Open Access Article
Open Access ArticleSulfonated carbon derived from the residue obtained after recovery of essential oil from the leaves of Cinnamomum longepaniculatum using Brønsted acid ionic liquid, and its use in the preparation of ellagic acid and gallic acid
Zaizhi Liu *ac,
Yanlong Qi
*ac,
Yanlong Qi b,
Mengling Guia,
Chunte Fengc,
Xun Wangc and
Yang Lei*c
b,
Mengling Guia,
Chunte Fengc,
Xun Wangc and
Yang Lei*c
aCollege of Life Sciences, Jiangxi Normal University, Nanchang 330022, China. E-mail: zaizhiliu@hotmail.com
bKey Laboratory of Synthetic Rubber, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Renmin Road, Changchun 5625, P. R. China
cKey Laboratory of Forest Plant Ecology, Ministry of Education, Northeast Forestry University, Harbin 150040, China. E-mail: yanglei@nefu.edu.cn
First published on 11th February 2019
Abstract
A Brønsted acid ionic liquid, 3-methyl-1-(4-sulfonylbutyl) imidazolium hydrogensulfate ([HO3S(CH2)4mim]HSO4), was used for the first time for the preparation of a sulfonated carbon catalyst. The catalyst was prepared from the residue obtained after recovery of the essential oil from the leaves of Cinnamomum longepaniculatum. The sulfonated carbon catalyst with an amorphous structure attained high acidic efficiency at a sulfonation temperature of 200 °C for 2 h of sulfonation time, and was characterised. SEM morphologies revealed that the carbon catalyst consisted of uniform carbon microspores. FTIR analysis, elemental analysis, and X-ray photoelectron spectroscopy revealed that the sulfonic acid group was successfully introduced on the surface of the sulfonated carbon catalyst. The result of TG analysis showed that the obtained sulfonated carbon catalyst has high thermal stability. Good acid and catalytic activity of the obtained sulfonated carbon catalyst were observed for the preparation of ellagic acid and gallic acid, which is comparable to those of diluted sulfuric acid and a sulfonated carbon catalyst that had been prepared with concentrated sulfuric acid. The excellent reusability of the sulfonated carbon catalyst was also confirmed by repeated experimental trials. In summary, the sulfonated catalyst derived from the residue obtained after recovery of essential oil from the leaves of C. longepaniculatum is an economic, eco-benign and promising substitute for traditional mineral acid catalysts for acidic catalysis in industrial applications.
1. Introduction
With diminishing fossil reserves and increasingly prominent environment problems, the comprehensive utilization of renewable resources and the production of platform chemicals have attracted great interest.1,2 As an important renewable resource for human survival and development, biomass accounts for a large proportion in nature and thus has a considerable potential to be used in the preparation of high value-added products through a series of varying bio-refinery procedures.3 So far, the use of biomass as raw materials to produce fine chemical products (e.g., catalytic materials) is becoming increasingly attractive for both academic research and practical purposes owing to their multiple advantages.4,5 In light of “green chemistry”, carbonaceous catalysts are versatile and have emerged as promising solid catalysts for chemical reactions.6,7 Cinnamomum longepaniculatum, belonging to the Lauraceae family, is rich in essential oil (main source of eucalyptol). For this reason, C. longepaniculatum has been widely cultivated and distributed in southwestern China as an economic crop, especially in the Yibin region (Sichuan province).8,9 Each year, a large amount of residues after the extraction of essential oil are produced and discarded as byproducts; hence, a study focusing on the biorefining of this valuable plant biomass to aid the industrial chemistry is urgently needed to achieve an integrated exploitation of C. longepaniculatum as a source of natural products. Therefore, the residue obtained after the recovery of essential oil from the leaves of C. longepaniculatum would be an ideal raw material for post-processing of carbonaceous catalysts.Ellagic acid and gallic acid are two important natural polyphenols with many bioactivities, which include antioxidant,10 preservative effects,11 antimycobacterial activity,12 anti-inflammatory and antitumor effects,13 and hence, they are widely used in food, pharmaceutical and cosmetic industries. Ellagic acid and gallic acid are present in the natural world in their free forms or as derivatives, and vary based on tree species;14 they are generally found as their derivatives (e.g., gallotannins and ellagitannins) in plant extracts.15 Conventionally, these two compounds are obtained mainly through several extraction methods involving heating or consumption of volatile organic solvents as well as acid hydrolysis of their derivatives with mineral acids as catalysts.16 Apart from the limitations of these methods,17 the use of homogeneous inorganic acid as catalysts in the hydrolysis procedures still has many disadvantages, such as the naturally corrosive threat on equipments, the high production of undesirable byproducts, sensitivity to water, tedious isolation process and the large input on the treatment of the acid-containing residues.18–21
