CrossRef.
- X. Li, H. Lv, J. Dai, L. Ma, X. C. Zeng, X. Wu and J. Yang, J. Am. Chem. Soc., 2017, 139, 6290 CrossRef CAS PubMed.
- C. Hermosa, J. Vicente Álvarez, M.-R. Azani, C. J. Gómez-García, M. Fritz, J. M. Soler, J. Gómez-Herrero, C. Gómez-Navarro and F. Zamora, Nat. Commun., 2013, 4, 1709 CrossRef PubMed.
- J. Li, X. Li, H.-J. Zhai and L.-S. Wang, Science, 2003, 299, 864 CrossRef CAS PubMed.
- H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC.
- H. Schmidbaur, Gold Bull., 2000, 33, 3 CrossRef CAS.
- H. Schmidbaur, Gold Bull., 1990, 23, 11 CrossRef CAS.
- A. Muñoz-Castro and R. G. Maturana, J. Phys. Chem. C, 2014, 118, 21185 CrossRef.
- R. G. Maturana, M. P. Vargas and A. Muñoz-Castro, J. Phys. Chem. A, 2012, 116, 8737 CrossRef CAS PubMed.
- A. I. Yanson, G. R. Bollinger, H. E. van den Brom, N. Agrait and J. M. van Ruitenbeek, Nature, 1998, 395, 783 CrossRef CAS.
- R. H. M. Smit, C. Untiedt, A. I. Yanson and J. M. van Ruitenbeek, Phys. Rev. Lett., 2001, 87, 266102 CrossRef CAS PubMed.
- E. Z. da Silva, F. D. Novaes, A. J. R. da Silva and A. Fazzio, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 115411 CrossRef.
- A. Thakur, A. Kumar and P. K. Ahluwalia, AIP Conf. Proc., 2013, 1512, 190 CrossRef CAS.
- V. G. Pol, E. Koren and A. Zaban, Chem. Mater., 2008, 20, 3055 CrossRef CAS.
- C. Zhu, J. Liang, B. Wang, J. Zhu and Z. Cao, Phys. Chem. Chem. Phys., 2012, 14, 12800 RSC.
- I. Aviv and Z. Gross, Chem. Commun., 2007, 20, 1987 RSC.
- V. S. Shetti, U. R. Prabhu and M. Ravikanth, J. Org. Chem., 2010, 75, 4172 CrossRef CAS PubMed.
- D. T. Gryko, Eur. J. Org. Chem., 2002, 2002, 1735 CrossRef.
- I. Aviv-Harel and Z. Gross, Chem.–Eur. J., 2009, 15, 8382 CrossRef CAS PubMed.
- C. Zhu, J.-X. Liang and Z. Cao, J. Phys. Chem. C, 2018, 122, 20781 CrossRef CAS.
- C. Zhu and J.-X. Liang, New J. Chem., 2015, 39, 3624 RSC.
- C. Zhu, J. Liang and Z. Cao, J. Phys. Chem. C, 2013, 117, 13388 CrossRef CAS.
- C. Zhu and Z. Cao, Acta Chim. Sin., 2013, 71, 1527 CrossRef CAS.
- W. Liu, X. Huang, M.-J. Cheng, R. J. Nielsen, W. A. Goddard and J. T. Groves, Science, 2012, 337, 1322 CrossRef CAS PubMed.
- S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS PubMed.
- W. Nam, Acc. Chem. Res., 2007, 40, 522 CrossRef CAS PubMed.
- G. Golubkov, J. Bendix, H. B. Gray, A. Mahammed, I. Goldberg, A. J. DiBilio and Z. Gross, Angew. Chem., Int. Ed., 2001, 40, 2132 CrossRef CAS PubMed.
- E. Vogel, S. Will, A. S. Tilling, L. Neumann, J. Lex, E. Bill, A. X. Trautwein and K. Wieghardt, Angew. Chem., 1994, 106, 771 CrossRef CAS.
- H. Schlaich, G. G. Lindner, J. Feldmann, E. O. Göbel and D. Reinen, Inorg. Chem., 2000, 39, 2740 CrossRef CAS PubMed.
- A. E. Meier-Callahan, H. B. Gray and Z. Gross, Inorg. Chem., 2000, 39, 3605 CrossRef CAS PubMed.
- S. Will, J. Lex, E. Vogel, V. A. Adamian, E. Van Caemelbecke and K. M. Kadish, Inorg. Chem., 1996, 35, 5577 CrossRef CAS PubMed.
- G. Golubkov and Z. Gross, Angew. Chem., 2003, 115, 4645 CrossRef.
- J. Liang, C. Zhu and Z. Cao, Phys. Chem. Chem. Phys., 2013, 15, 13844 RSC.
- C. Zhu and J.-X. Liang, J. Power Sources, 2015, 283, 343 CrossRef CAS.
- E. Rabinovich, I. Goldberg and Z. Gross, Chem.–Eur. J., 2011, 17, 12294 CrossRef CAS PubMed.
- H. He, P. Zapol and L. A. Curtiss, J. Phys. Chem. C, 2010, 114, 21474 CrossRef CAS.
- C. Ouyang, Y. Du, S. Shi and M. Lei, Phys. Lett. A, 2009, 373, 2796 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558 CrossRef CAS.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef.
- D. Mac-Leod Carey, C. Morales-Verdejo, A. Muñoz-Castro, F. Burgos, D. Abril, C. Adams, E. Molins, O. Cador, I. Chávez, J. M. Manríquez, R. Arratia-Pérez and J. Y. Saillard, Polyhedron, 2010, 29, 1137 CrossRef CAS.
- A. Muñoz-Castro, D. Mac-Leod Carey, C. Morales-Verdejo, I. Chávez, J. M. Manríquez and R. Arratia-Pérez, Inorg. Chem., 2010, 49, 4175 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2019 |