Open Access Article
Open Access ArticleHomogeneous hydroformylation of long chain alkenes catalyzed by water soluble phosphine rhodium complex in CH3OH and efficient catalyst cycling
Yan-li Liu,
Jian-gui Zhao,
Yuan-jiang Zhao,
Hui-Min Liu,
Hai-yan Fu,
Xue-li Zheng *,
Mao-lin Yuan
*,
Mao-lin Yuan *,
Rui-xiang Li
*,
Rui-xiang Li and
Hua Chen
and
Hua Chen
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, P. R. China. E-mail: cdscyml@163.com; zhengxueli@scu.edu.cn; Fax: +86-28-8541-2904
First published on 6th March 2019
Abstract
The hydroformylation of long chain alkenes catalyzed by a water soluble Rh/TPPTS complex (TPPTS: sodium salt of sulfonated triphenylphosphine) in methanol was investigated. The mixture of rhodium precursor HRh(CO)(TPPTS)3, ligand TPPTS, methanol and a long chain alkene becomes a single phase under reaction conditions, which make the hydroformylation reaction proceed homogeneously. Both the conversion of long chain alkene and the selectivity to aldehydes (including the aldehydes forming methylacetals) could reach up to 97.8% and 97.6%, respectively, with 3323 h−1 of TOF (TOF: turnover frequency is defined as the moles of converted alkene per mole of Rh per hour). After the solvent methanol was removed under the reaction temperature, two phases were formed automatically. The colourless product phase could be efficiently separated from the precipitate rhodium catalyst phase by centrifuge. The catalyst was reused for five times without obvious loss of rhodium and the catalytic activity. The rhodium leaching in product mixture was less than 0.03% of the total rhodium.
1. Introduction
For more than 80 years, the chemical industry has relied on the hydroformylation reaction to convert alkenes, CO and H2 into aldehydes which were widely used in making plasticizers, pharmaceuticals, lubricants, amines and so on.1–10 Nowadays, the production capacity is beyond 12 million tons annually.11,12 Rhodium-based homogeneous catalyst was found to be far more active to hydroformylation of alkene because of its superior activity, allowing the process at a lower temperature and pressure, and the ability to control the selectivity.13 However, the traditional homogeneous catalysis confronts difficulties related to the separation of products from a catalyst as well as the recycling of the catalyst.14 Especially with regard to long chain alkenes, the thermal separation of the products with high boiling points would put thermal stress for the temperature-sensitive catalyst and result in slow degradation of the catalyst under these conditions.Aqueous-organic biphasic catalysis possessing many advantages such as convenient separation of product, environmentally friendly and economy,15–22 was successfully applied to the hydroformylation of ethene and propene on plant because their solubilities in the aqueous phase are sufficient to allow the hydroformylation to occur at an acceptable rate without phase-transfer limitations. Nevertheless, long chain alkenes (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C6) have significantly lower solubility in the aqueous solution of the catalyst, which correspondingly leads to lower reaction rates in aqueous-organic biphasic systems, making it difficult and economically unviable. Many approaches have been developed to overcome the barrier from phase transfer in aqueous-organic biphasic systems such as using cosolvents, surfactants and ionic liquids to increase the miscibility or the interfacial area between two solvents, but they have some disadvantages, such as emulsion or a higher catalyst leaching.23–27 Therefore, seeking for a catalytic system which could cover the advantage of homogeneous (good reactivity) and biphasic catalysis (easy separation) is highly desirable.28 As well known, the amount of rhodium and ligand used in hydroformylation is quite low, so it may be expected that a low boiling point polar solvent (i.e., methanol) could be selected to dissolve water soluble phosphine rhodium complex, ligand and alkene under the reaction temperature and could be helpful for the formation of homogeneous hydroformylation. Once the hydroformylation finished, the low boiling polar solvent was evacuated under lower temperature than that of product aldehydes. Rhodium catalyst precipitated as the form of solid since it is difficult to dissolve in the product aldehydes, and then the resulting mixture became solid (catalyst)–liquid (aldehydes) two phases. Therefore, the catalyst could be readily separated and reused for the next cycle.