Recently, sulfonated carbonaceous catalysts appeared as promising “green solid catalysts” for the application in acidic catalysis22,23 because of their remarkable and stable solid acid characteristics, low cost, and easy availability through simple treatments.24–26 Nonetheless, a previous preparation process of sulfonated carbonaceous catalysts involves the post-processing of a carbon material for activation with concentrated sulfuric acid or other sulfonation compounds.27,28 Brønsted acid ionic liquids were first synthesized in 2002 by Cole et al.29 and then researches conducted using them rapidly caught attention, mainly because Brønsted acid ionic liquids (especially functionalized with –SO3H group) not only exhibit strong Brønsted acidity, but also possess other remarkable characteristics just as other ionic liquids.30 To date, no attempt has been reported on the use of a Brønsted acid ionic liquid to substitute the traditional –SO3H group donors (e.g., sulfuric acid) to prepare sulfonated carbonaceous catalysts for acidic hydrolysis purposes.
In our previous study, a Brønsted acid ionic liquid 3-methyl-1-(4-sulfonylbutyl) imidazolium hydrogensulfate ([HO3S(CH2)4mim]HSO4) was used as the dual extractant and catalyst for obtaining ellagic acid and gallic acid from crude extracts of Eucalyptus globulus leaves. However, the requirement for the purchase of [HO3S(CH2)4mim]HSO4 and recycling it from crude extracts in the industrial processes were the main concerns. In the present study, the residue obtained after recovery of the essential oil from the leaves of C. longepaniculatum was used as the raw material and [HO3S(CH2)4mim]HSO4 was first introduced in the preparation process of the sulfonated carbonaceous catalyst to replace the traditionally used concentrated sulfuric acid or other sulfonation compounds.
The synthesized sulfonated carbonaceous catalyst was successively characterized by X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), N2 adsorption–desorption isotherms, Fourier Transform Infrared Spectroscopy (FTIR), Thermogravimetric (TG) analysis, Elemental Analysis (EA), and X-ray Photoelectron Spectroscopy (XPS). The synthesized sulfonated carbonaceous catalyst was used to replace the liquid acidic catalysts in the preparation of ellagic acid and gallic acid by hydrolysis of their derivatives from E. globulus leaves. The performance of the prepared sulfonated carbonaceous catalyst was compared to that of the diluted sulfuric acid. Catalyst ability of the recycled sulfonated carbonaceous catalyst was also investigated to study its reusability.
2. Materials and methods
2.1. Chemicals and materials
Chromatographically pure (purity ≥ 95%) ellagic acid (CAS no. 476-66-4) and gallic acid (CAS no. 149-91-7) were obtained from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) as standards. [HO3S(CH2)4mim]HSO4 was provided from Chengjie Chemical Co. Ltd., (Shanghai, China) and used without further purification. Chromatographic grade methanol and acetic acid for the HPLC analysis, and spectral grade potassium bromide used for FTIR analysis were bought from Aladdin Reagent Co. Ltd., (Shanghai, China). Other chemicals used were of analytical grade and were purchased from Beijing Chemical Reagents Co. Ltd., (Beijing, China). A Milli-Q Water Purification system (Millipore, Waltham, MA, USA) was used for the depurated distilled water. Sample solutions were filtered using a 0.45 μm nylon membrane before subjecting to HPLC analysis.Dried C. longepaniculatum and E. globulus leaves were picked in autumn from Yibin city (Sichuan, China) and authorized by Professor Kailin Mo (Sichuan Academy of Forestry, China). All the samples were mashed and sieved into a proper size (≤0.425 mm) and kept in a desiccator at ambient temperature before use. E. globulus crude extracts were extracted as described by Shin et al.31 The residue obtained after recovery of the essential oil from the leaves of C. longepaniculatum was pre-extracted of essential oil and then dried prior to the usage as raw materials for the preparation of sulfonated carbonaceous catalyst.
