Open Access Article
Open Access ArticleSilver sulfadiazine loaded zein nanofiber mats as a novel wound dressing
Sana Ullah,
Motahira Hashmi,
Muhammad Qamar Khan,
Davood Kharaghani,
Yuseke Saito,
Takayuki Yamamoto and
Ick Soo Kim *
*
Nano Fusion Technology Research Group, Institute of Frontier Fiber Engineering, Shinshu University, 3-15-1, Tokida, Ueda, Nagano 386-8567, Japan. E-mail: kim@shinshu-u.ac.jp; Fax: + 81-268-21-5482; Tel: + 81-268-21-5439
First published on 2nd January 2019
Abstract
In this report a novel antibacterial wound dressing was prepared and then characterized for required testing. We loaded silver sulfadiazine (AgSD) for the first time by electrospinning. AgSD was added in zein (0.3%, 0.4%, 0.5%, and 0.6% by weight) and was electrospun to fabricate nanofiber mats for wound dressings. Nanofiber mats were characterized by Fourier transform infrared spectroscopy (FTIR) to check if there was any chemical reaction between AgSD and zein. Morphological properties were analyzed by Scanning Electron Microscopy (SEM), which showed uniform nanofibers without any bead formation. The diameter of the nanofibers gradually decreased with an increase in the amount of AgSD, which can be associated with strong physical bonding between zein and AgSD. Thermal properties of nanofiber mats were analyzed by Thermogravimetric Analysis (TGA). X-Ray Diffraction (XRD) further demonstrated the crystalline structure of the nanofiber mats, and X-ray Photoelectron spectroscopy (XPS) was performed to confirm Ag and S contents in the prepared wound dressings. In order to investigate antibacterial properties, a disc diffusion method was employed. Bacillus and E. coli bacteria strains were used as Gram-positive and Gram-negative strains respectively. The antibacterial effectiveness of AgSD released from zein nanofibers was determined from the zone inhibition of the bacteria. The antibacterial activity of zein nanofibers loaded with drug was observed with both strains of bacteria in comparison to a control. Excellent antibacterial efficacy was attributed to the sample with 0.6% AgSD. Excellent release properties were also associated with the sample with 0.6% AgSD in zein nanofibers. Keeping in mind the abovementioned characteristics, prepared nanofiber mats would be effective for application in wound dressings.
Introduction
Wound dressings are pieces of fabric which are used to cover a cut or a wound. They can be woven, knitted or non-woven fabric. With the increasing demand for effective wound care technology, a number of researchers are carrying out research in the field of wound care systems. Nanofiber mats have been used as wound dressings for a couple of years. It is stated that about 60% of total nanofiber production is used for biomedical purposes only. This can be for wound dressings, self-healing features, drug delivery, or implants.1–4Silver has been among the top antibacterial drugs for decades. It has been used with a number of natural and synthetic polymers either in composite form or incorporated as nanoparticles. Silver sulfadiazine was investigated for the first time in 1974 as an effective drug for burn wound care. According to their results, sulfadiazine was not so effective for antibacterial activity in lower concentrations but they found it effective in combination with silver.5 In 1982 a study was carried out to investigate the inactivation of silver sulfadiazine by adding cerium salts. It was concluded that by the addition of cerium salt, inhibitory zone around the infected area became closer which ultimately resulted due to inactivation of silver sulfadiazine.6 A brief review was done on importance of silver nano-particles as effective antimicrobial agent. Due to availability of emerging antibacterial agents silver use was declined but it went up because of electrospinning method. In electrospinning metallic nano-particles can be used in useful manner.7 Nylon/silver composite membrane was prepared and characterized in 2010. It was observed that tensile strength of composite membrane increased as compared to nylon membranes.8 Non-woven polyvinyl alcohol (PVA), chitosan (CS), and sliver nitrate (AgNO3) was prepared and characterized for antibacterial activity, it was concluded that prepared blends had higher antibacterial activity.9 Silver nanoparticles (AgNPs) were also added in one step electrospinning of nylon 6, and characterized for antimicrobial activity as well as morphological and surface properties.10
Along with above mentioned literature, AgNPs have been used in various applications incorporating with different natural and synthetic polymers, for example, poly methyl methacrylate (PMMA), polyvinylpyrrolidone (PVP), zein, polyacrylonitrile (PAN), chitosan, and others as well.4,11–13
Zein is a natural polymer which is obtained from corn. It is biodegradable and biocompatible polymer. Due to its versatile biocompatible and biodegradable properties, zein has been widely used in biomedical applications. An ultrafine membrane of zein was prepared by electrospinning and investigated the factors which affect the structure and morphology of electrospun zein nanofibers.14 Zein prolamine nanofibers were also developed and characterized for thermal and morphological properties.15 Silver and zein composites were fabricated on two different pH conditions and then characterized for antibacterial activity. It was concluded that silver nanoparticles were effective for antibacterial activity.16 Zein has been used effectively in biomedical applications incorporated with different antibacterial drugs i.e., cyclodextrins, eudragit, ketoprofen (KET), and ferulic acid (FA).17–22 Zein incorporation with different natural and synthetic polymers has also been used as control drug delivery and tissue engineering.2,4,23–25
Electrospinning has been used for producing nonwoven fabric since first patent for electrospinning but it became popular in 21st century. Since then a number of researchers have been working on electrospinning of different polymers.26 In this paper, electrospinning was used to develop nanofiber mats of zein incorporated with a novel antibacterial drug silver sulfadiazine (AgSD). We are the first one to successfully load AgSD on electrospinning. AgSD has been used for wound care system in the form of hydro-gel or composite form but no one has loaded AgSD on electrospun nanofiber mats. The advantage of using this drug incorporation with natural, biocompatible and biodegradable polymer is that AgSD could occupy larger surface area. This research will be useful for industrial scale applications of AgSD loaded wound dressings.