C6) have significantly lower solubility in the aqueous solution of the catalyst, which correspondingly leads to lower reaction rates in aqueous-organic biphasic systems, making it difficult and economically unviable. Many approaches have been developed to overcome the barrier from phase transfer in aqueous-organic biphasic systems such as using cosolvents, surfactants and ionic liquids to increase the miscibility or the interfacial area between two solvents, but they have some disadvantages, such as emulsion or a higher catalyst leaching.23–27 Therefore, seeking for a catalytic system which could cover the advantage of homogeneous (good reactivity) and biphasic catalysis (easy separation) is highly desirable.28 As well known, the amount of rhodium and ligand used in hydroformylation is quite low, so it may be expected that a low boiling point polar solvent (i.e., methanol) could be selected to dissolve water soluble phosphine rhodium complex, ligand and alkene under the reaction temperature and could be helpful for the formation of homogeneous hydroformylation. Once the hydroformylation finished, the low boiling polar solvent was evacuated under lower temperature than that of product aldehydes. Rhodium catalyst precipitated as the form of solid since it is difficult to dissolve in the product aldehydes, and then the resulting mixture became solid (catalyst)–liquid (aldehydes) two phases. Therefore, the catalyst could be readily separated and reused for the next cycle.
Herein, methanol is chosen to improve the hydroformylation of long chain alkenes catalyzed by a water soluble Rh/TPPTS complex. This is the first example on taking advantages of both homogeneous reaction and heterogeneous separation of catalyst from product.
2. Experimental
2.1 Materials
Ligand TPPTS (sodium salt of sulfonated triphenylphosphine, Fig. 1) and rhodium precursor HRh(CO)(TPPTS)3 were prepared according to the literatures.29,30 All the solvents were purchased from Kelong Chemical Co. of Chengdu and used without any additional purification. 1-Hexene, 1-octene, 1-decene, 1-dodecene, cyclohexene, norbornene and dicyclopentadiene were purchased from Sigma-Aldrich. Hydrogen (H2, 99.99%) and carbon monoxide (CO, 99.9%) were obtained from Southwest Institute. The composition of reaction mixture was determined by means of gas chromatography (PANNA A91, KB-1: 30 m × 0.25 mm × 0.50 μm, FID). Rhodium leaching in the product mixture was analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES). 31P NMR spectra were recorded on the Bruker AVANCE III HD 400 M.2.2 Hydroformylation and catalyst recycling
Long chain alkene hydroformylation experiments were carried out in a 25 mL stainless steel autoclave equipped with a magnetic stirrer. Typically HRh(CO)(TPPTS)3, TPPTS, long chain alkene and a low boiling point polar solvent were loaded into the autoclave, which was sealed, purged with syngas (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO = 1.0
CO = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0) for three times and charged to the desired pressure and heated to the preset temperature. Then the stirrer was started and the time was recorded. After the reaction was complete, the autoclave was cooled down to room temperature, and the gas was carefully released. The products were analyzed by GC (PANNA A91 on a KB-1 capillary column 30 m × 0.25 mm × 0.50 μm, FID).
1.0) for three times and charged to the desired pressure and heated to the preset temperature. Then the stirrer was started and the time was recorded. After the reaction was complete, the autoclave was cooled down to room temperature, and the gas was carefully released. The products were analyzed by GC (PANNA A91 on a KB-1 capillary column 30 m × 0.25 mm × 0.50 μm, FID).
After hydroformylation, the methanol in the product mixture was removed via distillation under the reaction temperature and the remained mixture became two phases. The upper product phase was separated from the lower precipitate catalyst phase by centrifuge and filtration. Then the distillated methanol and a new portion of long chain alkene were introduced for the next catalytic reaction.