2.2. Preparation of the sulfonated carbon catalyst
The starting materials (20 g) of the residue obtained after recovery of the essential oil from the leaves of Cinnamomum longepaniculatum was initially carbonized with a heating speed of 30 °C min−1 to 400 °C for 6 h under N2 flow to prepare a black solid, namely, carbonaceous materials. The above black powder (2 g) was separately mixed with pure [HO3S(CH2)4mim]HSO4 or concentrated sulfuric acid and subsequently heated under a set temperature for a pre-set sulfonation time to introduce the –SO3H group onto the carbonaceous materials' surface, and then cooled to atmospheric temperature. The sulfonation reaction was performed in a Teflon autoclave under pure N2 atmosphere and a hot air oven was used for heating to the pre-set sulfonation temperature.32 The reaction mixtures were then washed three times by adding 100 mL of hot distilled water (80 °C), followed by the separation of black precipitates from the suspension by centrifugation. The washing processes were repeated until no impurities (e.g., sulfate ions) were determined in the washing water. The black solid was then placed in an oven and dried for 12 h at 80 °C. The parameters of [HO3S(CH2)4mim]HSO4 amount, sulfonation temperature and time were investigated by single factor experiments during the preparation process of the sulfonated carbon catalyst.2.3. Catalytic tests
The heat reflux method was carried out to test the catalytic capacity of the prepared sulfonated carbon catalysts using [HO3S(CH2)4mim]HSO4 or concentrated sulfuric acid and was compared to that of the diluted sulfuric acid for the hydrolysis of E. globulus crude extracts. Briefly, E. globulus leaves crude extracts solution was mixed with 5% of the prepared sulfonated carbon catalyst or the same acidity (pH 1.0) of diluted sulfuric acid to make a comparison and then treated for 4 h by an electric jacket at 1000 W. After the treatment was completed, the solutions (1 mL) were filtered and then analyzed by HPLC analysis to measure the yields of the two target compounds; their yields were expressed as milligram of ellagic acid and gallic acid per gram E. globulus leaf powder. The experiments were carried out in triplicate, and the yields of the two target compounds were denoted as mean ± SD. The HPLC analysis program for the analysis of the obtained ellagic acid and gallic acid was based on our previous study.14 The remaining reaction mixtures were isolated by centrifugation, and the precipitates were recovered through ethanol washing and drying at 80 °C before using for the reusability test.2.4. Physical measurement