Materials & methods
Materials
Zein polymer was purchased from the Sigma-Aldrich Corporation (Saint Louis, MO 63103, USA), that was in powder form and contained water content less than 8%. Ethanol was purchased from Sigma-Aldrich Corporation (Saint Louis, MO 63103, USA), with purity level of 99.5%. Glutaraldehyde (GA, 50% in aqueous solution) was purchased from MP Biomedical (Tokyo, Japan). Distilled water was used from laboratory. Silver(I) sulfadiazine (AgSD) was purchased from Sigma-Aldrich Corporation (Saint Louis, MO 63103, USA) in powder form with 98% purity.Method
Zein 25% by weight was dissolved in aqueous solution (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 75
water = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 by weight). Solution was kept on stirring for 2 hours and then AgSD was added to the zein solution by following proportions:
25 by weight). Solution was kept on stirring for 2 hours and then AgSD was added to the zein solution by following proportions:
Glutaraldehyde was added in the solution at 2.5 wt% for cross-linking of the solution. The prepared solution was then loaded to electrospinning to produce AgSD loaded zein nanofibers. Electrospinning was run on 15 kV voltage, distance between needle tip and collecting roller was kept 17 cm, and flow rate of 0.6 ml h−1. After the formation of nanofibers mat, it was taken off from collecting drum and was crosslinked by HCL foaming. The hydrochloric acid (HCL) foaming of zein nanofibers and zein/AgSD nanofibers was done at 30 °C for 60 s. In this reaction, GA and HCl acted as a chemical cross-linking agent and a catalyst, respectively. This cross-linking was done to improve crystalline structure of nanofibers. Nanofibers were dried at room temperature for 3 hours and kept in air tight bags for further characterization (Table 1).
| Samples | Proportions by weight | Sample code |
|---|---|---|
| Sample 1 | Zein 100%, AgSD 0% | Zein |
| Sample 2 | Zein 99.7%, AgSD 0.3% | 0.3% AgSD |
| Sample 3 | Zein 99.6%, AgSD 0.4% | 0.4% AgSD |
| Sample 4 | Zein 99.5%, AgSD 0.5% | 0.5% AgSD |
| Sample 5 | Zein 99.4%, AgSD 0.6% | 0.6% AgSD |
Characterizations
Bacillus and E. coli bacteria strain were used for the assessment of the antimicrobial properties by disc diffusion method. The samples were cut in round shape (disc diameter 8 mm) and placed on top of plates, which cultured separately with each bacteria strain and incubated overnight (12 hours) at 37 °C. The morphological properties of nanofiber mats were characterized by Scanning Electron Microscope (SEM) (JSM-5300, JEOL Ltd, Japan), which was accelerated with the voltage of 12 kV. The average diameters of nanofibers were measured by taking 50 readings from random nanofibers of each sample using image analysis software (Image J, version 