3. Results and discussion
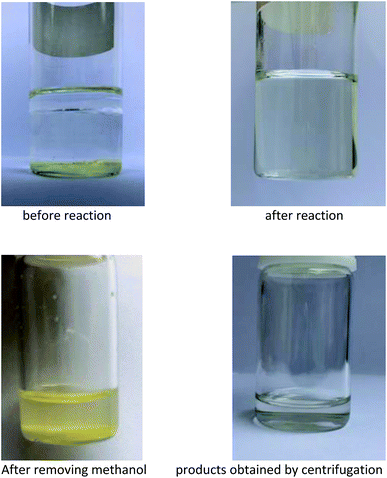
Long chain alkenes have significantly lower solubility in the aqueous solution of the catalyst, which correspondingly leads to lower reaction rates in biphasic catalytic system, and cosolvent was found to have a great impact on the hydroformylation of long chain alkenes catalyzed by water soluble phosphine rhodium complex.31 In this study, different low boiling polar solvents were investigated in the hydroformylation of 1-dodecene using HRh(CO)(TPPTS)3 as a rhodium precursor and TPPTS as a water soluble phosphine. Based on our previous study on the hydroformylation of 1-dodecene catalyzed by Rh/TPPTS,32 2.0 MPa of syngas and 80 °C was selected as initial reaction parameters. The side reactions mainly include 1-dodecene isomerization to generate internal dodecenes and 1-dodecene hydrogenation to dodecane, and then the acetalation reaction of the formed aldehydes to methylacetals.33 As presented in Table 1, the solvents and their amounts have great influence on the hydroformylation of 1-dodecene. Among them, methanol exhibited superior performance to other solvents, the reason might be that the rhodium precursor, ligand TPPTS and 1-dodecene had a better solubility in methanol under the reaction temperature, and thus creating a single phase in which the homogeneous hydroformylation of long chain alkenes proceeded smoothly. The conversion of 1-dodecene could reach up to 99.7% (Table 1, entry 6). The results also showed that when the amount of methanol increased from 1.0 mL to 5.0 mL, the activity increased, the reason is that the reaction system turned from a biphase into a homogeneous phase, which resulted in quick reaction. Further increasing the volume of methanol to 10.0 mL, the content of methylacetals in products is decreased due to the decrease of catalyst concentration. After hydroformylation, the reaction mixture presented a single phase (shown in Fig. 2). When methanol was removed from the reaction mixture under the reaction temperature, the remained mixture became two phases and the upper one was the product phase which could be separated efficiently from the bottom solid rhodium catalyst phase (shown in Fig. 2). The results suggested that the strategy to make homogeneous hydroformylation of long chain alkenes with water soluble Rh/TPPTS complex and easy separation of products is feasible, just through using an appropriate low boiling point polar solvent.| Entry | Solvent | Volume (mL) | Conv.b (%) | Ald.c (%) | Ace.d (%) | L/Be | TOFf (h−1) |
|---|---|---|---|---|---|---|---|
a Reaction conditions: Rh = 1.35 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 3333, 1-dodecene = 1.0 mL, P = 2.0 MPa (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1.0 H2 = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), T = 80.0 °C, t = 1.0 h.b Percent of converted alkene, determined by GC.c Content of aldehydes in products.d Content of methylacetals in products.e Ratio of the linear aldehyde to the branched aldehyde in products.f Turnover frequency defined as the moles of converted alkene per mole of Rh per hour. 1.0), T = 80.0 °C, t = 1.0 h.b Percent of converted alkene, determined by GC.c Content of aldehydes in products.d Content of methylacetals in products.e Ratio of the linear aldehyde to the branched aldehyde in products.f Turnover frequency defined as the moles of converted alkene per mole of Rh per hour. |
|||||||
| 1 | No | 0.0 | — | — | — | — | — |
| 2 | THF | 10.0 | — | — | — | — | — |
| 3 | CH3CN | 10.0 | — | — | — | — | — |
| 4 | CH2Cl2 | 10.0 | — | — | — | — | — |
| 5 | EtOH | 10.0 | 10.0 | 87.3 | 0.0 | 3.0 | 333 |
| 6 | MeOH | 10.0 | 99.7 | 92.7 | 6.5 | 2.5 | 3323 |
| 7 | MeOH | 1.0 | 3.3 | 84.2 | 0.0 | 2.1 | 110 |