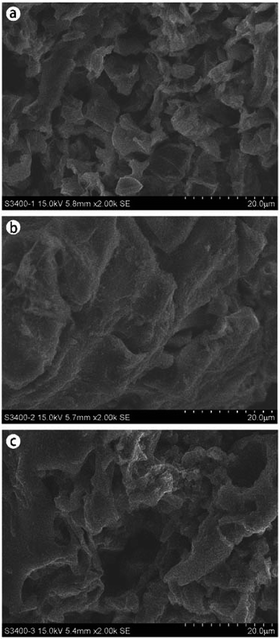
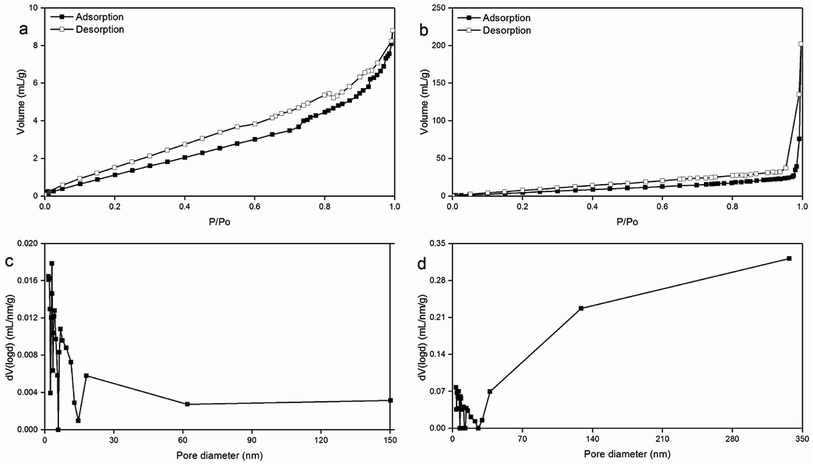
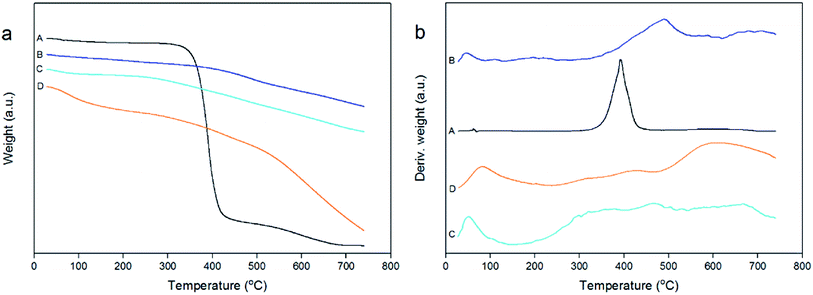
XRD patterns of the carbonaceous materials before and after the sulfonation treatment were collected using an X-ray diffractometer with Cu-Kα radiation (30 mA, 40 kV) at a step length of 0.02° over 2θ with a range from 5° to 60°. SEM morphologies were recorded on an S-3400N scanning electron microscope (HITACHI, Japan). The sulfonated carbon catalysts prepared using concentrated sulfuric acid and [HO3S(CH2)4mim]HSO4 were characterized by N2 adsorption–desorption isotherms using a Quantachrome Autosorb-iQ apparatus. The samples were prepared as KBr discs and the spectra were recorded using a Magna-IR 560 (Nicolet, USA), and the wavenumber range of the IR spectrometer was 4000–500 cm−1. TG analyses of carbonaceous materials and sulfonated carbon catalysts were performed on a STA 409PC thermo-gravimetrical analyzer (NETZSCH, Germany) and the heating program was conducted with the temperature increasing from 26 °C to 750 °C at a rate of 20 °C min−1 with the total run time of 36.20 min. EA of the carbon materials before and after the sulfonation treatment was studied using a VARIO ELcube Elemental Analyzer (Elementar, Germany) with the oxygen percentage calculated by content difference. XPS analysis was performed by an Axis THERMO X-ray photoelectron spectroscopy (Thermo Electron American) to detect the surface structures of the carbonaceous materials and sulfonated carbon materials. The acidic site contents of the sulfonated carbon catalysts that were prepared by [HO3S(CH2)4mim]HSO4 was determined by acid–base titration method as described by Xu et al.333. Results and discussion
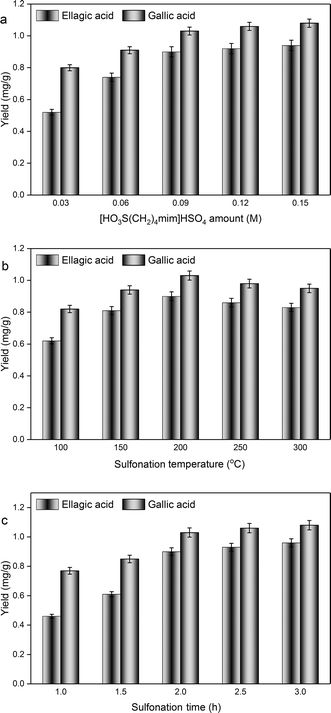
3.1. Single factor experiment
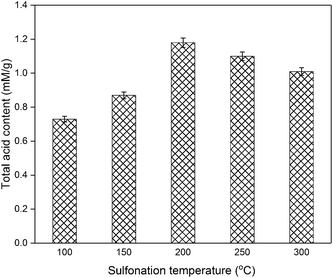
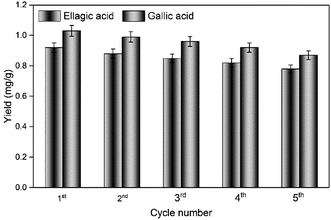
