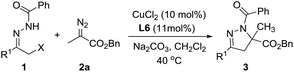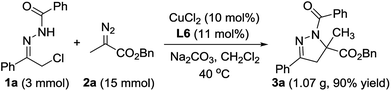Open Access Article
Open Access ArticleFormal [4 + 1] cycloaddition of in situ generated 1,2-diaza-1,3-dienes with diazo esters: facile approaches to dihydropyrazoles containing a quaternary center†
Bo Chen,
Wen-Dao Chu * and
Quan-Zhong Liu*
* and
Quan-Zhong Liu*
Chemical Synthesis and Pollution Control, Key Laboratory of Sichuan Province, College of Chemistry and Chemical Engineering, China West Normal University, No. 1, Shida Road, Nanchong 637002, P. R. China. E-mail: chuwendaonpo@126.com; quanzhongliu@cwnu.edu.cn
First published on 11th January 2019
Abstract
A Cu(II)/bisoxazoline ligand-promoted formal [4 + 1] cycloaddition of diazo esters with azoalkenes formed in situ has been developed. This strategy provides a potential protocol for the construction of dihydropyrazoles containing a quaternary center with good to excellent yields.
The efficient construction of quaternary carbon centers has remained a crucial issue in organic synthesis.1 Quaternary carbon centers are ubiquitous in various natural products, and pharmaceutically relevant compounds.2 Although significant efforts have been devoted to the effective construction of quaternary centers in recent years,1 new methodologies that could be advantageous in terms of functional-group tolerance, operational simplicity, and the use of easily obtained starting materials are still highly desired.
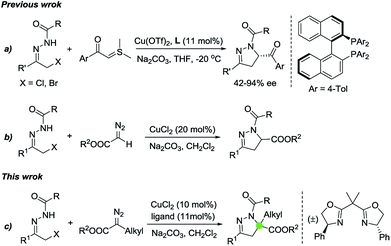
On the other hand, dihydropyrazoles represent a class of important heterocycles that occur in biologically active natural products and pharmaceuticals such as anti-amoebic, hypotensive, analgesic, anti-bacterial, anti-cancer, anti-depressant and nonsteroidal anti-inflammatory agents.3 Accordingly, great research efforts have been devoted toward their synthesis, and remarkable advances have been achieved in the construction of these nitrogen heterocycles. Representative synthetic strategies include formal [3 + 2] cycloaddition,4 [4 + 1] cycloaddition,5 catalytic asymmetric Fischer's pyrazoline synthesis via a sequential aza-Michael addition/cyclocondensation process,6 and photocatalytic radical cyclization.7,8 In comparison with the more ubiquitous family of [3 + 2] cycloadditions, [4 + 1] cycloannulations are relatively underutilized in these target-directed five-membered aza-heterocycles construction.5 In 2012, Bolm and coworkers reported the first example of asymmetric synthesis of dihydropyrazoles by formal [4 + 1] cycloaddition of in situ derived azoalkenes and sulfur ylides (Scheme 1a).5a Recently, diazo esters as 1,1-dipolar C1 synthons had also been utilized by the group of Favi to synthesize racemic dihydropyrazoles in a similar manner (Scheme 1b).5b However, none of these investigations has explored the possibility of accessing dihydropyrazoles containing a quaternary center. Herein, we present a Cu(II)/bisoxazoline ligand-promoted formal [4 + 1] cycloaddition of diazo esters with azoalkenes formed in situ, affording dihydropyrazoles containing a quaternary center with good to excellent yields (Scheme 1c).