| 8 | MeOH | 2.0 | 67.3 | 98.4 | 1.4 | 2.7 | 2243 |
| 9 | MeOH | 3.0 | 75.4 | 95.6 | 3.7 | 2.6 | 2513 |
| 10 | MeOH | 4.0 | 83.4 | 92.6 | 6.1 | 2.5 | 2780 |
| 11 | MeOH | 5.0 | 99.7 | 77.4 | 20.1 | 2.1 | 3323 |
| 12 | MeOH | 6.0 | 99.6 | 79.3 | 18.3 | 2.3 | 3320 |
| 13 | MeOH | 8.0 | 99.8 | 83.5 | 15.5 | 2.4 | 3327 |
| 14 | MeOH | 12.0 | 90.1 | 92.8 | 6.5 | 2.5 | 3020 |
The molar ratio of phosphine to rhodium was one of the important factors affecting the activity and regioselectivity of hydroformylation. It can be seen from Table 2 that the conversion of 1-dodecene rose fast with the increase in the molar ratio of phosphine to rhodium, however, the content of aldehydes in products increased initially and then decreased. Also, as the molar ratio of phosphine to rhodium increased to 25, the content of methylacetals in products increased from 5.2 to 13.5%. While further increasing the molar ratio of P/Rh to 30, the content of methylacetals in products did not ascend since the excess ligand could not be fully dissolved in 10.0 mL of methanol. It is well known that the presence of phosphine ligand would affect the coordination equilibrium of catalytic active species.34 When the molar ratio of P/Rh increased, HRh(CO)(TPPTS)2 catalytic active species increased, which resulted into higher reactivity. The optimum molar ratio of P/Rh was 20. The conversion of 1-dodecene and the selectivity to aldehydes (including the aldehydes converting to methylacetals) could reach up to 99.7% and 99.2%, respectively.
| Entry | P/Rh | Conv.b (%) | Ald.c (%) | Ace.d (%) | L/Be | TOFf (h−1) |
|---|---|---|---|---|---|---|
a Reaction conditions: Rh = 1.35 × 10−3 mmol, S/C = 3333, 1-dodecene = 1.0 mL, methanol = 10.0 mL, P = 2.0 MPa (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1.0 H2 = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), T = 80.0 °C, t = 1.0 h.b See Table 1.c See Table 1.d See Table 1.e See Table 1.f See Table 1. 1.0), T = 80.0 °C, t = 1.0 h.b See Table 1.c See Table 1.d See Table 1.e See Table 1.f See Table 1. |
||||||
| 1 | 0.0 | 55.0 | 62.5 | 5.2 | 2.7 | 1833 |
| 2 | 5.0 | 74.1 | 62.1 | 5.6 | 2.7 | 2470 |
| 3 | 10.0 | 99.5 | 83.5 | 5.9 | 2.5 | 3317 |
| 4 | 15.0 | 99.1 | 88.9 | 6.3 | 2.5 | 3303 |
| 5 | 20.0 | 99.7 | 92.7 | 6.5 | 2.5 | 3323 |
| 6 | 25.0 | 99.1 | 82.7 | 13.5 | 2.4 | 3303 |
| 7 | 30.0 | 99.1 | 82.8 | 13.5 | 2.4 | 3303 |
The effect of temperature on the hydroformylation of 1-dodecene was also investigated and the results were shown in Table 3. It seemed that the reaction temperature also displayed a significant role in this hydroformylation. The conversion of 1-dodecene increased sharply when the reaction temperature increased from 60 to 80 °C (Table 3, entries 1, 2 and 6). This may be due to the fact that the solubilities of rhodium precursor, TPPTS and 1-dodecene increase with the rise of reaction temperature. Meanwhile, higher temperature is favorable for hydroformylation and the formation of catalytic active species.35 Further enhancing the reaction temperature to 100 °C has an obvious increase of content of methylacetals in product (Table 3, entry 10, 58.0%). In addition, the effect of reaction time on the 1-dodecene hydroformylation was obvious, the initial rate is much higher (see Table 3, entry 3), prolonging the reaction time was favourable for the formation of methylacetals (Table 3, entry 8, 53.8%). In order to obtain higher content of aldehydes in product mixture, different base was added in the hydroformylation to prevent the formation of acetal side products and triethylamine showed a good result (Table 3, entry 11), the conversion and the selectivity to aldehydes could reach up to 99.6% and 94.5%, respectively, and the TOF could achieve 3320 h−1.