Based on these results, the parameters for the preparation of C. longepaniculatum sulfonated carbon catalyst using [HO3S(CH2)4mim]HSO4 were as follows: 0.09 M of [HO3S(CH2)4mim]HSO4, 200 °C as the sulfonation temperature, and 2 h as the sulfonation time. Under these conditions, the prepared sulfonated carbon catalyst possessed high acidic capacity with yields of 0.90 ± 0.03 mg g−1 for ellagic acid and 1.03 ± 0.02 mg g−1 for gallic acid, which is considerably higher than those prepared with diluted sulfuric acid (0.98 ± 0.03 mg g−1 for ellagic acid and 1.16 ± 0.04 mg g−1 for gallic acid) and concentrated sulfuric acid (0.94 ± 0.02 mg g−1 for ellagic acid and 1.10 ± 0.03 mg g−1 for gallic acid).
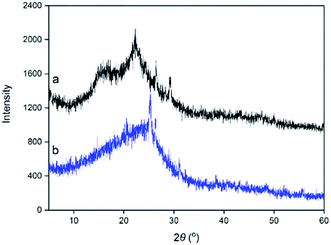
3.2. Structural characterization of the obtained C. longepaniculatum sulfonated carbon catalysts
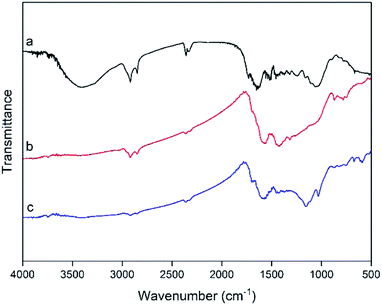
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of carboxyl (–COOH), while the band at 1600 cm−1 can be assigned to the carbon–carbon double bond (C
O) of carboxyl (–COOH), while the band at 1600 cm−1 can be assigned to the carbon–carbon double bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of the poly-aromatic rings in the carbonaceous materials and sulfonated carbon catalyst.39 The peak around 634 cm−1 was attributed to S
C) of the poly-aromatic rings in the carbonaceous materials and sulfonated carbon catalyst.39 The peak around 634 cm−1 was attributed to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and the vibration bands at 1155 cm−1 and 1032 cm−1 were assigned to symmetrical stretching vibration bands in O
O stretching vibration, and the vibration bands at 1155 cm−1 and 1032 cm−1 were assigned to symmetrical stretching vibration bands in O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and SO32− stretching vibrations, which clearly indicated that the prepared sulfonated carbon catalyst surface was functionalized by the use of [HO3S(CH2)4mim]HSO4.40–43 The significant peak at around 1450 cm−1 illustrated the presence of CH2 bending mode, and the aliphatic areas (CH2–CH2 chains) were sufficiently abundant, as confirmed in the infrared spectrum.44 Therefore, the FTIR spectrum clearly suggests that after the sulfonation process using [HO3S(CH2)4mim]HSO4, –SO3H groups have been successfully introduced into the C. longepaniculatum leaves-derived catalyst.