1.4.3). The chemical reaction between zein and AgSD were analyzed by FTIR with an ATR Prestige-21 (Shimadzu, Japan). ATR fingerprints were recorded from 400 cm−1 to 4000 cm−1. Wide angle X-ray diffractions (WAXRD) spectra were performed for the evaluation of the crystal structure at 25 °C with nanofiber samples using a Rotaflex RT300 mA (Rigaku manufacturer, Osaka, Japan) and Nickel-filtered Cu Kα radiation was used for measurements, along with an angular angle of 5 ≤ 2θ ≤ 80°. Thermal degradation of zein and AgSD/zein nanofibers was performed by thermogravimetric analysis using Thermo-plus TG 8120 (Rigaku Corporation, Osaka, Japan). It was operated in a static mode under air atmosphere at a heating rate of 10 °C min−1 and a temperature range of 0–500 °C. The XPS analysis was examined by using a Shimadzu-Kratos AXIS-ULTRA HAS SV (Shimadzu, Kyoto, Japan). The release behavior of AgSD from zein nanofibers was characterized by inductively coupled plasma (ICP) atomic emission spectrometer (SHIMADZU/ICPS, 10000IV, Japan) over a period of 72 hours by immersing the nanofibers in deionized water and stirring very slowly at room temperature.Results and discussion
Morphological properties
Morphological properties of prepared nanofiber mats were assessed by SEM. The SEM images in Fig. 1, showed that nanofibers of pure zein were smooth and do not contain any beads on described conditions. Addition of AgSD caused a little change in morphology as diameter is decreased and nanofiber structure was also changed. But nanofiber mats with addition of AgSD were also smooth as it can be shown in figure.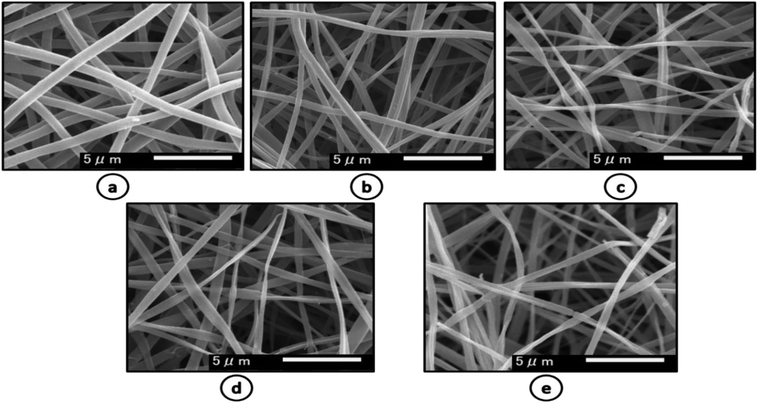 | ||
| Fig. 1 SEM images of (a) pure zein (b) 0.3% AgSD in zein (c) 0.4% AgSD in zein (d) 0.5% AgSD (e) 0.6% AgSD in zein. | ||
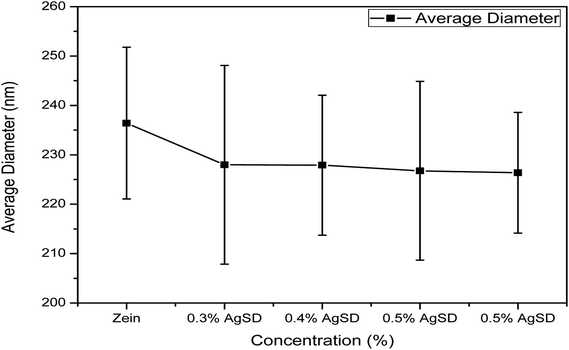
Diameter interpretation
As we mentioned above that diameter of nanofibers was changed with addition of AgSD in to the zein. Fig. 2 shows quantitative interpretation of average diameters of nanofibers. The average of 50 nanofibers from each sample showed a little change in diameters. Average diameters of zein, 0.3% AgSD, 0.4% AgSD, 0.5% AgSD, and 0.6% AgSD are 236.42 nm, 227.98 nm, 227.90 nm, 226.76 nm, and 226.36 nm. It was observed that all the diameters were in descending order with increase in AgSD content. Usually addition of nanoparticles or additives increase the diameter of nanofibers but in this case results were opposite which can be associated with packing of zein backbone due to strong physical bonding between zein and AgSD.EDX analysis