| Entry | T (°C) | t (h) | Conv.b (%) | Ald.c (%) | Ace.d (%) | L/Be | TOFf (h−1) |
|---|---|---|---|---|---|---|---|
a Reaction conditions: Rh = 1.35 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 3333, 1-dodecene = 1.0 mL, methanol = 10.0 mL, P = 2.0 MPa (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1.0 H2 = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0).b See Table 1.c See Table 1.d See Table 1.e See Table 1.f See Table 1.g 30 μL of triethylamine was added. 1.0).b See Table 1.c See Table 1.d See Table 1.e See Table 1.f See Table 1.g 30 μL of triethylamine was added. |
|||||||
| 1 | 60.0 | 1.0 | 12.3 | 88.4 | 2.2 | 1.9 | 410 |
| 2 | 70.0 | 1.0 | 28.8 | 94.5 | 2.0 | 2.5 | 960 |
| 3 | 80.0 | 0.25 | 40.2 | 97.5 | 0.0 | 2.7 | 5359 |
| 4 | 80.0 | 0.5 | 61.0 | 94.5 | 2.2 | 2.4 | 4066 |
| 5 | 80.0 | 0.75 | 77.4 | 91.5 | 5.4 | 2.5 | 3440 |
| 6 | 80.0 | 1.0 | 99.7 | 92.7 | 6.5 | 2.5 | 3323 |
| 7 | 80.0 | 2.0 | 99.8 | 74.1 | 23.4 | 2.1 | 1663 |
| 8 | 80.0 | 4.0 | 99.7 | 43.7 | 53.8 | 1.6 | 831 |
| 9 | 90.0 | 1.0 | 99.7 | 65.9 | 30.4 | 2.0 | 3323 |
| 10 | 100.0 | 1.0 | 99.6 | 36.5 | 58.0 | 1.3 | 3320 |
| 11g | 100.0 | 1.0 | 99.6 | 94.5 | 0.0 | 2.5 | 3320 |
In considering the fact that the excellent catalytic performance of the studied catalytic system during 1-dodecene hydroformylation, the hydroformylation of other long chain alkenes and cycloolefins in the studied catalytic system were also investigated. As presented in Table 4, excellent conversion of alkenes was maintained. Results further revealed that the studied catalytic system can also be applied to the hydroformylation of other long chain alkenes and cycloolefin, proving the generality of this catalytic system in hydroformylation.