O and SO32− stretching vibrations, which clearly indicated that the prepared sulfonated carbon catalyst surface was functionalized by the use of [HO3S(CH2)4mim]HSO4.40–43 The significant peak at around 1450 cm−1 illustrated the presence of CH2 bending mode, and the aliphatic areas (CH2–CH2 chains) were sufficiently abundant, as confirmed in the infrared spectrum.44 Therefore, the FTIR spectrum clearly suggests that after the sulfonation process using [HO3S(CH2)4mim]HSO4, –SO3H groups have been successfully introduced into the C. longepaniculatum leaves-derived catalyst.
| Samples | C% | H% | O% | S% |
|---|---|---|---|---|
| Carbonaceous materials | 74.52 ± 2.32 | 3.60 ± 0.12 | 21.61 ± 0.54 | 0.27 ± 0.01 |
| Sulfonated carbon catalyst prepared by [HO3S(CH2)4mim]HSO4 | 66.46 ± 1.86 | 3.32 ± 0.08 | 26.95 ± 0.60 | 3.27 ± 0.06 |
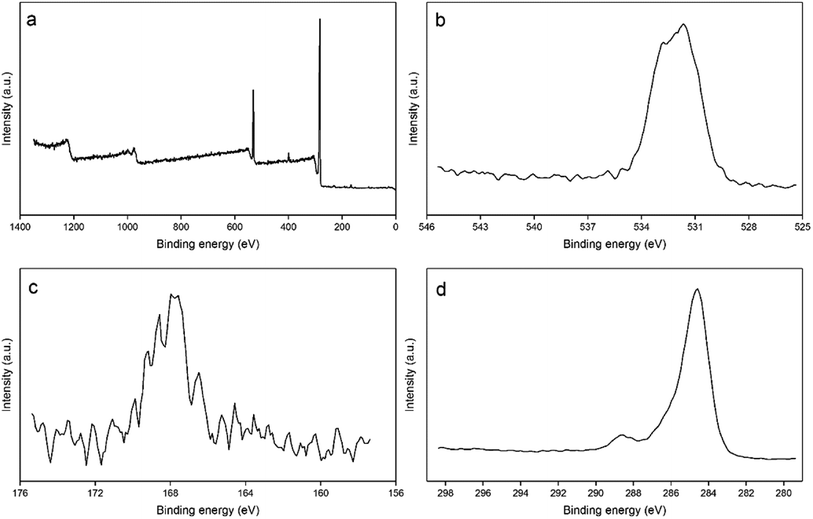
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. Fig. 7c shows the high resolution spectra of S 2p. The two sulfur peaks with binding energies at 164.0 and 165.2 eV can be ascribed to the S 2p3/2 and S 2p1/2 of thiophenic sulphur (–C–S–C–), respectively.47 Fig. 7d displays the high resolution spectra of C 1s. The main characteristic peak at 284.5 eV can be assigned to carbon atoms with sp2 hybridization. Based on the literature,48 the peaks at 288.6 eV and 284.6 eV belong to C–C and C–O, respectively.
O, respectively. Fig. 7c shows the high resolution spectra of S 2p. The two sulfur peaks with binding energies at 164.0 and 165.2 eV can be ascribed to the S 2p3/2 and S 2p1/2 of thiophenic sulphur (–C–S–C–), respectively.47 Fig. 7d displays the high resolution spectra of C 1s. The main characteristic peak at 284.5 eV can be assigned to carbon atoms with sp2 hybridization. Based on the literature,48 the peaks at 288.6 eV and 284.6 eV belong to C–C and C–O, respectively.
4. Conclusions
An attempt was carried out towards the utilization of the residue obtained after the recovery of essential oil from the C. longepaniculatum leaves for the preparation of sulfonated carbon catalyst by using [HO3S(CH2)4mim]HSO4. The sulfonation conditions for the preparation of the C. longepaniculatum sulfonated carbon catalyst were as follows: 0.09 M of [HO3S(CH2)4mim]HSO4, 200 °C as the sulfonation temperature, and 2 h as the sulfonation time. XRD results revealed that the prepared sulfonated carbon catalyst has an amorphous carbon structure. The –SO3H functionalization on the obtained sulfonated carbon catalyst surface was characterized by XRD, SEM, N2 adsorption–desorption isotherms, FTIR spectroscopy, XPS and EA analysis. TG analysis indicated that the obtained sulfonated carbon catalyst has a high thermal stability. The obtained sulfonated carbon catalyst was used in the catalysis of hydrolysable tannins contained in Eucalyptus globulus leaves to prepare ellagic acid and gallic acid. The results revealed that the obtained sulfonated carbon catalyst from the residue obtained after the recovery of essential oil from the leaves of Cinnamomum longepaniculatum possesses high catalytic activity and high reuse capacity.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors thank the Fundamental Research Fund by the National Natural Science Foundation of China (31860189) for financial support.References
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC.
- L. R. Lynd, P. J. Weimer, W. H. van Zyl and I. S. Pretorius, Microbiol. Mol. Biol. Rev., 2002, 66, 506 CrossRef CAS PubMed.
- M. Tao, H. Guan, X. Wang, Y. Liu and R. F. Louh, Fuel Process. Technol., 2015, 138, 355 CrossRef CAS.
- S. Chen, S. Tang, Y. Sun, G. Wang, H. Chen, X. Yu, Y. Su and G. Chen, Materials, 2018, 11, 1407 CrossRef PubMed.
- T. Teng, T. C. Hsiao and C. C. Chung, Materials, 2018, 11, 1315 CrossRef PubMed.
- D. Mun, A. T. H. Vo, B. Kim, Y. G. Shul and J. K. Cho, Catal. Commun., 2017, 96, 32 CrossRef CAS.
- D. R. Lathiya, D. V. Bhatt and K. C. Maheria, Bioresource Technology Reports, 2018, 2, 69 CrossRef.
- C. Tao, Afr. J. Pharm. Pharmacol., 2013, 7, 1148 CrossRef.
- Z. Luo, W. Li, Q. Wei and T. Luo, J. Sichuan Norm. Univ., 2001, 24, 317 CAS.
- Y. Amakura, M. Yoshimura, N. Sugimoto, T. Yamazaki and T. Yoshida, Biosci., Biotechnol., Biochem., 2009, 73, 1060–1065 CrossRef CAS PubMed.
- B. Song, Shaanxi Huagong, 1988, 3, 14 Search PubMed.
- P. Fyhrquist, I. Laakso, S. Garcia Marco, R. Julkunen-Tiitto and R. S. Hiltunen, S. Afr. J. Bot., 2014, 90, 1 CrossRef CAS.
- M. Albrecht, W. Jiang, J. Kumi-Diaka, E. P. Lansky, L. M. Commersall, A. Patel, R. Mansel, I. Neeman, A. A. Geldof and M. J. Campbell, J. Med. Food, 2004, 7, 274 CrossRef CAS PubMed.
- Z. Liu, Z. Chen, F. Han, X. Kang, H. Gu and L. Yang, Ind. Crops Prod., 2016, 81, 152 CrossRef CAS.
- I. Mueller-Harvey, Anim. Feed Sci. Technol., 2001, 91, 3 CrossRef CAS.
- S. H. Häkkinen, S. O. Kärenlampi, H. M. Mykkänen, I. M. Heinonen and A. R. Törrönen, Eur. Food Res. Technol., 2000, 212, 75–80 CrossRef.
- J. M. Cruz, H. Domínguez and J. C. Parajó, Food Chem., 2005, 90, 503 CrossRef CAS.
- S. Gamez, J. J. Gonzalez-Cabriales, J. A. Ramirez, G. Garrote and M. Vazquez, J. Food Eng., 2006, 74, 78 CrossRef CAS.
- K. Weerasai, V. Champreda, C. Sakdaronnarong, A. Shotipruk and N. Laosiripojana, Food Bioprod. Process., 2018, 110, 136 CrossRef CAS.
- P. Lenihan, A. Orozco, E. O’ Neill, M. N. M. Ahmad, D. W. Rooney and G. M. Walker, Chem. Eng. J., 2010, 156, 395 CrossRef CAS.
- K. Karimi, S. Kheradmandinia and M. J. Taherzadeh, Biomass Bioenergy, 2006, 30, 247 CrossRef CAS.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2011, 13, 520 RSC.
- Y. Jiang, X. Li, X. Wang, L. Meng, H. Wang, L. Wang, X. Wang and X. Mu, Green Chem., 2012, 14, 2162 RSC.
- V. L. Budarin, J. H. Clark, R. Luque and D. J. Macquarrie, Chem. Commun., 2007, 6, 634 RSC.
- S. Dora, T. Bhaskar, R. Singh, D. V. Naik and D. K. Adhikari, Bioresour. Technol., 2012, 120, 318 CrossRef CAS PubMed.
- B. L. A. Prabhavathi Devi, K. N. Gangadhar, P. S. Sai Prasad, B. Jagannadh and R. B. N. Prasad, ChemSusChem, 2009, 2, 617 CrossRef CAS PubMed.
- Y. Wang, F. Delbecq, W. Kwapinski and C. Len, Mol. Catal., 2017, 438, 167 CrossRef CAS.
- P. Ergenekon, E. Gürbulak and B. Keskinler, Chem. Eng. Process., 2011, 50, 16 CrossRef CAS.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis Jr., J. Am. Chem. Soc., 2002, 124, 5962 CrossRef CAS PubMed.
- A. Pourjavadi, S. H. Hosseini and R. Soleyman, J. Mol. Catal. A: Chem., 2012, 365, 55 CrossRef CAS.
- Y. Shin, M. S. Shon, G. N. Kim and S. C. Lee, Food Sci. Biotechnol., 2014, 23, 1689 CrossRef CAS.
- K. Ngaosuwan, J. G. Goodwin Jr. and P. Prasertdham, Renewable Energy, 2016, 86, 262 CrossRef CAS.
- Q. Xu, Y. Xia, D. Yin, C. Fan and Z. Xu, New Carbon Mater., 2011, 26, 103 CAS.
- R. Liu, X. Wang, X. Zhao and P. Feng, Carbon, 2008, 46, 1664 CrossRef CAS.
- J. R. Kastner, J. Miller, D. P. Geller, J. Locklin, L. H. Keith and T. Johnson, Catal. Today, 2012, 190, 122 CrossRef CAS.
- S. Niu, Y. Ning, C. Lu, K. Han, H. Yu and Y. Zhou, Energy Convers. Manage., 2018, 163, 59 CrossRef CAS.
- B. Manoj and A. G. Kunjomana, Int. J. Electrochem. Sci., 2012, 7, 3127 CAS.
- V. Boonamnuayvitaya, C. Chaiya and W. Tanthapanichakoon, J. Chem. Eng. Jpn., 2004, 37, 1504 CrossRef CAS.
- W. W. Mar and E. Somsook, Procedia Eng., 2012, 32, 212 CrossRef CAS.
- S. Suganuma, K. Nakajima, M. Kitano, H. Kato, A. Tamura, H. Kondo, S. Yanagawa, S. Hayashi and M. Hara, Microporous Mesoporous Mater., 2011, 143, 443 CrossRef CAS.
- Z. Jia, Z. Li, S. Li, Y. Li and R. Zhu, J. Mol. Liq., 2016, 220, 56 CrossRef CAS.
- S. Imaizumi, H. Matsumoto, M. Ashizawa, M. Minagawa and A. Tanioka, RSC Adv., 2012, 2, 3109 RSC.
- K. Nakajima and M. Hara, ACS Catal., 2012, 2, 1296 CrossRef CAS.
- A. C. Zaman, J. Mol. Liq., 2018, 249, 892 CrossRef CAS.
- I. Ogino, Y. Suzuki and S. R. Mukai, Catal. Today, 2018, 314, 62 CrossRef CAS.
- C. Zhang, Z. Fu, Y. Liu, B. Dai, Y. Zou, X. Gong, Y. Wang, X. Deng, H. Wu, Q. Xu, K. R. Stevena and D. Yin, Ind. Eng. Chem. Res., 2012, 14, 1928 CAS.
- J. Li, Y. Wang, B. Lu, Y. Wang, T. Deng and X. Hou, Appl. Catal., A, 2018, 566, 140 CrossRef CAS.
- J. Zhou, H. Li, G. Lin and H. Zhang, Acta Phys.-Chim. Sin., 2010, 26, 3080 CAS.
| This journal is © The Royal Society of Chemistry 2019 |