EDX analysis was done for the quantitative measurement of Ag and sulfur content in the nanofibers. Fig. 3 shows that Ag and sulfur content increased as concentration of AgSD in the nanofiber increased. So, it was a direct check on the presence of Ag and sulfur as active ingredients of the prepared nanofibers mats. It was also observed in the EDX analysis that both Ag and S showed ascending trend, as the percentage of AGSD was increased in nanofibers mat, the quantity of Ag and S also increased in EDX analysis.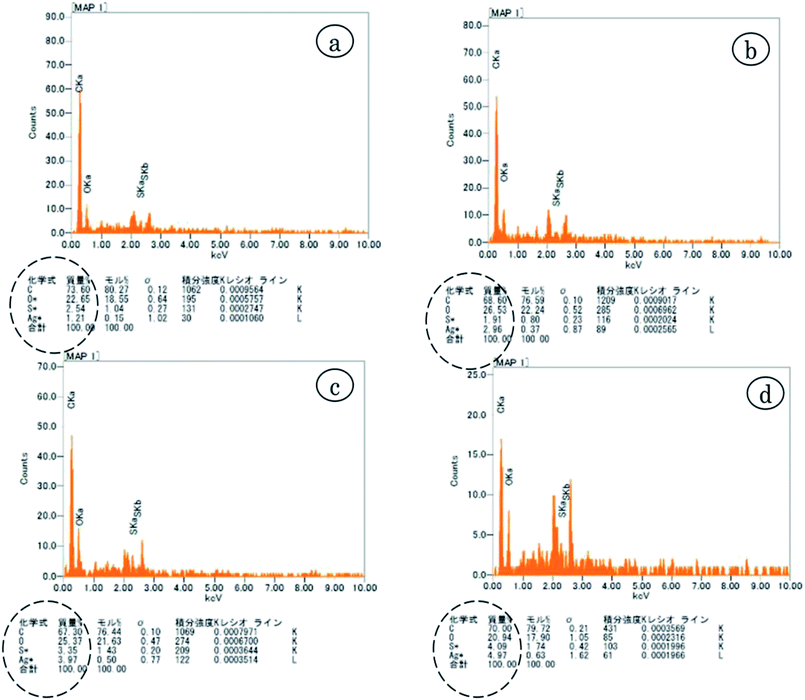 | ||
| Fig. 3 EDX analysis of (a) 0.3% AgSD in zein (b) 0.4% AgSD in zein (c) 0.5% AgSD in zein (d) 0.6% AgSD in zein. | ||
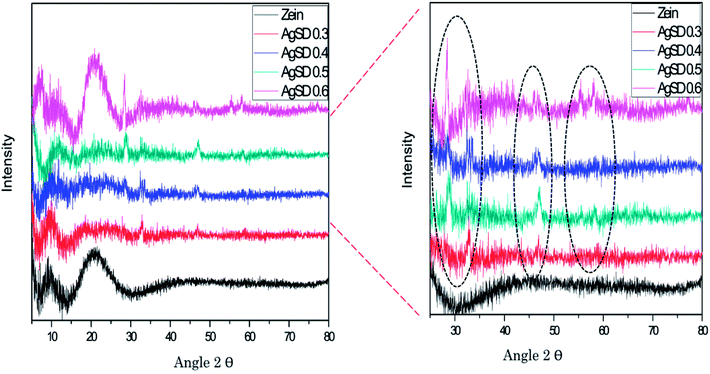
X-ray diffraction (XRD)
XRD was done to confirm crystalline structure of nanofiber mats (Fig. 4). Pure zein nanofibers showed peak at 2θ = 9°, and a wide peak at 2θ = 20°.while zein did not exhibit any peak up to 2θ = 80°. Also from literature it was confirmed that pure zein powder also exhibits the same trend which is clear evidence that zein had retained its structure in nanofiber form too. It was also observed that in pure zein, shorter peaks represented the intra-helix average backbone distance (α-helix structure of pure zein) while broader spacing was related intra-helix packing between the neighboring chains.Addition of AgSD in zein presented sharp peaks at 2θ = 28°, 2θ = 31°, 2θ = 47°, 2θ = 56°, and 2θ = 58°. Except from the two peaks (2θ = 9° and 2θ = 20°), all other peaks represents the presence of AgSD and all these peaks were associated with AgSD. From XRD graph it was also observed that structural stability of zein did not changed by the addition of AgSD, although AgSD showed their peaks in nanofiber mats of zein/AgSD. Presence of AgSD in zein nanofibers were also proven by XPS analysis.
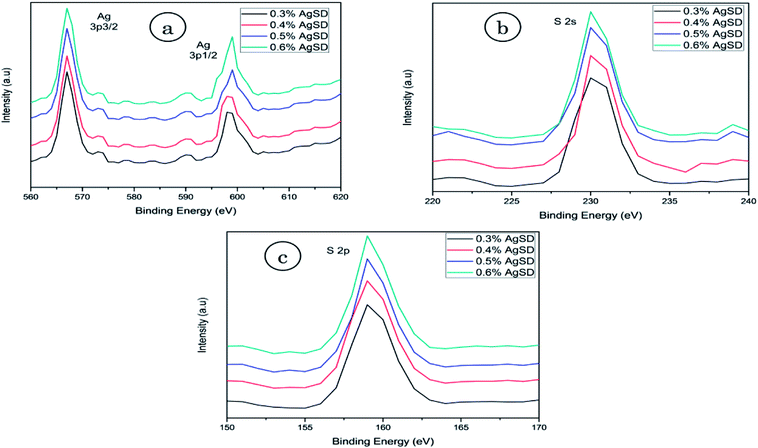
X-ray photoelectron spectroscopy (XPS)
XPS analysis was done to confirm the presence of AgSD in nanofiber mats (Fig. 5). As AgSD consists of Ag (silver) and S (sulfur), so XPS results for Ag (3p) and sulfur (2p and 2s) were obtained. Ag 3p spectra had two peaks at 568.0 eV (Ag 3p3/2) and 601.0 eV (Ag 3p1/2) with a slit of 33 eV. Those peaks were related to metallic Ag in nanofibers. The standard peaks for Ag 3p3/2 and Ag 3p1/2 were recorded 570.0 eV and 604.0 eV respectively. Spectra of S 2s had shown peak at binding energy of 230.0 eV and spectra of S 2p at 163.0 eV while standard peaks for S 2s and S 2p were recorded at 231.0 eV and 164.5 eV. It was also observed that intensity of described peaks were higher as concentration of AgSD was increased in zein nanofibers. From all of these results it was clear evidence of silver and sulfur presence in the nanofiber mats as all above mentioned peaks are related to Ag and S.Fourier-transform infrared spectroscopy (FTIR)
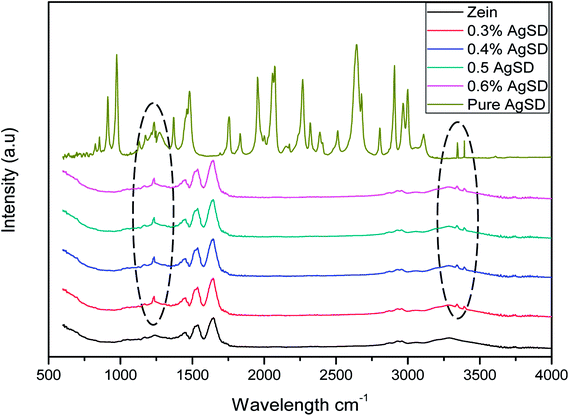
FTIR spectra of pure zein and composite nanofibers of Ag/zein are shown in Fig. 6. It was observed that AgSD had shown a sharp peak at 1231.6 cm−1 which is associated with the main component of AgSD that is SO2 asymmetric stretching. Along with this peak, specimen containing AgSD had also shown peaks on 3341.52 cm−1 and 3391.07 cm−1 which were associated with stretching band of amine (NH2). It was also observed that addition of AgSD did not change the spectra of pure zein, which was indication that there was no chemical reaction between zein and AgSD.27 The band corresponding to the stretching of the N–H and O–H bonds of the amino acids of the protein appeared between 2800 and 3500 cm−1. Another peak was observed at 1650 cm−1, which was corresponding to stretching of the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of amide groups belonging to the peptide groups. The peak at 1540 cm−1 was associated with angular deformational vibrations of the N–H bond, and finally, the band at 1230 cm−1 was associated with the axial deformational vibration of the C–N bond.
O) of amide groups belonging to the peptide groups. The peak at 1540 cm−1 was associated with angular deformational vibrations of the N–H bond, and finally, the band at 1230 cm−1 was associated with the axial deformational vibration of the C–N bond.
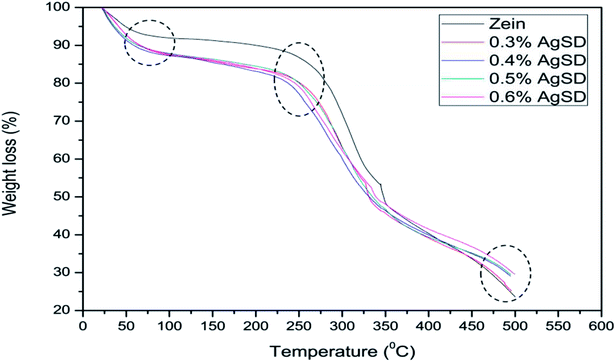
Thermogravimetric analysis (TGA)
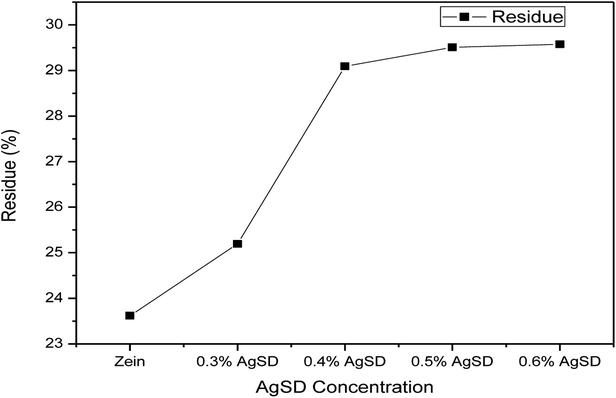
Thermogravimetric analysis was done to analyze the effect of AgSD on thermal degradation of zein. TGA graph is divided in three parts. Where 1st part (up to 100 °C) shows removal of moisture, 2nd part shows the thermal degradation onset and the last part shows combustion zone where we analyze the residual amount. It can be shown in Fig. 7 that 1st part of graph showed higher moisture absorption in zein/AgSD composite nanofibers mats as graph went downward because of removal of moisture contents in nanofiber mats. 2nd part of graph, which declares the thermal degradation onset, showed that onset of pure zein nanofibers was at 270 °C and that was for zein/AgSD nanofibers started from 260 °C which showed slight lower thermal stability by addition of AgSD to pure zein but not much affected. Offset of thermal degradation curve for both pure zein and AgSD added samples was found to be at same temperatures. Last part of TGA graph showed different residual percentage for each sample. As concentration of AgSD increased in zein, the residue was also increased which clearly presented the presence of AgSD in zein nanofibers.Fig. 8 shows broad view of residual amount of zein nanofibers and zein/AgSD nanofibers. Pure zein nanofibers had 23.31% residue content at 500 °C, residue contents for 0.3% AgSD, 0.4% AgSD, 0.5% AgSD, and 0.6% AgSD were 25.16%, 29.089%, 29.50%, and 29.58% respectively. So, it was concluded that addition of AgSD in zein caused increase in residual contents of nanofibers.
Anti-bacterial activity test
In order to investigate of antibacterial properties, disc diffusion method was carried out as shown in Fig. 9.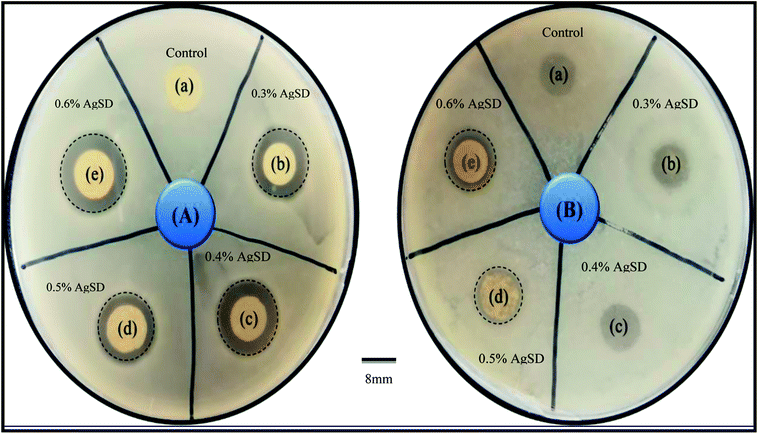 | ||
| Fig. 9 Antibacterial activity test by disk diffusion method (A) E. coli (B) Bacillus ((a) control, (b) 0.3% AgSD in zein, (c) 0.4% AgSD in zein, (d) 0.5% AgSD in zein, (e) 0.6% AgSD in zein). | ||
Bacillus and E. coli bacteria strain were used as Gram-positive and Gram-negative respectively. The antibacterial effectiveness of AgSD released from zein nanofibers was determined from the zone inhibition of the bacteria. The antibacterial activity of zein nanofibers loaded by drug observed with both strains of bacteria in comparison to control. Excellent antibacterial efficacy was attributed to sample with 0.6% AgSD. It was observed that increasing the amount of drug had a significant effect on the antibacterial activity of nanofibers mat. For example, some of the samples were degraded by bacterial action due to insufficient amount of drug in those samples (Fig. 1 B). Herein, from the results it was concluded that, the sample with a drug concentration of 0.6% showed the excellent antibacterial activity in comparison to control and other samples. Where, samples (a), (b), (c), (d), (e) represent pure zein, 0.3% AgSD, 0.4% AgSD, 0.5% AgSD, and 0.6% AgSD respectively.
Investigation of release properties
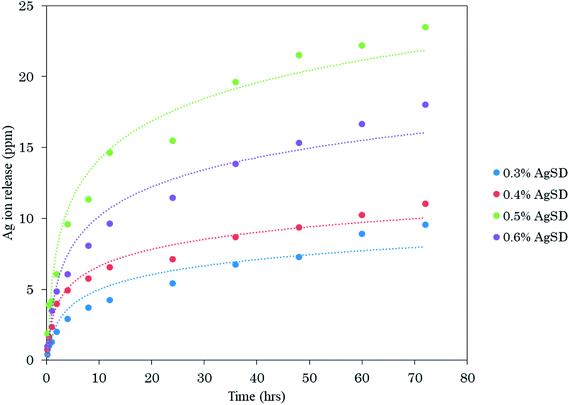
In order to analyze release behavior of Ag from zein nanofibers. 0.5 g of AgSD/zein nanofibers (each sample with different AgSD concentration) was immersed in 50 ml of deionized water and keep on stirring (slowly) for 72 hours and analyzed by ICP. It was observed (Fig. 10) that release of Ag was gradually increased with increasing concentration of AgSD in zein nanofibers. It was also observed that samples were not dissolved in deionized water over 72 hours. Sample with 0.6% AgSD concentration showed a maximum of 23.5 ppm release of Ag ion. Samples with 0.3%, 0.4%, and 0.5% AgSD concentration showed 9.54 ppm, 11.01 ppm, and 18.05 ppm of Ag ion. From release test, it was observed that prepared nanofiber mats has good release properties and can be effective for wound care applications.Conclusions
AgSD loaded zein nanofibers were successfully prepared by above mentioned methods. From antibacterial activity test it was concluded that prepared nanofiber mats will be effective in wound care applications. Nanofibers with 0.6% AgSD in zein, had shown excellent antibacterial activity for both Gram positive as well as Gram negative bacteria, so from this result we recommend 0.6% concentration of drug for further applications. Release test was carried out by ICP which also showed excellent release properties of sample having 0.6% concentration of AgSD as compared to other samples with low concentration of AgSD. SEM images had shown uniform morphology with no beads formation at 25% of zein concentration. After analysis of XPS and EDX, it was concluded that AgSD did not disturbed the crystalline structure of zein and was present in the nanofibers. FTIR study had shown that there was no chemical reaction involved between zein and AgSD. Thermal properties of AgSD/zein nanofibers were also analyzed which declared that nanofibers containing AgSD were also stable above 260 °C. The degradation temperature was not increased by the addition of AgSD which was sign that degradability of zein was not much disturbed due to presence of AgSD in nanofibers. Finally, from all above mentioned results and discussions it was concluded that AgSD loaded zein nanofibers have great potential to be used as wound dressing, and will be applicable on industrial scale manufacturing of nonwoven wound dressings.Conflicts of interest
There are no conflicts to declare.References
- D. Kharaghani, Y. Kee Jo, M. Q. Khan, Y. Jeong, H. J. Cha and I. S. Kim, Electrospun antibacterial polyacrylonitrile nanofiber membranes functionalized with silver nanoparticles by a facile wetting method, Eur. Polym. J., 2018, 108, 69–75, DOI:10.1016/j.eurpolymj.2018.08.021.
- H. Lee, G. Xu and D. Kharaghani, et al., Electrospun tri-layered zein/PVP-GO/zein nanofiber mats for providing biphasic drug release profiles, Int. J. Pharm., 2017, 531(1), 101–107, DOI:10.1016/j.ijpharm.2017.08.081.
- M. Q. Khan, D. Kharaghani and N. Nishat, et al., The development of nanofiber tubes based on nanocomposites of polyvinylpyrrolidone incorporated gold nanoparticles as scaffolds for neuroscience application in axons, Text. Res. J., 2018, 004051751880118, DOI:10.1177/0040517518801185.
- U. Dashdorj, M. K. Reyes and A. R. Unnithan, et al., Fabrication and characterization of electrospun zein/Ag nanocomposite mats for wound dressing applications, Int. J. Biol. Macromol., 2015, 80, 1–7, DOI:10.1016/j.ijbiomac.2015.06.026.
- C. L. Fox and S. M. Modak, Mechanism of Silver Sulfadiazine Action on Burn Wound Infections Mechanism of Silver Sulfadiazine Action on Burn Wound Infections, Antimicrob. Agents Chemother., 1974, 5(6), 582–588, DOI:10.1128/AAC.5.6.582.
- I. Alan Holder, In vitro inactivation of silver sulphadiazine by the addition of cerium salts, Burns, 1982, 8(4), 274–277, DOI:10.1016/0305-4179(82)90009-2.
- M. Rai, A. Yadav and A. Gade, Silver nanoparticles as a new generation of antimicrobials, Biotechnol. Adv., 2009, 27(1), 76–83, DOI:10.1016/j.biotechadv.2008.09.002.
- L. Francis, F. Giunco, A. Balakrishnan and E. Marsano, Synthesis, characterization and mechanical properties of nylon-silver composite nanofibers prepared by electrospinning, Curr. Appl. Phys., 2010, 10(4), 1005–1008, DOI:10.1016/j.cap.2009.12.025.
- A. T. Hang, B. Tae and J. S. Park, Non-woven mats of poly(vinyl alcohol)/chitosan blends containing silver nanoparticles: fabrication and characterization, Carbohydr. Polym., 2010, 82(2), 472–479, DOI:10.1016/j.carbpol.2010.05.016.
- B. Pant, H. R. Pant and D. R. Pandeya, et al., Characterization and antibacterial properties of Ag NPs loaded nylon-6 nanocomposite prepared by one-step electrospinning process, Colloids Surf., A, 2012, 395, 94–99, DOI:10.1016/j.colsurfa.2011.12.011.
- A. GhavamiNejad, A. Rajan Unnithan and A. Ramachandra Kurup Sasikala, et al., Mussel-Inspired Electrospun Nanofibers Functionalized with Size-Controlled Silver Nanoparticles for Wound Dressing Application, ACS Appl. Mater. Interfaces, 2015, 7(22), 12176–12183, DOI:10.1021/acsami.5b02542.
- C. H. Yang, L. S. Wang and S. Y. Chen, et al., Microfluidic assisted synthesis of silver nanoparticle–chitosan composite microparticles for antibacterial applications, Int. J. Pharm., 2016, 510(2), 493–500, DOI:10.1016/j.ijpharm.2016.01.010.
- B. Maharjan, M. K. Joshi, A. P. Tiwari, C. H. Park and C. S. Kim, In-situ synthesis of AgNPs in the natural/synthetic hybrid nanofibrous scaffolds: Fabrication, characterization and antimicrobial activities, J. Mech. Behav. Biomed. Mater., 2017, 65, 66–76, DOI:10.1016/j.jmbbm.2016.07.034.
- T. Miyoshi, K. Toyohara and H. Minematsu, Preparation of ultrafine fibrous zein membranes via electrospinning, Polym. Int., 2005, 54(8), 1187–1190, DOI:10.1002/pi.1829.
- S. Torres-Giner, E. Gimenez and J. M. Lagaron, Characterization of the morphology and thermal properties of zein prolamine nanostructures obtained by electrospinning, Food Hydrocolloids, 2008, 22(4), 601–614, DOI:10.1016/j.foodhyd.2007.02.005.
- B. Zhang, Y. Luo and Q. Wang, Development of silver–zein Composites as a promising antimicrobial agent, 2010:pp. 2366–2375 Search PubMed.
- F. Kayaci and T. Uyar, Electrospun zein nanofibers incorporating cyclodextrins, Carbohydr. Polym., 2012, 90(1), 558–568, DOI:10.1016/j.carbpol.2012.05.078.
- K. Karthikeyan, S. Guhathakarta, R. Rajaram and P. S. Korrapati, Electrospun zein/eudragit nanofibers based dual drug delivery system for the simultaneous delivery of aceclofenac and pantoprazole, Int. J. Pharm., 2012, 438(1–2), 117–122, DOI:10.1016/j.ijpharm.2012.07.075.
- Y. N. Jiang, H. Y. Mo and D. G. Yu, Electrospun drug-loaded core-sheath PVP/zein nanofibers for biphasic drug release, Int. J. Pharm., 2012, 438(1–2), 232–239, DOI:10.1016/j.ijpharm.2012.08.053.
- L. Y. Huang, C. Branford-White, X. X. Shen, D. G. Yu and L. M. Zhu, Time-engineeringed biphasic drug release by electrospun nanofiber meshes, Int. J. Pharm., 2012, 436(1–2), 88–96, DOI:10.1016/j.ijpharm.2012.06.058.
- J. M. Yang, Z. L. sheng, D. G. Yu and J. Liu, Coaxial electrospinning with acetic acid for preparing ferulic acid/zein composite fibers with improved drug release profiles, Colloids Surf., B, 2013, 102, 737–743, DOI:10.1016/j.colsurfb.2012.09.039.
- W. Huang, T. Zou, S. Li, J. Jing, X. Xia and X. Liu, Drug-Loaded Zein Nanofibers Prepared Using a Modified Coaxial Electrospinning Process, AAPS PharmSciTech, 2013, 14(2), 675–681, DOI:10.1208/s12249-013-9953-1.
- R. Paliwal and S. Palakurthi, Zein in controlled drug delivery and tissue engineering, J. Controlled Release, 2014, 189, 108–122, DOI:10.1016/j.jconrel.2014.06.036.
- A. R. Unnithan, G. Gnanasekaran, Y. Sathishkumar, Y. S. Lee and C. S. Kim, Electrospun antibacterial polyurethane-cellulose acetate-zein composite mats for wound dressing, Carbohydr. Polym., 2014, 102(1), 884–892, DOI:10.1016/j.carbpol.2013.10.070.
- X. Hu, S. Liu, G. Zhou, Y. Huang, Z. Xie and X. Jing, Electrospinning of polymeric nanofibers for drug delivery applications, J. Controlled Release, 2014, 185(1), 12–21, DOI:10.1016/j.jconrel.2014.04.018.
- S. López-Alcaide, M. Nakamura, R. Macip-Ríos and E. Martínez-Meyer, Does behavioural thermoregulation help pregnant Sceloporus adleri lizards in dealing with fast environmental temperature rise?, J. Herpetol., 2014, 24(1), 41–47, DOI:10.1097/00042752-200605000-00013.
- E. Corradini, P. S. Curti, A. B. Meniqueti, A. F. Martins, A. F. Rubira and E. C. Muniz, Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials, Int. J. Mol. Sci., 2014, 15(12), 22438–22470, DOI:10.3390/ijms151222438.
| This journal is © The Royal Society of Chemistry 2019 |