| Entry | Alkene | Conv.b (%) | Ald.c (%) | Ace.d (%) | L/Be | TOFf (h−1) |
|---|---|---|---|---|---|---|
a Reaction conditions: Rh = 1.35 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 3333, P = 2.0 MPa (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1.0 H2 = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), T = 80.0 °C, t = 1.0 h.b See Table 1.c See Table 1.d See Table 1.e See Table 1.f See Table 1.g Rh = 2.70 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 1667, P = 2.0 MPa (CO 1.0), T = 80.0 °C, t = 1.0 h.b See Table 1.c See Table 1.d See Table 1.e See Table 1.f See Table 1.g Rh = 2.70 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 1667, P = 2.0 MPa (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1.0 H2 = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), T = 120.0 °C, t = 4.0 h.h Rh = 2.70 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 1667, P = 2.0 MPa (CO 1.0), T = 120.0 °C, t = 4.0 h.h Rh = 2.70 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 1667, P = 2.0 MPa (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1.0 H2 = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :1 :1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) .0), T = 120.0 °C, t = 4.0 h.i Rh = 2.70 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 1667, P = 2.0 MPa (CO .0), T = 120.0 °C, t = 4.0 h.i Rh = 2.70 × 10−3 mmol, [P]/[Rh] = 20.0, S/C = 1667, P = 2.0 MPa (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1.0 H2 = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), T = 120.0 °C, t = 4.0 h. 1.0), T = 120.0 °C, t = 4.0 h. |
||||||
| 1 | 1-Hexene | 97.8 | 90.6 | 7.0 | 2.3 | 3260 |
| 2 | 1-Octene | 98.0 | 93.8 | 5.2 | 2.5 | 3267 |
| 3 | 1-Decene | 99.0 | 95.1 | 3.9 | 2.4 | 3300 |
| 4 | 1-Dodecene | 99.7 | 92.7 | 6.5 | 2.5 | 3323 |
| 5g | Cyclohexene | 93.5 | 92.3 | 0.0 | — | 390 |
| 6h | Norbornene | 92.5 | 94.6 | 0.0 | — | 385 |
| 7i | Dicyclopentadiene | 93.0 | 95.5 | 0.0 | — | 388 |
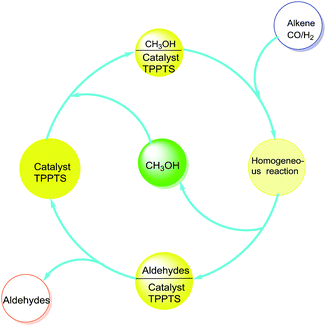
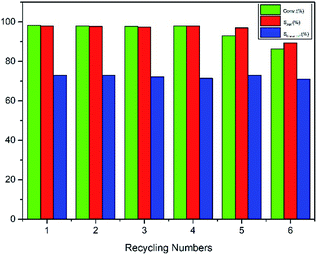
Encouraged by these excellent results, the recycling efficiency of the studied catalytic system used in 1-hexene hydroformylation was explored. After hydroformylation, the reaction mixture presented a single phase. The remained mixture became two phases when methanol was recycled from reaction mixture by distillation under the reaction temperature. The upper colorless product phase was easily separated from the lower solid catalyst phase after centrifuge. The above recycled methanol, solid rhodium catalyst and a new portion of alkene were then added to the autoclave to start the next cycle (see Fig. 3). In order to avoid the effect of operational loss of rhodium precursor and ligand TPPTS in the catalyst recycling experiments, the amount of Rh and the molar ratio of P/Rh were enlarged to 4.05 × 10−3 mmol and 50.0, respectively. Results in Fig. 4 indicated that the catalyst could be reused for five runs without evident loss in activity and selectivity for aldehydes (including the aldehydes converting to methylacetals). However, the activity began to decrease after five cycles. The main reason may be that the ligand TPPTS was partly oxidized with air during the catalyst separation from products, which was proved by the 31P NMR characterization of the precipitate catalyst residue after hydroformylation (see Fig. 5, OTPPTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPPTS oxide). Also, another reason resulted from the operational loss of rhodium precursor and TPPTS was inevitable during their separation. Fortunately, the leaching of Rh in the organic phase was below ppm. As demonstrated in Fig. 2, when methanol was removed from the mixture after hydroformylation, the resulting mixture showed a liquid–solid biphasic state, which might hint that the employment of methanol indeed realized homogeneous hydroformylation and heterogeneous separation of products from catalysts. This strategy developed an innovation for the hydroformylation of long chain olefins. Ideally, the catalyst could be recycled more runs if the operational loss and the TPPTS oxidation with air of catalyst could be avoided. Further modification of the operation process is ongoing.