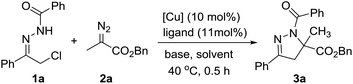
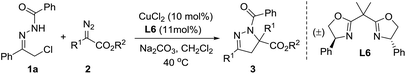
At the outset of this investigation, we employed hydrazone 1a and diazo ester 2a as the substrates (Table 1). Preliminary screening showed that the ligand has a remarkable effect on the reaction. For instance, the reaction with phosphine ligands gave the desired dihydropyrazole 3a in low yields (Table 1, entry 2–4). It was found that the reaction proceeded efficiently when bisoxazoline L6 was employed as ligand, leading to the desired product 3a in 98% yield (Table 1, entry 7). Subsequently, different bases and solvents were then explored (Table 1, entries 7–16), Na2CO3 and CH2Cl2 was the best choice.
| Entry | [Cu] | Ligand | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction was run under the following conditions: a solution of 1a (0.1 mmol), 2a (0.5 mmol), base (0.5 mmol), Cu cat. (10 mol%), and ligand (11 mol%) in anhydrous solvent (1 mL) was stirred at 40 °C under nitrogen atmosphere for 0.5 h.b Yields refer to isolated products. | |||||
| 1 | CuCl2 | None | Na2CO3 | CH2Cl2 | None |
| 2 | CuCl2 | L1 | Na2CO3 | CH2Cl2 | 18 |
| 3 | CuCl2 | L2 | Na2CO3 | CH2Cl2 | 6 |
| 4 | CuCl2 | L3 | Na2CO3 | CH2Cl2 | 22 |
| 5 | CuCl2 | L4 | Na2CO3 | CH2Cl2 | 5 |
| 6 | CuCl2 | L5 | Na2CO3 | CH2Cl2 | 6 |
| 7 | CuCl2 | L6 | Na2CO3 | CH2Cl2 | 98 |
| 8 | CuCl2 | L6 | K2CO3 | CH2Cl2 | 15 |
| 9 | CuCl2 | L6 | Cs2CO3 | CH2Cl2 | 26 |
| 10 | CuCl2 | L6 | NaOH | CH2Cl2 | Trace |
| 11 | CuCl2 | L6 | KOtBu | CH2Cl2 | Trace |
| 12 | CuCl2 | L6 | Et3N | CH2Cl2 | Trace |
| 13 | CuCl2 | L6 | Na2CO3 | THF | 83 |
| 14 | CuCl2 | L6 | Na2CO3 | Toluene | Trace |
| 15 | CuCl2 | L6 | Na2CO3 | CH3CN | 5 |
| 16 | CuCl2 | L6 | Na2CO3 | Hexane | 12 |
With the optimized conditions in hand, we next explored the substrate scope of the heterodienes. A series of hydrazones 1a–l bearing electron-neutral, -deficient or -rich aromatic substituents were smoothly reacted with diazo ester 2a to give the corresponding dihydropyrazoles 3a–l in 76−98% yield (Table 2, entry 1–12). Also α-bromo N-benzoyl hydrazone 1o reacted well, and 88% yield were achieved (Table 2, entry 15). In contrast, 2-naphthyl-substituted hydrazone 1m and aliphatic hydrazone 1n only gave a small quantity of product 3m and 3n (Table 2, entry 13–14).
| Entry | 1 | X | R1 | Yieldb of 3 (%) |
|---|---|---|---|---|
| a Reaction was run under the following conditions: a solution of 1 (0.1 mmol), 2a (0.5 mmol), Na2CO3 (0.5 mmol), CuCl2 (10 mol%), and L6 (11 mol%) in anhydrous CH2Cl2 (1 mL) was stirred at 40 °C under nitrogen atmosphere for 0.5 h.b Yields refer to isolated products. | ||||
| 1 | 1a | Cl | Ph | 3a, 98 |
| 2 | 1b | Cl | 2-Br–Ph | 3b, 82 |
| 3 | 1c | Cl | 2-F–Ph | 3c, 78 |
| 4 | 1d | Cl | 2-CH3–Ph | 3d, 76 |
| 5 | 1e | Cl | 3-Cl–Ph | 3e, 93 |
| 6 | 1f | Cl | 3-OCH3–Ph | 3f, 92 |
| 7 | 1g | Cl | 3-CH3–Ph | 3g, 89 |
| 8 | 1h | Cl | 4-Cl–Ph | 3h, 98 |
| 9 | 1i | Cl | 4-F–Ph | 3i, 94 |
| 10 | 1j | Cl | 4-OCH3–Ph | 3j, 98 |
| 11 | 1k | Cl | 4-NO2–Ph | 3k, 92 |
| 12 | 1l | Cl | 4-CH3–Ph | 3l, 98 |
| 13 | 1m | Cl | 2-Naphthyl | 3m, trace |
| 14 | 1n | Cl | n-Bu | 3n, trace |
| 15 | 1o | Br | Ph | 3o, 88 |
Next, the scope of the reaction was extended by conducting the reaction with various diazo esters (Table 3). Variation of the ester R2 group (entries 1 and 2) had little influence on the yield of product 3. The significant steric effect of R1 has been observed. Methyl and ethyl groups gave excellent results (entries 2–3), while the more bulky groups gave only a trace of products (entries 4–5).
| Entry | 2 | R1 | R2 | Yieldb of 3 (%) |
|---|---|---|---|---|
| a Reaction was run under the following conditions: a solution of 1a (0.1 mmol), 2 (0.5 mmol), Na2CO3 (0.5 mmol), CuCl2 (10 mol%), and L6 (11 mol%) in anhydrous CH2Cl2 (1 mL) was stirred at 40 °C under nitrogen atmosphere for 0.5 h.b Yields refer to isolated products. | ||||
| 1 | 2a | Me | Bn | 3a, 98 |
| 2 | 2b | Me | Et | 3p, 98 |
| 3 | 2c | Et | Et | 3q, 92 |
| 4 | 2d | Bn | Bn | 3r, trace |
| 5 | 2e | Ph | Et | 3s, trace |
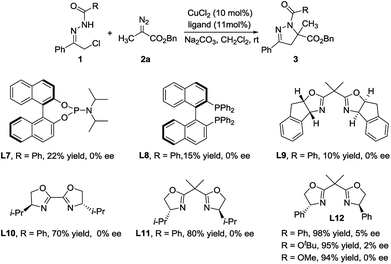
We next attempted to investigate asymmetric variant of this Cu(II)-catalyzed formal [4 + 1] cycloaddition reaction of diazo esters with azoalkenes formed in situ (Scheme 2). An extensive screening of chiral phosphine ligands (L7, L8), bisoxazoline ligands (L9–12) and different reaction conditions had been implemented. Unfortunately, only up to 5% ee was obtained when L12 was employed as chiral ligand, albeit with excellent yield (98%).
To show the synthetic potential of this strategy, we have carried out a gram scale synthesis of 3a (Scheme 3). Under the optimized reaction conditions, the reaction with 3 mmol of 1a proceeded smoothly with 5 equiv. of 2a, affording 1.07 g of 3a (90% yield).
In summary, we have developed a Cu(II)/bisoxazoline ligand-promoted formal [4 + 1] cycloaddition of diazo esters with azoalkenes formed in situ, affording dihydropyrazoles containing a quaternary center with good to excellent yields. The reaction involves the use of stable, readily available starting materials and is operationally simple.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financial supported by the National Natural Science Foundation of China (No. 21572183 and 21801208).Notes and references
- For selected reviews, see: (a) K. Fuji, Chem. Rev., 1993, 93, 2037–2066 CrossRef CAS; (b) J. C. Douglas and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5363–5367 CrossRef PubMed; (c) J. Christoffers and A. Baro, Quaternary Stereocenters-Challenges and Solutions for Organic Synthesis, Wiley-VCH, Weinheim, 2005 CrossRef; (d) B. M. Trost and C. Jiang, Synthesis, 2006, 369–396 CrossRef CAS; (e) M. Shimizu, Angew. Chem., Int. Ed., 2011, 50, 5998–6000 CrossRef CAS PubMed; (f) B. M. Wang and Y. Q. Tu, Acc. Chem. Res., 2011, 44, 1207–1222 CrossRef CAS PubMed; (g) J. P. Das and I. Marek, Chem. Commun., 2011, 47, 4593–4623 RSC; (h) M. Büschleb, S. Dorich, S. Hanessian, D. Tao, K. B. Schenthal and L. E. Overman, Angew. Chem., Int. Ed., 2016, 55, 4156–4186 CrossRef.
- (a) D. J. Newman and G. M. Cragg, J. Nat. Prod., 2012, 75, 311–335 CrossRef CAS PubMed; (b) D. J. Newman and G. M. Cragg, J. Nat. Prod., 2016, 79, 629–661 CrossRef CAS PubMed and references within; (c) Y. Tu, C. Jeffries, H. Ruan, C. Nelson, D. Smithson, A. A. Shelat, K. M. Brown, X.-C. Li, J. P. Hester, T. Smillie, I. A. Khan, L. Walker, K. Guy and B. Yan, J. Nat. Prod., 2010, 73, 751–754 CrossRef CAS.
- (a) M. Kissane and A. R. Maguire, Chem. Soc. Rev., 2010, 39, 845–883 RSC; (b) C.-H. Küchenthal and W. Maison, Synthesis, 2010, 719–740 Search PubMed; (c) A. Sahoo, S. Yabanoglu, B. N. Sinha, G. Ucar, A. Basu and V. Jayaprakash, Bioorg. Med. Chem. Lett., 2010, 20, 132–136 CrossRef CAS PubMed; (d) M. Johnson, B. Younglove, L. Lee, R. LeBlanc, H. Holt Jr, P. Hills, H. Mackay, T. Brown, S. L. Mooberry and M. Lee, Bioorg. Med. Chem. Lett., 2007, 17, 5897–5901 CrossRef CAS PubMed; (e) M. A. Ali and M. Shaharyar, Bioorg. Med. Chem., 2007, 15, 1896–1902 CrossRef CAS PubMed; (f) J. H. M. Lange and C. G. Kruse, Curr. Opin. Drug Discovery Dev., 2004, 7, 498 CAS.
- (a) S. Kanemasa and T. Kanai, J. Am. Chem. Soc., 2000, 122, 10710–10711 CrossRef CAS; (b) R. Shintani and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 10778–10779 CrossRef CAS; (c) M. P. Sibi, L. M. Stanley and C. P. Jasperse, J. Am. Chem. Soc., 2005, 127, 8276–8277 CrossRef CAS PubMed; (d) A. Suárez, C. W. Downey and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 11244–11245 CrossRef PubMed; (e) T. Kano, T. Hashimoto and K. Maruoka, J. Am. Chem. Soc., 2006, 128, 2174–2175 CrossRef CAS PubMed; (f) M. P. Sibi, L. M. Stanley and T. Soeta, Adv. Synth. Catal., 2006, 348, 2371–2375 CrossRef CAS; (g) M. P. Sibi, L. M. Stanley and T. Soeta, Org. Lett., 2007, 9, 1553–1556 CrossRef CAS PubMed; (h) L. Gao, G. S. Hwang, M. Y. Lee and D. H. Ryu, Chem. Commun., 2009, 5460–5462 RSC; (i) H. Suga, Y. Furihata, A. Sakamoto, K. Itoh, Y. Okumura, T. Tsuchida, A. Kakehi and T. Baba, J. Org. Chem., 2011, 76, 7377–7387 CrossRef CAS PubMed; (j) T. Arai and Y. Ogino, Molecules, 2012, 17, 6170–6178 CrossRef CAS PubMed; (k) T. Imaizumi, Y. Yamashita and S. Kobayashi, J. Am. Chem. Soc., 2012, 134, 20049–20052 CrossRef CAS PubMed; (l) M. Rueping, M. S. Maji, H. B. Kücük and I. Atodiresei, Angew. Chem., Int. Ed., 2012, 51, 12864–12868 CrossRef CAS PubMed; (m) G. Wang, X. Liu, T. Huang, Y. Kuang, L. Lin and X. Feng, Org. Lett., 2013, 15, 76–79 CrossRef CAS PubMed; (n) A. L. Gerten, M. C. Slade, K. M. Pugh and L. M. Stanley, Org. Biomol. Chem., 2013, 11, 7834–7837 RSC; (o) T. Arai, Y. Ogino and T. Sato, Chem. Commun., 2013, 49, 7776–7778 RSC; (p) T. Hashimoto, Y. Takiguchi and K. Maruoka, J. Am. Chem. Soc., 2013, 135, 11473–11476 CrossRef CAS PubMed; (q) M. Hori, A. Sakakura and K. Ishihara, J. Am. Chem. Soc., 2014, 136, 13198–13201 CrossRef CAS PubMed; (r) X. Hong, H. B. Kücük, M. S. Maji, Y.-F. Yang, M. Rueping and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 13769–13780 CrossRef CAS PubMed; (s) X. Wang, Y.-m. Pan, X.-c. Huang, Z.-y. Mao and H.-s. Wang, Org. Biomol. Chem., 2014, 12, 2028–2032 RSC; (t) D.-Y. Zhang, L. Shao, J. Xu and X.-P. Hu, ACS Catal., 2015, 5, 5026–5030 CrossRef CAS.
- (a) J.-R. Chen, W.-R. Dong, M. Candy, F.-F. Pan, M. Jörres and C. Bolm, J. Am. Chem. Soc., 2012, 134, 6924–6927 CrossRef CAS PubMed; (b) O. A. Attanasi, L. D. Crescentini, G. Favi, F. Mantellini, S. Mantenuto and S. Nicolini, J. Org. Chem., 2014, 79, 8331–8338 CrossRef CAS PubMed; (c) Z. Wang, Y. Yang, F. Gao, Z. Wang, Q. Luo and L. Fang, Org. Lett., 2018, 20, 934–937 CrossRef CAS PubMed.
- (a) H. Yanagita and S. Kanemasa, Heterocycles, 2007, 71, 699–709 CrossRef CAS; (b) S. Müller and B. List, Angew. Chem., Int. Ed., 2009, 48, 9975–9978 CrossRef PubMed; (c) S. Müller and B. List, Synthesis, 2010, 2010, 2171–2178 CrossRef; (d) O. Mahé, I. Dez, V. Levacher and J.-F. Brière, Angew. Chem., Int. Ed., 2010, 49, 7072–7075 CrossRef PubMed; (e) N. R. Campbell, B. Sun, R. P. Singh and L. Deng, Adv. Synth. Catal., 2011, 353, 3123–3128 CrossRef CAS PubMed; (f) M. Fernández, E. Reyes, J. L. Vicario, D. Badía and L. Carrillo, Adv. Synth. Catal., 2012, 354, 371–376 CrossRef; (g) O. Mahé, I. Dez, V. Levacher and J.-F. Brière, Org. Biomol. Chem., 2012, 10, 3946–3954 RSC.
- (a) X.-Q. Hu, J.-R. Chen, Q. Wei, F.-L. Liu, Q.-H. Deng, A. M. Beauchemin and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 12163–12167 CrossRef CAS; (b) Q. Wei, J.-R. Chen, X.-Q. Hu, X.-C. Yang, B. Lu and W.-J. Xiao, Org. Lett., 2015, 17, 4464–4467 CrossRef CAS PubMed; (c) J. Cheng, P. Xu, W. Li, Y. Cheng and C. Zhu, Chem. Commun., 2016, 52, 11901–11904 RSC; (d) Q.-Q. Zhao, J. Chen, D.-M. Yan, J.-R. Chen and W.-J. Xiao, Org. Lett., 2017, 19, 3620–3623 CrossRef CAS PubMed; (e) J.-m. Yu, G.-P. Lu and C. Cai, Chem. Commun., 2017, 53, 5342–5345 RSC.
- For other methods for synthesis of dihydropyrazoles, see: (a) C. B. Tripathi and S. Mukherjee, Org. Lett., 2014, 16, 3368–3371 CrossRef CAS PubMed; (b) X. Wu, M. Wang, G. Zhang, Y. Zhao, J. Wang and H. Ge, Chem. Sci., 2015, 6, 5882–5890 RSC; (c) M.-N. Yang, D.-M. Yan, Q.-Q. Zhao, J.-R. Chen and W.-J. Xiao, Org. Lett., 2017, 19, 5208–5211 CrossRef CAS PubMed; (d) J. Zhao, M. Jiang and J.-T. Liu, Org. Chem. Front., 2018, 5, 1155–1159 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and compound characterisation data, including X-ray crystal structures of 3h. CCDC 1840892. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra08909d |
| This journal is © The Royal Society of Chemistry 2019 |