TPPTS oxide). Also, another reason resulted from the operational loss of rhodium precursor and TPPTS was inevitable during their separation. Fortunately, the leaching of Rh in the organic phase was below ppm. As demonstrated in Fig. 2, when methanol was removed from the mixture after hydroformylation, the resulting mixture showed a liquid–solid biphasic state, which might hint that the employment of methanol indeed realized homogeneous hydroformylation and heterogeneous separation of products from catalysts. This strategy developed an innovation for the hydroformylation of long chain olefins. Ideally, the catalyst could be recycled more runs if the operational loss and the TPPTS oxidation with air of catalyst could be avoided. Further modification of the operation process is ongoing.
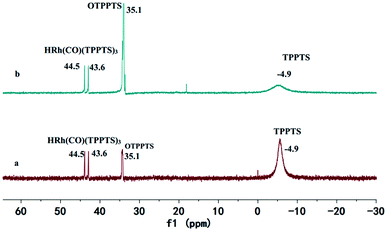 | ||
| Fig. 5 31 P NMR of rhodium catalyst before and after reaction (a) before reaction; (b) after product separation. | ||
4. Conclusion
In conclusion, a highly efficient catalytic recycling system of long chain alkenes hydroformylation has been established based on water soluble phosphine rhodium catalyst and methanol. The presence of methanol showed excellent hydroformylation efficiency for a wide scope of alkenes since the reaction could proceed homogeneously. The high catalytic efficiency mainly relies on the solubility capacity of solvent methanol for water soluble rhodium precursor HR(CO)(TPPTS)3, ligand TPPTS and substrate alkene. When the studied catalytic system was applied in the hydroformylation of other long chain alkenes, up to 3260 h−1 of TOF could be obtained with higher than 99.0% of selectivity to aldehydes (including the aldehydes forming methylacetals). The addition of a little base such as triethylamine could prevent the acetalation of the formed aldehydes with methanol. No significant loss of catalytic efficiency and rhodium was observed for more than five cycles during the catalyst recycling experiments. Further studies are in progress with the aim to explore the application of the studied system in other reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the financial support from the National Natural Science Foundation of China (No. 201202108, 21871187), from the Opening Project of Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education (No. LZJ1402), from the Sichuan University Outstanding Scholar Research Fund (No. 2015SCU04A05, 2018SCUH0079) and from the Key Program of Sichuan Science and Technology Project (No. 2018GZ0312, 2019YFG0146). We are grateful to the Centre of Testing & Analysis and the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, Sichuan University for the analysis work.Notes and references
- O. Roelen, German Patent DE 849548, 1938/1952.
- R. Franke, D. Selent and A. Börner, Chem. Rev., 2012, 112, 5675 CrossRef CAS PubMed.
- E. V. Gusevskaya, J. Jimenez-Pinto and A. Börner, ChemCatChem, 2014, 6, 382 CrossRef CAS.
- P. Eilbracht, L. Bärfacker, C. Buss, C. Hollmann, B. E. Kitsos-Rzychon, C. L. Kranemann, T. Rische, R. Roggenbuck and A. Schmidt, Chem. Rev., 1999, 99, 3329 CrossRef CAS PubMed.
- Y. Liu, Z. Li, B. Wang and Y. Zhang, Catal. Lett., 2016, 146, 2252 CrossRef CAS.
- X. Jin, K. Zhao, F. Kong, F. Cui, Q. Liu and Y. Zhang, Catal. Lett., 2014, 144, 192 CrossRef CAS.
- L. Zhang, C. Li, X. Zheng, H. Fu, H. Chen and R. Li, Catal. Lett., 2014, 144, 1074 CrossRef CAS.
- F. Agbossou, J. F. Carpentier and A. Mortreux, Chem. Rev., 1995, 95, 2485 CrossRef CAS.
- A. C. Brezny and C. R. Landis, J. Am. Chem. Soc., 2017, 139, 2778 CrossRef CAS PubMed.
- B. Cornils, W. A. Herrmann and M. Rasch, Angew. Chem., Int. Ed. Engl., 1994, 33, 2144 CrossRef.
- M. Haumann and A. Riisager, Chem. Rev., 2008, 108, 1475 CrossRef PubMed.
- A. Kämper, P. Kucmierczyk, T. Seidensticker, A. J. Vorholt, R. Franke and A. Behr, Catal. Sci. Technol., 2016, 6, 8072 RSC.
- Y. Jiao, M. S. Torne, J. Gracia, J. W. (Hans) Niemantsverdrietbc and P. W. N. M. van Leeuwen, Catal. Sci. Technol., 2017, 7, 1404 RSC.
- S. Lu and H. Alper, J. Am. Chem. Soc., 2003, 125, 13126 CrossRef CAS PubMed.
- Y. Wang, J. Chen, M. Luo, H. Chen and X. Li, Catal. Commun., 2006, 7, 979 CrossRef CAS.
- T. Vanbésien, A. Sayede, E. Monflier and F. Hapiot, Catal. Sci. Technol., 2016, 6, 3064 RSC.
- S. Chen, Y. Wang, W. Yao, X. Zhao, G. VO-Thanh and Y. Liu, J. Mol. Catal. A: Chem., 2013, 378, 293 CrossRef CAS.
- B. Cornils and E. Wiebus, Chem. Ing. Tech., 1994, 66, 916 CrossRef.
- B. Cornils and E. G. Kuntz, J. Organomet. Chem., 1995, 502, 177 CrossRef CAS.
- B. Cornils, W. A. Herrmann and R. W. Eckl, J. Mol. Catal. A: Chem., 1997, 116, 27 CrossRef CAS.
- B. Cornils, J. Mol. Catal. A: Chem., 1999, 143, 1 CrossRef CAS.
- S. Siangwata, N. Baartzes, B. C. E. Makhubela and G. S. Smith, J. Organomet. Chem., 2015, 1, 7 Search PubMed.
- F. Hapiot, H. Bricout, S. Menuel, S. Tilloy and E. Monflier, Catal. Sci. Technol., 2014, 4, 1899 RSC.
- F. Hapiot, S. Menuel, H. Bricout, S. Tilloy and E. Monflier, Appl. Organomet. Chem., 2015, 29, 580 CrossRef CAS.
- H. Fu, M. Li, H. Chen and X. Li, J. Mol. Catal. A: Chem., 2006, 259, 156 CrossRef CAS.
- X. Jin, K. Zhao, F. Kong, F. Cui and D. Yang, Catal. Lett., 2013, 143, 839 CrossRef CAS.
- M. Chevry, T. Vanbésien, S. Menuel, E. Monflier and F. Hapiot, Catal. Sci. Technol., 2017, 7, 114 RSC.
- Q. Sun, Z. Dai, X. Liu, N. Sheng, F. Deng, X. Meng and F. S. Xiao, J. Am. Chem. Soc., 2015, 137, 5204 CrossRef CAS PubMed.
- W. A. Herrmann, C. W. Kohlpaintner, H. Bahrmann and W. Knokol, J. Mol. Catal., 1992, 73, 191 CrossRef CAS.
- H. Chen, H. Liu, Y. Li, F. Cheng and X. Li, J. Mol. Catal., 1994, 8, 124 CAS.
- J. Wei, J. Lang, H. Fu, R. Li, X. Zheng, M. Yuan and H. Chen, Transition Met. Chem., 2016, 41, 599 CrossRef CAS.
- Y. Zhao, Y. Liu, J. Wei, H. Fu, X. Zheng, M. Yuan, R. Li and H. Chen, Catal. Lett., 2018, 148, 438 CrossRef CAS.
- P. Wang, H. Liu, Y. Li, X. Zhao, Y. Lu and Y. Liu, Catal. Sci. Technol., 2016, 6, 3854 RSC.
- H. Chen, Y. Li, B. Chen, Q. Yang and X. Li, J. Mol. Catal., 1994, 8, 347 CAS.
- A. C. Brezny and C. R. Landis, J. Am. Chem. Soc., 2017, 139, 2778 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |