 Open Access Article
Open Access ArticleElectro-membrane processes for organic acid recovery
L. Handojo
a,
A. K. Wardani
a,
D. Regina
a,
C. Bella
a,
M. T. A. P. Kresnowati
a and
I. G. Wenten
 *ab
*ab
aDepartment of Chemical Engineering, Institut Teknologi Bandung, Jl. Ganesha 10, Bandung 40132, Indonesia. E-mail: igw@che.itb.ac.id
bResearch Center for Nanosciences and Nanotechnology, Institut Teknologi Bandung, Jl. Ganesha 10, Bandung 40132, Indonesia
First published on 11th March 2019
Abstract
With an increase in the organic acid requirement, the production of organic acids has been increased over the years. To achieve cost-effective production of organic acids, efficient recovery processes are needed. Electro-membrane processes, including electrodialysis (ED), electrometathesis (EMT), electro-ion substitution (EIS), electro-electrodialysis (EED), electrodialysis with bipolar membrane (EDBM), and electrodeionization (EDI), are promising technologies for the recovery of organic acids. In the electro-membrane processes, organic acids are separated from water and other impurities based on the electro-migration of ions through ion-exchange membranes. These processes can recover various types of organic acids from the fermentation broth with high recovery yield and low energy consumption. In addition, the integration of fermentation and the electro-membrane process can improve the acid recovery with lower byproduct concentration and energy consumption.
Introduction
Organic acids are organic compounds composed of a weak acid group, ranging widely from carboxylic (pKa = 3) to phenolic groups (pKa = 9).1 Due to their biodegradability, there has been an increase in the applications of organic acids in the last decade; for example, lactic acid has significant potential for applications in biodegradable plastics and bio-adaptable medical sutures;2–4 succinic acid plays an important role as a precursor molecule in the synthesis of biodegradable polyester resins, dyestuffs, and pharmaceuticals;5 citric acid is widely used as a flavoring agent for foods and beverages as well as a cleaning and polishing agent,6–8 and formic acid is mainly used in the textile and paper industries.9,10In general, there are two routes for the production of organic acids: chemical synthesis and carbohydrate fermentation. The chemical synthetic route is limited by its production capacity because it is associated with the byproduct of another process11 and has high manufacturing costs; moreover, carbohydrate fermentation can utilize various raw materials and produce organic acids with a higher degree of safety; thus, it has mainly been chosen to produce organic acids in the recent years.12 A wide variety of renewable resources, such as silage, grains, syrups, molasses, and cheese whey, can be used as raw materials in carbohydrate fermentation. However, the fermentation broth obtained from these resources usually consists of various ingredients, and thus, its separation and purification are necessary to achieve high purity of the organic acids.
About 50–70% of the cost of the production of organic acid is generated by the recovery of the fermentation product.13,14 Therefore, various technologies, such as precipitation, extraction, adsorption, ion-exchange system, membrane, etc.,15–23 have been developed for the efficient recovery of organic acids. However, some of these conventional technologies, especially extraction, adsorption, and ion-exchange, require further concentration steps, produce dangerous waste, require hazardous solvents, and consume high energy.12 Therefore, the recovery of organic acids has been shifted from conventional technologies to electro-membrane processes including electrodialysis (ED), electrometathesis (EMT), electro-ion substitution (EIS), electro-electrodialysis (EED), electrodialysis with bipolar membranes (EDBM), and electrodeionization (EDI). The electro-membrane process separates organic acids from water and other impurities based on the electro-migration of ions through ion-exchange membranes. Thus, it can provide high quality product in a short time without salt introduction or discharge.3
Fig. 1 shows the number of publications on the recovery of organic acids since 1980. It can be seen that the number of publications on the recovery of organic acids has increased over years, with the highest number of publications in 2010. Based on technology, the publications on the recovery of organic acids via extraction was dominant with up to 51%. However, since 2000, there has been an increase in the number of publications on the recovery of organic acids via electro-membrane processes due to their ability to recover organic acids with high yield and without the requirement of solvent addition. In the earlier studies, ED was the only electro-membrane process applied for organic acid recovery. Compared to conventional processes, ED requires lower energy consumption and eliminates the use of solvent. However, the purity of the acid product from the ED process is relatively low. Therefore, many researchers have tried to develop the ED process via stack modification (i.e. EMT, EIS, and EED), membrane modification (i.e. EDBM), resin addition (i.e. EDI), and integration with other processes.
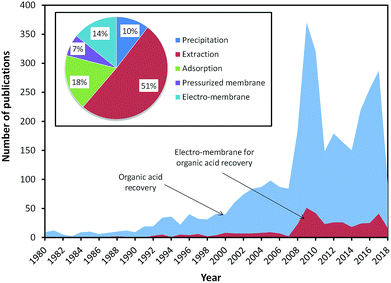 | ||
| Fig. 1 The number of reported studies related to the search term organic acid recovery, as indexed by Scopus (TITTLE-ABS-KEY (terms); September 2018). | ||
A review on the recovery of organic acids via electro-membrane processes was published by Huang et al. in 2007.12 However, their review mainly focused on the ED configuration and its economic evaluation. Thus, for a better understanding of this process, we aim to provide a more comprehensive review of the recovery of organic acids using electro-membrane processes. The conventional processes for the recovery of organic acids are summarized to give a better overview on the state of the art of organic acid recovery. Meanwhile, the discussion on electro-membrane processes includes not only their configuration, but also their performances and design development on the recovery of various organic acids. In addition, the issues with electro-membrane processes and their possible solutions are discussed.
Conventional processes for organic acid recovery
Separation and purification of fermentation broth are the primary determinants for the cost-effective production of organic acids. Calcium precipitation using Ca(OH)2 or CaO is one of the most used methods to recover organic acids from fermentation broth. This method has been used for recovery of citric acid,25,26 lactic acid,27 and succinic acid.5,28,29 Calcium precipitation offers the advantage of being directly performed on existing equipment, technology and infrastructure, and thus it has very low technological barriers and risks.24 Beside calcium precipitation, the recovery of succinic acids also has been conducted by ammonia precipitation. Succinic acid was recovered up to 93.3% using ammonia precipitation.30 Generally, precipitation produces a low amount of waste by-products since the used reagent can be recycled. However, this method requires high energy consumption and equipment erosion due to the low pH and high temperature in the reagent recycle process.Liquid–liquid extraction has also been widely used for the recovery of organic acids. The liquid–liquid extraction system consists of two separate liquid phases, where the organic acid transfers from one phase to the other based on the solubility differences between the phases.31 In liquid–liquid extraction, the selection of an appropriate solvent is fundamental to obtain a high organic acid recovery rate. There are seven important criteria for solvent selection: (a) good distribution coefficient for organic acid uptake, (b) high separation capacity, (c) high selectivity, (d) easy product backwashing with water, (e) low tendency to emulsify, (f) low cost, and (g) non-toxic.32 Several organic solvents have been widely used, such as butyl alcohol, acetone and tributyl phosphate or with certain amines.23,33–36 Among them, amine extraction is one of the most prospective methods for the separation of carboxylic or hydroxyl-carboxylic acid from aqueous solution.32,37,38
In several cases, the distribution of organic acids in the extraction phase seems to be unsatisfactory, and thus large quantities of extraction agent are required. Consequently, some researchers developed reactive extraction to solve this problem. The product from fermentation is firstly converted into a compound without carboxyl groups and then recovered by liquid–liquid extraction.24 This method has been used to recover succinic acid in several studies.5,39,40 The results showed that more than 95% succinic acid was recovered by reactive extraction. This method also has been applied for lactic acid,20,41–44 acetic acid,45–48 carboxylic acid,49,50 and propionic acid21,51,52 since these organic acids are poorly extractable by common organic solvents due to their hydrophilic nature.
The other alternative for the recovery of organic acids is the use of solid sorbents that are selective for organic acids. To obtain high recovery yield, the sorbent must have a high separation capacity for the acid and specificity for the product.24,41 Alumina, activated carbon, silica, and zeolite molecular sieves are examples of sorbents widely used to recover organic acids from fermentation broth. To adsorb succinic acid, alumina53 and a high-silica zeolite (SiO2/Al2O3 = 218)54 were chosen as sorbents. Meanwhile, for lactic acid recovery, adsorption by silicalite molecular sieves15 and activated carbon55 was used. In addition, activated carbon also has been utilized to recover acetic acid, butyric acid, fumaric acid, and propionic acid.18,19 Several researchers also studied the use of ion exchange resins to adsorb organic acids. Various types of ion exchange resins such as alkaline-type anion exchange resins,56,57 weak base anion-exchange resins (Amberlite IRA-92,58 IRA-400,17,59–61 and IRA-900 (ref. 62)) and poly(4-vinylpyridine) (PVP) resins55,63–65 have been used. The studies showed that a weakly basic ion exchange resin was the best ion exchange resin for the purification of organic acids.
Membrane technologies have also proven for the advanced recovery of organic acids. Compared to other conventional technologies, pressure-driven membranes offer the advantages of process continuity and high selectivity. For small molecules such as organic acids and salts, nanofiltration (NF) membranes can be used to separate them efficiently. NF membranes consist of a dense, ultrathin skin layer on microporous polymeric supports, and mostly with charged groups on the membrane surface.66 The rejection mechanism in NF membranes is a combination of size sieving, solution-diffusion, and Donnan exclusion.67 NF has been used for the recovery of lactic acid by several researchers.68–71 These studies showed that NF was able to recover highly purified lactic acid by removing 85% of mineral ions such as Na+, K+, Mg2+, and Ca2+ and 90% of residual carbohydrates. Several researchers also tried to combine NF with other processes such as microfiltration and ultrafiltration to improve the recovery efficiency.72–75 In addition, membrane distillation (MD) has also been studied to recover organic acids. The organic acid is separated from impurities by heating and evaporating the liquid and allowing the vapor to pass through a microporous hydrophobic membrane. Then the vapors are allowed to condense into liquid by cooling on the other side of the membranes.76 Ban et al.77 used this method to recover various types of organic acids, including glycolic acid, glyoxylic acid lactic acid, pyruvic acid, malonic acid, and glutaric acid. Their results showed that the rejection rate for all the abovementioned organic acids except pyruvic acid was above 97.0%. Generally, MD is able to recover organic acids with a high recovery rate; however, its thermal energy efficiency is very low.
Table 1 shows the recovery yield of organic acids using various conventional technologies. It can be seen that most of them were successfully utilized to recover organic acids with recovery yields greater than 60%. However, each technology has advantages and disadvantages, as summarized in Table 2. Calcium precipitation offers the advantage of the ability to be directly used on existing equipment, technology, and infrastructure, and thus has very low technological barriers and risks. However, it has low selectivity and produces CaSO4 sludge. Liquid–liquid extraction offers some advantages, such as high purity product and low energy consumption, but the use of hazardous solvents leads to environmental problems. Moreover, adsorption using solid sorbents or ion exchange resins is a reliable technology, but it requires large amounts of chemicals for the regeneration of the ion exchange resins and adjustment of the feed pH to increase the sorption efficiency. Meanwhile, pressure-driven membranes have several advantages, such as process continuity and easy scale-up, compared to conventional technologies; however fouling is still the main limitation in membrane separation processes.
| Acid | Method | Recovery yield (%) | Ref. |
|---|---|---|---|
| Acetic acid | Extraction | 66–97 | 35, 45, 46, 48 and 78 |
| Adsorption | 94 | 79 | |
| Pressure-driven membrane | 88 | 80 | |
| Citric acid | Extraction | 90 | 37 |
| Formic acid | Extraction | 87 | 81 |
| Fumaric acid | Extraction | 70.7 | 22 |
| Adsorption | 85–93 | 19 and 62 | |
| Lactic acid | Extraction | 37–97 | 20, 23, 31, 33, 43 and 82–84 |
| Adsorption | 74–95 | 15, 17, 65 and 85 | |
| Precipitation | 92 | 27 | |
| Pressure-driven membrane | 60–100 | 74, 75 and 80 | |
| Picolinic acid | Extraction | 75–96.6 | 38 and 86 |
| Propionic acid | Extraction | 75 | 21 |
| Adsorption | 64 | 18 | |
| Pyruvic acid | Extraction | 40–82 | 87 and 88 |
| Succinic acid | Extraction | 67–95 | 40 and 89–91 |
| Precipitation | 93.3 | 30 | |
| Adsorption | 96–99 | 57 and 62 | |
| Tartaric acid | Extraction | 90 | 23 |
| Adsorption | 75–99 | 92 and 93 |
| Technology | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Precipitation | Very low technological barriers and risks | Resulting CaSO4 sludge (notorious for solid pollution) | 12, 14 and 24 |
| Low selectivity | |||
| Extraction | High purity product | Undesirable distribution coefficients | 14, 24 and 94 |
| Low energy consumption | Environmental problems due to the use of hazardous solvents | ||
| Expensive extraction agent and diluent | |||
| Adsorption | Easy to operate | Short lifetime of adsorbents | 12 and 18 |
| Low capacity | |||
| Ion exchange | Easy to operate | Consumes a great amount of acid, base, and water to regenerate ion exchange resins | 12 |
| Pressure-driven membrane | High selectivity | Fouling formation | 95 |
| Easy to operate | |||
| Easy to scale-up | |||
| Electro-membrane processes | High purity product | Fouling formation | 96 and 97 |
| No need salt introduction or discharge |
Electro-membrane processes for organic acid recovery
The improvement of technologies for organic acids recovery is very important since it is associated with fermentation process effectiveness and production costs. The conventional technologies generally need further concentration steps, produce dangerous waste, require hazardous solvents, and consume high energy. To solve those problems, organic acid recovery has shifted to electro-membrane processes. In electro-membrane processes, organic acids are separated from water and other impurities based on the electro-migration of ions through ion-exchange membranes. These processes are able to recover various types of organic acids from fermentation broth with high purity and yield without requiring the use of solvent.Electrodialysis (ED)
One of the promising technologies to obtain high purity organic acids without the requirement of any solvent is electrodialysis or ED. ED is an electrochemical separation process with the driving force of electrical potential difference to separate ionic species through ion exchange membranes. It has been applied for the production of table salt, organic acid recovery, heavy metal recovery, and sugar demineralization.98–101 ED mainly consists of three compartments, including diluate, concentrate, and electrode compartments. The compartments are separated by anion and cation exchange membranes arranged between an anode and cathode,99 as shown in Fig. 2(a).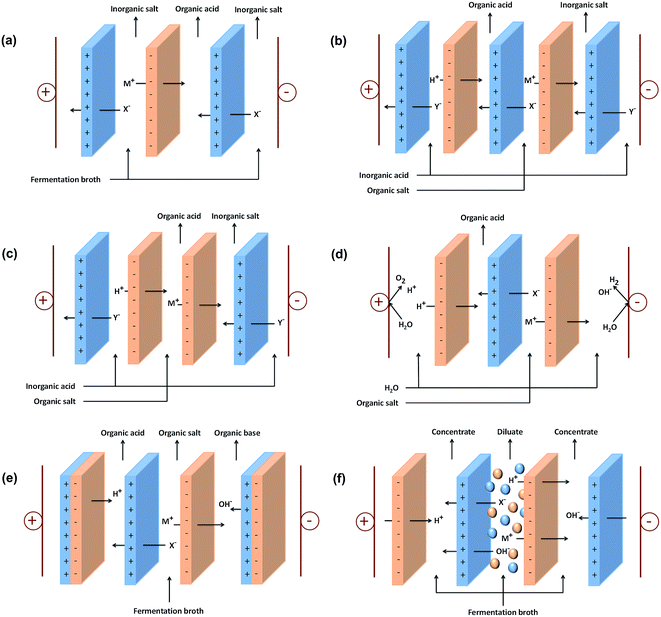 | ||
| Fig. 2 Schematic of the stack configuration for: (a) ED, (b) EMT, (c) EIS, (d) EED, (e) EDBM, and (f) EDI to produce organic acids. | ||
The ion-exchange membranes are made of a macromolecular material that carries ionic groups.102 The cation exchange membranes (CEM) possess negatively charged fixed groups and allow the migration of cations, while anion exchange membranes (AEM) possess positively charged fixed groups and allows the migration of anions. When a sufficient electrical field is applied on the solution, the mobile ions are replaced by same charged ions (counter ion) from the solution and the membrane matrix will repel ions that have the same charge as the membrane matrix (co-ion). This mechanism allows the selective passage of ions.103
To recover organic acids from fermentation broth, the feed solution is circulated on each compartment of the ED system, while a potential difference is applied between the two electrodes. Due to the potential difference, positive ions (cations) migrate to the negative electrode (cathode), while the negative ions (anions) migrate to the positive electrode (anode). After some time, organic acids as ionic species are stripped from the diluate compartment and collected in the concentrate compartment. Uncharged molecules are not affected by this driving force, and hence organic acids can be separated from the uncharged components in the solution.104
Electrometathesis (EMT)
EMT is a modified ED process, which has been used for the recovery of citric acid105 and lactic acid.94,106 Generally, an EMT process consists of four compartments formed by two alternately arranged anion exchange membranes and two cation exchange membranes with two types of feed streams and outputs, as shown in Fig. 2(b). EMT can realize double composition reactions that hardly occur in other conditions than that in ED systems. In EMT, inorganic acids such as HCl and H2SO4 are necessary for the conversion of organic salts into organic acids.12 The addition of strong acid such as HCl in the product compartment helps to facilitate the transport of ions.107Electro-ions substitution (EIS)
EIS is another modified ED technique that consists of two adjacent cation-selective (or anion-selective) membranes, one anion-selective (or cation-selective) membrane, and three compartments, as shown in Fig. 2(c). EIS can realize substitution between ions of the same sign. The H+ needed for ion substitution is supplied by an additional feed stream using an inorganic acid, such as H2SO4. At the beginning of the EIS process, a current efficiency of more than 100% can be reached due to H+/M+ Donnan dialysis. However, more M+ in the acid stream will compete with H+, and hence decrease the current efficiency. To suppress this competition, a cation-selective membrane with a higher H+/M+ permselectivity can be added.12Electro-electrodialysis (EED)
EED is an alternative methods that can be used to convert organic salt into organic acid due to its effective functional integration of ion exchange membranes and water splitting formed at the cathode and anode by reduction and oxidation.108 A schematic representation of the EED process is presented in Fig. 2(d). In EED, the anion exchange membrane is essential since the acids are displaced by permselective transport of acid anions through the anion exchange membrane and electrodialysis of protons from the electrochemical reactions that occur at the electrodes.109,110 This method was mainly used for the production of formic acid.110,111 Formate anions (HCOO−) pass through the anion exchange membrane into the anolyte compartment. In this compartment, formic acid is formed by combining formate anions with the protons produced by anodic water oxidation. Meanwhile, H+ is retained in the catholyte compartment and reacts with hydroxide ions to form water.Besides formic acid, EED also has been used for the recovery of lactic acid,112 acetic acid,113 salicylic acid,114 glutamic acid,115 and propionic acid.116 Generally, the overall electrical current efficiencies of EED are much higher than that from conventional ED. However, the concentration ratio of the concentrated solutions is low, even less than 1.0.117 Therefore, some studies developed two-phase electro-electrodialysis (TPEED) to improve the recovery ratio of the produced acid. TPEED has been used to recover citric acid117 and lactic acid.112,118 This method successfully increased the acid concentration ratio with half the energy consumption for EED.
Electrodialysis with bipolar membranes (EDBM)
To improve the separation performance of ED, several researchers combined the conventional ED and water dissociation of bipolar membranes, exploring a new avenue for the development of conventional ED. A bipolar membrane is a combination of a cation-selective and an anion-selective membrane; however, it has distinctive functions from that mono-polar membranes. Under a reverse potential bias, bipolar membranes can realize the dissociation of solvent molecules.12 EDBM can split and separate water into H+ and OH− ions and the membranes can operate at about 80% of the theoretical thermodynamic efficiency.119 This technology also enables H+ to be transported to the acid anion to form the free acid, while the OH− ion is transported to the cation compartment to form the free base. Therefore, separation efficiency and acid purity can be increased by the addition of bipolar membrane. Besides, the effluent (glucose, calcium, magnesium, etc.) from EDBM can be reused for fermentation after simple processing. However, the cost of membranes is still the main limitation for the large-scale application of EDBM.120For application in organic acid recovery, EDBM is mainly operated using a three-compartment configuration, as shown in Fig. 2(e). This configuration consists of brine, base, and acid-containing streams.121 The fermentation broth passes through the membrane stack in the brine compartment, which is placed between an anion and a cation exchange membrane. The compartment between the cation membrane and the anion side of the bipolar membrane is filled with the base stream, where hydroxide is generated. Meanwhile, the acid stream is placed between the anion membrane and the cation side of the bipolar membrane, where acid is generated. The anions are transported from the brine compartment to the acid compartment through the anion membrane, while cations are transported from the brine compartment to the base compartment through the cation membrane. In this configuration, hydrochloric acid and Na/K hydroxide can be recovered in the acid and base compartments, respectively.102
Electrodeionization (EDI)
EDI is a modified ED process with the presence of ion exchange resins between the anion and cation exchange membranes, as illustrated in Fig. 2(f). The presence of ion exchange resins enhances the ionic conductivity of a dilute solution by absorbing and concentrating the ions on the resin beads. Besides, the water splitting reaction on the resins surface enables in situ regeneration of the ion exchange resins and aids in the transport of ionic species across the ion exchange membranes.7 The water splitting reaction produces protons and hydroxyl ions at the resin–resin and resin–membrane interfaces. These ions continuously replace the adsorbed ions on the ion exchange resins surface; thus, resulting in more conductive ion exchange resins pathway.122 Thus, EDI offers a continuous process where ion exchange resins can be continuously regenerated by a direct current electric field.123 Due to its ability to produce high purity products, EDI is mainly applied for the production of ultrapure water.124–126 However, several studies on the recovery of organic acids using EDI also have been established, such as the recovery of citric acid,7 amino acid,127 butyric acid,128,129 and lactic acid.123Performance of organic acid recovery using electro-membrane processes
For high productivity, fermentation-based processes require maintenance of near neutral pH and addition of alkali in most cases for the production of organic acids. The addition of alkali leads to the formation of organic salt instead of organic acid. To overcome this problem, electro-membrane processes have attracted the attention of several researchers since they do not require the addition of acid or alkali.Table 3 summarizes the reported results for various electro-membrane processes for the recovery of organic acids. Among the various organic acids, many researchers have focused on the recovery of lactic acid due to its multifunctional applications as a preservative in the food, pharmaceutical, leather, and textile industries, and a chemical feedstock.130 The recovery of lactic acid has been conducted using ED, EIS, EDBM, and EDI. The results showed that these electro-membrane processes were able to recover more than 90% of lactic acid with a final concentration of up to 185 g L−1.
| Organic acid | Technology | Operation condition | Results | Ref. |
|---|---|---|---|---|
| Acetic acid | ED | Membrane effective area: 10 cm2 | Current efficiency: 80–98% | 131 |
| Voltage: 5, 10, 15 V | Recovery rate: 24.05–40.82% (configuration 1) and 91–394% (configuration 2) | |||
| EED | Membrane effective area: 32 cm2 | Removal efficiency: >90% | 113 | |
| EDBM | Membrane effective area: 98 cm2 | Acid recovery: up to 70% | 132 | |
| Acid concentration: 0.2% | Current efficiency: 40% | |||
| Voltage: 30 V | ||||
| Amino acid | ED | Membrane effective area: 36 cm2 | Recovery rate: up to 63% | 133 |
| pH: 12.5 | Current efficiency: up to 83% | |||
| Acid concentration: 25, 50, 75, 100 mM | Energy consumption: 3 kW h kg−1 | |||
| EDBM | Lysine concentration: 146.19 M | Acid concentration increase 35–50 times | 134 | |
| Arginine concentration: 174.21 M | ||||
| Histidine concentration: 155.16 M | ||||
| Membrane effective area: 20 cm2 | ||||
| Current density: 2–10 mA cm−2 | ||||
| EDBM | Methionine concentration: 24.65 g L−1 | Acid purity: 99.98% | 135 | |
| Membrane effective area: 945 cm2 | Current efficiency: 75.10% | |||
| Current density: 150–300 mA cm−2 | Energy consumption: 2.156–3.265 kW h kg−1 | |||
| EDI | Membrane effective area: 50 cm2 | Current efficiency: 60–85% | 127 | |
| Voltage: 20 V | Energy consumption: 1–1.6 kW h kg−1 | |||
| Resin filling: 0, 5, 10, 20 mL | ||||
| Butyric acid | ED | Current: 0.05 A | Acid purity: 85% | 129 |
| Current efficiency: 52% | ||||
| EDI | Membrane effective area: 10 cm2 | Recovery rate: 82–89% | 128 | |
| Voltage: 3.6–4.7 V | Current efficiency: 81–85% | |||
| pH: 2–4.5 | Energy consumption: 0.197–0.204 kW h kg−1 | |||
| EDI | Membrane effective area: 0.001 m2 | Acid purity: 92% | 129 | |
| Current: 0.05 A | Current efficiency: 59% | |||
| Citric acid | ED | Membrane effective area: 3900 cm2 | Optimum p: 7.5 | 136 |
| Voltage: 10, 15, 20 V | Optimum voltage: 20 V | |||
| Flow rate: 4, 8, 12 mL min−1 | Optimum flow rate: 4 mL min−1 | |||
| pH: 2, 4.5, 7.5 | ||||
| EDBM | Membrane effective area: 50 cm2 | Final acid concentration: up to 0.65 mol dm−3 | 137 | |
| Acid concentration: 0.1 mol dm−3 | ||||
| Current density: 52, 78, 104 mA cm−2 | ||||
| EDBM | Membrane effective area: 220 cm2 | Current efficiency: 73.7–100% | 138 | |
| Current density: 30, 40, 50 mA cm−2 | Energy consumption: 4–8 kW h kg−1 | |||
| EDI | Membrane effective area: 50 cm2 | Current efficiency: 40–96% | 7 | |
Acid concentration: 500–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm 000 ppm |
Energy consumption: 1.16 kW h kg−1 | |||
| Flow rate: 1–4 L h−1 | ||||
| Formic acid | EED | Temperature: 30 °C | Concentration ratio: 1.2–1.5 | 111 |
| Current density: 8–29 mA cm−2 | Current efficiency: 70–140% | |||
| EED | Membrane effective area: 7.07 cm2 | Current efficiency: >100% | 110 | |
| Current density: 3, 5, 10, 15 mA cm2 | Formic acid transport through the membranes: 0.004–0.120 M | |||
| Temperature: 20–40 °C | ||||
| Acid concentration: 0.05–0.5 M | ||||
| Anolyte and catholyte concentration: 0.1 M | ||||
| Fumaric acid | EDBM | Membrane effective area: 0.0064 m2 | Recovery ratio: up to 75% | 139 |
| Current density: 90, 120, 150 mA cm−2 | Current efficiency: 80–90% | |||
| Acid concentration: 1.45–2.90 g L−1 | Energy consumption: 5–13 kW h kg−1 | |||
| Circulation flow rate: 6.2 L h−1 | ||||
| Glyceric acid | ED | Membrane effective area: 550 cm2 | Acid recovery: 75–95% | 140 |
| Acid concentration: 32.3–130.2 g L−1 | Current efficiency: 87.2–100% | |||
| pH: 7 | Energy consumption: 0.19–0.31 kW h kg−1 | |||
| Lactic acid | ED | Membrane effective area: 58 and 180 cm2 | Current efficiency: 45–83% | 141 |
| Voltage: 1.5 V | Final acid concentration: up to 157 g L−1 | |||
| Current: 1.4 A | Energy consumption: 0.26–0.87 kW h kg−1 | |||
| ED | Membrane effective area: 200 cm2 | Acid recovery: 96.4–98.7% | 3 | |
| Acid concentration: 80–100 g dm−3 | Current efficiency: 77.3–83.0% | |||
| Energy consumption: 1.2–3.8 | ||||
| Current: 8–12 A | ||||
| ED | Feed: 0.1 N lactic acid and 0.1 N sodium sulfate | Final acid concentration: up to 4 g L−1 | 97 | |
| Flow rate: 150 mL min−1 | ||||
| ED | Membrane effective area: 57.6 and 180 cm2 | Current efficiency: 66–84% | 142 | |
| Voltage: 1.5 V | Final acid concentration: up to 173 g L−1 | |||
| Current density: 7.8 mA cm2 | Energy consumption: 0.24–0.32 kW h kg−1 | |||
| ED | Voltage: 0–15 V | Acid recovery: 40–100% | 16 | |
| Acid concentration: 40 g L−1 | Energy consumption: 0.163–0.910 kW h kg−1 | |||
| ED | Membrane effective area: 100 cm2 | Acid recovery: up to 97% | 143 | |
| Acid concentration: 0, 1, 5 g L−1 | Energy consumption: 0.25 kW h kg−1 | |||
| Voltage: 10, 15, 20 | ||||
| Temperature: 32 °C | ||||
| ED | Voltage: 7 V | Mineral removal: 90% | 144 | |
| Current density: 10–300 A m−2 | Energy consumption: 0.004–0.014 kW h kg−1 | |||
| EIS | Membrane effective area: 25 cm2 | Final acid concentration: up to 9 g L−1 | 97 | |
| Feed: 0.1 N lactic acid and 0.1 N sodium sulfate | ||||
| Flow rate: 150 mL min−1 | ||||
| EDBM | Membrane effective area: 57.6 and 180 cm2 | Current efficiency: 61–92% | 142 | |
| Voltage: 12 V | Final acid concentration: up to 173 g L−1 | |||
| Current density: 67.7 mA cm−2 | Energy consumption: 0.84–1.38 kW h kg−1 | |||
| EDI | Membrane effective area: 90 cm2 | Final acid concentration: up to 185 g L−1 | 123 | |
| Voltage: 0–70 V | ||||
| Current density: 0–45 mA cm−2 | ||||
| Acid concentration: 0–80 g L−1 | ||||
| Malic acid | EDBM | Membrane effective area: 0.02 m2 | Current efficiency: 30% | 145 |
| Acid concentration: 24.4 g L−1 | Energy consumption: 1.15–1.27 kW h kg−1 | |||
| Circulation flow rate: 300 L h−1 | ||||
| Propionic acid | EED | Membrane effective area: 20 cm2 | Current efficiency: 90.5–99.2% | 116 |
| Acid concentration: 40 g L−1 | Final acid concentration: 150 g L−1 | |||
| Current density: 70 mA cm−2 | ||||
| EDBM | Membrane effective area: 20 cm2 | Current efficiency: 85.2–100% | 116 | |
| Acid concentration: 40 g L−1 | Final acid concentration: 145 g L−1 | |||
| Current density: 70 mA cm−2 | ||||
| Salicylic acid | EDBM | Feed acid concentration: 1 M | Current efficiency: 80–90% | 121 |
| Flow rate: 90 L h−1 | Final acid concentration: up to 45 M | |||
| Circulation rate: 4.6 cm s−1 | Energy consumption: 14–38 W h m2 kg−1 | |||
| Current density: 30, 50, 75 mA cm−2 | ||||
| EDBM | Membrane effective area: 7.07 cm2 | Current efficiency: 99.6% | 146 | |
| Acid concentration: 0.05–0.4 mol L−1 | Energy consumption: 2.1 W h m2 kg | |||
| Current density: 14–50 mA cm−2 | ||||
| Succinic acid | ED | Membrane effective area: 178 cm2 | Final acid concentration: 63–77.6 g L−1 | 147 |
| Acid concentration: 51.5 g L−1 | Current efficiency: 76.2–78.9% | |||
| ED | Membrane effective area: 64 cm2 | Current efficiency: 15–25% | 148 | |
| Voltage: 0–20 V | Total carboxylate basis: 50–60% | |||
| EDBM | Membrane effective area: 80 cm2 | Final acid concentration: 0.25–0.60 M | 149 | |
| Acid concentration: 0.05 M | Current efficiency: 90% | |||
| Circulation rate: 15 L h−1 | Energy consumption: 1–4 kW h kg−1 | |||
| Current density: 12.5, 25, 37.5 mA cm−2 | ||||
| EDBM | Membrane effective area: 80 cm2 | Current efficiency: 96.8% | 149 | |
| Current density: 12.5–37.5 mA cm−2 | Energy consumption: <4 kW h kg−1 | |||
| EDBM | Membrane effective area: 207 cm2 | Current efficiency: 75.4% | 150 | |
| Current density: 90 and 120 A m−2 | Energy consumption: 1.5–3.2 kW h kg−1 | |||
| Acid concentration: 43, 100, and 200 g L−1 | ||||
| EDBM | Current density: 90 and 120 mA cm−2 | Current efficiency: 14.3–19% | 151 | |
| Acid concentration: 15 and 16.9 g dm−3 | Final acid concentration: 13 and 15.7 g dm−3 | |||
| Feed flow rate: 100 dm2 h−1 | ||||
| Tartaric acid | ED | Temperature: 25–40 °C | Current efficiency: 33–65% | 152 |
| Feed: 10 kg m−3 tartaric acid and 60 kg m−3 glucose | Final acid concentration: 170–300 kg m−3 | |||
| Energy consumption: 5.103–12.103 kJ kg−1 |
The electro-membrane process also gave good results for the recovery of other organic acids. ED and EDBM have been used for almost all organic acids. ED was able to recover 63–65% amino acid133 and tartaric acid,152 85% of butyric acid, and more than 90% of glyceric acid140 and lactic acid.3,16,143 Meanwhile, EDBM has been used for the recovery of acetic acid,132 amino acid,134,135 citric acid,137,138 fumaric acid,139 lactic acid,142 malic acid,145 propionic acid,116 salicylic acid,121,146 and succinic acid.149 Most of the studies on EDBM mainly focused on the current efficiency, where the results showed that the current efficiency for organic acid recovery was affected by the EDBM stack configuration and feed characteristics.
Besides ED and EDBM, several researchers used EED for the recovery of acetic acid, formic acid, and propionic acid. Koter113 used EED for the separation of acetic acid, which resulted in a high acetic acid retention efficiency (>90%) when the process was conducted at current densities lower than the limiting current density. Akgemci et al.110 and Luo et al.111 investigated the current efficiency of EED for formic acid recovery and obtained a current efficiency of more than 100%. The current efficiency of 90–99% was obtained for the recovery of propionic acid by Boyaval et al.116 Meanwhile, EDI has been used for the recovery of amino acid,127 butyric acid,128,129 citric acid,7 and lactic acid.123 The results showed that EDI is a potential technology for the recovery of organic acids with a high current efficiency and recovery rate.
In general, electro-membrane processes are feasible to recover organic acids with a high acid recovery ratio and low energy consumption. However, each electro-membrane process has advantages and disadvantages, as presented in Table 4. ED and EED are simple processes without the need for acid; however, their recovery rate and product concentration are relatively lower than other electro-membrane processes. EMT and EIS can realize double composition reactions but require strong acid to facilitate the transport of ions. Meanwhile, EDBM and EDI can obtain high acid purity. However, EDBM has not been industrialized widely due to the high price of bipolar membranes, while EDI requires pretreatment.
| Technology | Advantages | Disadvantages |
|---|---|---|
| ED | Simple process, low energy consumption | Relatively low recovery rate |
| EMT | Can realize double composition reactions | Acid requirement |
| EIS | Can realize double composition reactions | Low current efficiency, acid requirement |
| EED | High current efficiency, can be operated continuously | Low product concentration, low membrane stability and selectivity |
| EDBM | High separation efficiency, high acid purity | Expensive bipolar membrane price |
| EDI | High recovery rate, self-regeneration, can be operated continuously | Requires pretreatment |
Development of electro-membrane process designs for organic acid recovery
The stack configuration plays an important role in the efficiency and recovery yield of electro-membrane processes. Several modifications of the electro-membrane configuration have been conducted to improve the recovery yield of organic acids for both the laboratory and industrial scales. Besides, the combined process of organic acid production and separation, which is mostly called the in situ separation process, also has been proposed to avoid product inhibition and degradation.Modification of electro-membrane configuration
In his patent, Datta153 designed an efficient and potentially economical process for lactic acid production. This process was configured to have desalting ED, water splitting electrodialysis (WSED), and ion-exchange purification steps. It was able to produce concentrated lactic acid with less than 0.1% of proteinaceous impurities from carbohydrate fermentation. In this process, there was no by-product gypsum and only a small amount of by-product salt was obtained from the ion-exchange regeneration. In addition, this process could be operated continuously, and thus can be scaled up for large-volume production.In 1997, an EDBM plant for the recovery of organic acids was constructed in France.154 This plant produces 2600 ton organic acids per year with 98% purity. The process requires 0.88 kW h kg−1 of energy to produce acids. Generally, EDBM has the main problem of intolerance to multivalent cations such as Ca2+ and Mg2+, which form insoluble hydroxides at the critical interface of the bipolar membrane where the ions separate. The concentration of divalent cations is usually limited to about 1 ppm, while fermentation broths mostly contain multivalent cations with concentrations of up to 1000 ppm. Therefore, Datta and Henry11 used desalting ED to remove the multivalent cations and concentrate the lactate salt, followed by treatment in a WSED unit with bipolar membranes to produce concentrated lactic acid and alkali for recycling. WSED is a general purpose unit operation for converting water-soluble salts to their corresponding acids and bases.155 The results showed that 98–99% of divalent ions were rejected with high recovery yield (>95%) and low power consumption, giving approximately 0.33 kW h kg−1 of lactate. However, when the feed solution contains metal ions, such as calcium and magnesium, fouling occurs faster in WSED due to the precipitation of metal ions in the cation-exchange membrane.97
Another attempt was also investigated to increase the recovery efficiency by molding ion exchange resins into a porous resin wafer (RW) and inserting it into the electro-membrane stack.122,156,157 This modification is usually called resin wafer electrodeionization (RW-EDI). Compared to conventional EDI, RW-EDI provides simpler assembly and efficient operation. RW-EDI demonstrates a very stable removal rate due to the rigidity of the porous wafer. Besides, the presence of a resin wafer enhances the electrical conductivity in the solutions and allows thinner EDI systems at reduced energy consumption, and regeneration of the resin wafers can occur within the cell through water splitting.158 Therefore, the use of resin wafer broadens the applications of EDI, such as for esterification,159 enzyme-based conversion and recovery of organic acids,156 and capture of carbon dioxide from flue gas.160 Datta et al.122 reported that RW-EDI successfully removed greater than 99% and 95% of sulfuric acid and acetic acid, respectively, while sugar retention was greater than 98%.
Furthermore, Lopez et al.158,161 used ionic liquids instead of a resin wafer. They used two types of ionic liquid, including 1-ethyl-3-methylimidazolium-trifluoromethanesulfonate ([EMIM][OTf]) and 1-butyl-3-methylimidazolium acetate ([BMIM][OAc]). By using ionic liquids as the wafer in EDI stacks, they were able to obtain a recovery rate of 99%, while the current efficiencies reached 37–90% with energy consumption rates of approximately 1.25–2.80 kW h kg−1 acid recovered.
In situ separation processes
Many fermentation processes have low productivity and yield due to product inhibition or hydrolysis of the product by further catalytic reactions.162 This problem can be solved by optimizing technological parameters, for example by keeping the dissolved product concentration as low as possible in the fermentation medium. Thus, the in situ separation process is promising for selectively removing the fermentation product from the vicinity of the biocatalyst as soon as it is formed and also increasing the efficiency of recovery processes.123The in situ separation process is a combination of product formation and separation of organic acids. This process has been studied by several researchers with various schemes and designs. Arora et al.156 compared two schemes of combined bioreactor and EDI. The first scheme was called side-stream bioreactor, while the second was named immobilized separative bioreactor, as shown in Fig. 3. In the first scheme, the reaction takes place in a traditional bioreactor containing biocatalysts (enzymes/cells), sugars, and the organic acid products all in solution, which is then fed to the EDI separation unit. In the second scheme, the biocatalyst is immobilized directly in the RW-EDI to enable simultaneous reaction and separation. The results showed that immobilized separative bioreactor had a stable reaction rate, while the reaction rate decreased as a function of pH in the side-stream bioreactor.
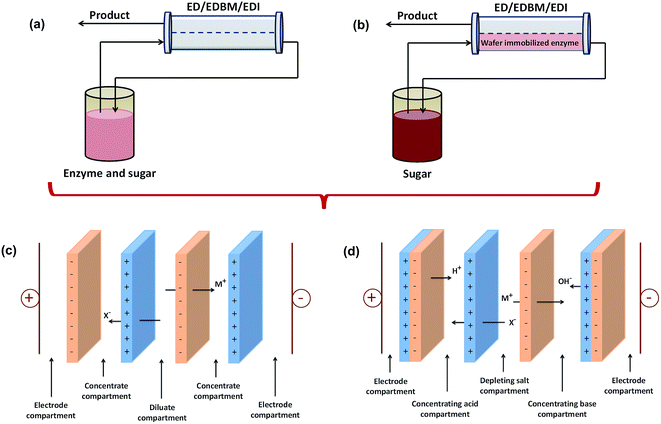 | ||
| Fig. 3 Schematic of (a) a side-stream bioreactor, (b) an immobilized separative bioreactor, (c) the stack configuration for EDF, and (d) the stack configuration of MEDCC. | ||
Gao et al.163 studied the use of electrodialysis fermentation (EDF) for lactic acid production. In EDF, the fermentation broth is continuously fed to the ED to concentrate the organic acid. The solution with a high concentration of organic acid is collected as the product, while the solution with a low organic acid concentration is returned to the fermentation reactor. Using EDF, the acid productivity was 1.5 times higher than the convention fermentation-ED process. The yield also increased by above 30% and glucose transport decreased from 0.46 to 0.05. Hirata et al.164 also used an EDF system equipped with a glucose concentration controller (GC controller). The GC controller was added to control the glucose concentration to be stable and low in the fermentation broth. Meanwhile, Danner et al.165 studied integrated continuous cell recycle cultivation using ultra-filtration membrane bioreactor (MBR) systems. The permeate from the MBR was streamed to an ED to recover and concentrate lactic acid. The recovery yield of lactic acid was stable at around 80% with the energy consumption of 0.49 kW h kg−1 of lactic acid.
Kumar et al. used an electro-membrane reactor where ion substitution and separation of acids occurred simultaneously for the recovery of glutamic acid115 and amino acid.166 Their studies resulted in a high recovery of glutamic acid and amino acid close to 96% and 85%, respectively. Meanwhile, Liu et al.167 investigated the potential of an integrated EDBM and biochemical process named the microbial electrodialysis and chemical-production cell (MEDCC) to produce malic acid. In general, MEDCC has same operating system with EDF; however, MEDCC uses bipolar membranes, as shown in Fig. 3(d). Liu et al.167 showed that MEDCC could convert 0.3 M malate into 0.23 M malic acid with a low energy consumption of 0.34 kW h kg−1. This energy consumption was only 10–30% of that in the reported EDBM. The same method was also used by Wang et al.168,169 for the production of lactic acid. The productivity of lactic acid was 1.76 g L−1 h−1 and the yield coefficient was 56.77%. In addition, Luo et al.170 also used MEDCC for the production of citric acid. The maximum citric acid production of 0.443 ± 0.096 M was achieved within 96 h operation. The energy consumption was 0.81 ± 0.03 kW h kg−1. Lu et al.171 also used MEDCC to produce formic acid. The minimum electricity consumption to produce 0.34 ± 0.04 kW h kg−1 of formic acid in the MEDCC with 72 cm of the anode fiber length was only 3.1–18.8% of that in the EDBM.
In addition, Zhu et al.172 developed a new of bioelectrochemical system called a microbial reverse-electrodialysis chemical-production cell (MRCC). This system was developed to produce acid and alkali using energy derived from organic matter (acetate) and salinity gradients (NaCl solutions representative of seawater and river water). A bipolar membrane was placed next to the anode to prevent Cl− contamination and acidification of the anolyte, and to produce protons for HCl recovery. The MRCC reactor produced electricity (908 mW m−2) as well as concentrated acidic and alkaline solutions, and therefore did not require an external power supply. The results showed that the acid and alkali-production efficiencies based on the generated current were 58 ± 3% and 25 ± 3%, respectively.
Fig. 4 shows a comparison of the energy consumption for various electro-membrane processes for organic acids, including separated downstream processes and in situ separation processes. It can be seen that the in situ separations such as EDF and MEDCC require much lower energy consumption, and thus are more promising to be developed on an industrial scale. However, the maximum current density of EDF and MEDCC is much lower than that of ED, which greatly limits their application in practice.
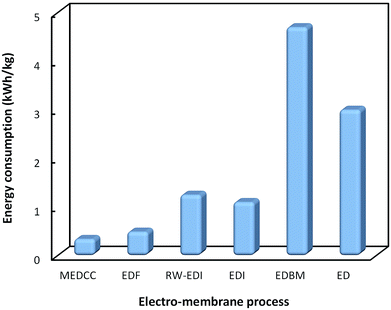 | ||
| Fig. 4 Comparison of energy consumption for various electro-membrane processes for the production of organic acids. | ||
Integrated separation processes
Fermentation operates most efficiently and effectively at near neutral pH, and hence produces the acid salt instead of the acid itself. Besides, the presence of impurities such as water, particles, microbial cells, unconverted carbon sources, and inorganic ions cannot be avoided. Therefore, the pretreatment of feed solution is important before pumping it into the electro-membrane stack to ensure stable operation and process performance. Press filtration and UF membrane are the alternative technologies to remove particles or colloids (bacteria, cellulose, proteins, or suspended solid chemicals) and keep the electro-membrane stack free from serious membrane fouling.142,173 Activated carbon can be used to clear up coloring matter to prevent fouling or the appearance of unfavorable byproducts.174 Meanwhile, chelating resins are able to selectively remove multivalent inorganic ions (especially, Ca2+, Mg2+, Ba2+, Mn2+, Zn2+, Fe3+, and Sr2+) to prevent scale formation.142,175,176Boyaval et al.177 produced lactic acid in a continuous process comprising three distinct operations in a bioreactor, UF module, and ED cell. UF recycled all or part of the biomass and separated low molecular weight metabolites, such as sodium lactate, which resulted from lactose fermentation. The product of UF was then extracted and concentrated continuously by ED. This process was able to yield lactate solution in a high concentration of up to 130 g L−1. Meanwhile, Sikder et al.71 demonstrated that combined NF-EDBM in downstream purification is able to replace multiple purification steps with only two steps, while yielding monomer grade lactic acid from a mixture of unconverted sugars and lactic acid. NF also led to nearly total discoloration of the fermentation broth.
Bailly et al.176 proposed a complete organic acid production using a membrane for clarification, concentration and conversion. The fermentation broth was first clarified by cross-flow MF. Divalent cations were then removed from the clarified broth since they act as a poison in the process. ED was further used prior to EDBM to increase the concentration of ionic species comprising the organic acid salt. Meanwhile, Norddahl178 combined UF and a two-step ED process. The first step used conventional ED membranes, while the second ED process used a bipolar membrane. A bipolar membrane was applied to separate the salts formed into lactic acid, inorganic acids, and ammonium hydroxide solution. By combining UF and two-step ED, the overall recovery rate of lactic acid was quite high at about 85–90%. Madzingaidzo et al.174 also combined ED and EDBM. ED was used for sodium lactate purification, while the recovery of lactic acid was done using EDBM. Sodium lactate with a feed concentration of 125 g L−1 was concentrated by ED to a maximum of 150 g L−1. The lactate solution was then passed through EDBM to obtain free lactic acid. The EDBM unit was able to obtain 160 g L−1 free lactic acid while color and other chemical impurities were significantly reduced. Later, further development based on this process involved adjustment of the pH to below the pKa-value of lactic acid (3.86).179 As a result, the free lactate ions combined with hydrogen ions to form lactic acid having no net electrical charge. In 2018, Prochaska et al.151 proposed an integrated system consisting of UF, EDBM, and three steps reactive extraction for the removal of succinic acid. UF acted as pre-clarification to remove high molecular contaminants. Meanwhile, EDBM allowed the acidification of the broth to be eliminated. The succinic acid present in the aqueous stream after EDBM was then removed in a three-step reactive extraction at more than 90% efficiency.
The downstream process on the industrial scale is mostly more complicated than the laboratory scale. As example, lactic acid is separated and substantially purified from fermentation broths by several membrane-based unit operations. MF or UF are used for cell separation and recycle, while NF is essential for separation of the lactic acid from other broth components using low rejection (LR) membranes.180 Meanwhile, to concentrate the lactate, RO or a combination of high rejection (HR) and low rejection (LR) NF can be used. Further, ED is added for the simultaneous separation and concentration of lactate. The lactate product of ED is still in the salt form, and hence, EDBM is required to form lactic acid in the acid form and allow the recycle of the alkali used for neutralizing the fermentation broth. By adding EDBM to the process, the alkali cost is minimized, while the waste products (e.g., calcium sulfate) generated in conventional downstream processes for organic acids are eliminated.176
Future prospect and challenges
The increasing demand for organic acids in various applications has led to further exploration for their efficient production. The development of recovery processes is one of the crucial approaches to obtain efficient organic acid production with high quality product. The separation methods that have been studied for the recovery of organic acids mainly include precipitation, extraction, adsorption, and nanofiltration. Unfortunately, the existing processes still have many obstacles that need to be addressed. Precipitation and liquid–liquid extraction need a large amount of chemical agents and energy. They produce a large amount of water effluent as well as solid residue and involve phase changes that lead to a degradation in the quality of citric acid. Meanwhile, adsorption has a short lifetime of adsorbents and low capacity.Electro-membrane processes are the promising technologies to replace the conventional technologies for the organic acid downstream process. Electro-membrane processes such as ED, EMT, EIS, EED, EDBM, and EDI can recover organic acids from their fermentation broth without the introduction of salt or discharge. Besides, their energy consumption is much lower than conventional technologies. However, concentration polarization and fouling are still main issues in electro-membrane processes. The concentration polarization is a result of the difference between the transport number of the ions in the membrane and in the feed solution. These phenomena then lead to an accumulation of ions on the membrane surface in concentrate compartment, while ion depletion occurs on the membrane surface in the diluate compartment.181 As a consequence, the operating currents of electro-membrane processes are restricted by the occurrence of concentration polarization.
In addition, fouling also undeniably occurs during membrane operation. Fouling is associated with the accumulation of substances on the membrane surface or within the membrane pores.182 Fouling not only leads to a decline in productivity, but also requires an additional energy supply to keep the membrane performance constant.183–186 In electro-membrane processes, fouling occurs due to the accumulation of colloids and organic substances. Colloidal fouling is mainly deposited on the AEM since most of the colloids treated by electro-membrane processes are negatively charged, which leads to interactions with positively charged ion-exchange groups of AEM. Meanwhile, organic substances stick to the surface of all types of membranes and/or lodge themselves inside the membrane. In colloidal fouling, the interaction of the foulant and membrane is only electrostatic in nature, while organic fouling on the IEM surface and inside the IEM may be due to electrostatic and hydrophobic interactions.187
Fouling and concentration polarization are inevitable but can be controlled. The usage of suitable strategies allows for a longer membrane life and lower operational costs. Various attempts can be undertaken to control fouling and concentration polarization, such as selection of appropriate pretreatment, modification of the membrane, and optimization of operation conditions by increasing the pH and cross flow velocity, or decreasing the initial flux. Selection of an appropriate pretreatment can be used to inhibit the fouling and concentration polarization by reducing the concentration of impurities in the feed water. Meanwhile, optimization of the operation can minimize the interaction between impurities/foulant and the membrane surface. As an example, an increase in cross flow velocity can reduce fouling due to the disruption of the fouling layer by a strong hydrodynamic shear rate.188 In addition, the properties of membrane materials plays an important role in fouling formation. Hydrophobic membranes are more prone to fouling formation than hydrophilic membranes. Therefore, in several cases, hydrophilic modification is required. Hydrophilic modification can be conducted by blending the original polymer with hydrophilic materials or the addition of hydrophilic polymer layers on the active surface of the membrane.189–193
Furthermore, there is still a need for further research to develop processes that are simple to carry out and allow the purification of organic acids directly from the fermentation broth. The integrated process of fermentation and product recovery, which is usually called in situ separation, is an alternative to solve this problem. The integration of fermentation and the electro-membrane process can increase the recovery yield to above 30%, while decreasing the energy consumption by at least 90%. However, this separation process still has room for improvement to make it industrially feasible, including the development of membrane materials, process design, and optimization of operating conditions.
Conflicts of interest
There are no conflicts to declare.References
- D. d. B. Richter, N.-H. Oh, R. Fimmen and J. Jackson, in The Rhizosphere, ed. J. L. Whitbeck, Academic Press, Burlington, 2007, pp. 179–200 Search PubMed.
- W. Zhao, X. Sun, Q. Wang, H. Ma and Y. Teng, Biomass Bioenergy, 2009, 33, 21–25 CrossRef CAS.
- Y. H. Kim and S. H. Moon, J. Chem. Technol. Biotechnol., 2001, 76, 169–178 CrossRef CAS.
- R. Datta, S. P. Tsai, P. Bonsignore, S. H. Moon and J. R. Frank, FEMS Microbiol. Rev., 1995, 16, 221–231 CrossRef CAS.
- T. Kurzrock and D. Weuster Botz, Biotechnol. Lett., 2010, 32, 331–339 CrossRef CAS PubMed.
- H. Takahashi, K. Ohba and K. Kikuchi, J. Membr. Sci., 2003, 222, 103–111 CrossRef CAS.
- I. N. Widiasa, P. D. Sutrisna and I. G. Wenten, Sep. Purif. Technol., 2004, 39, 89–97 CrossRef CAS.
- A. Shishikura, K. Kanamori, H. Takahashi and H. Kinbara, J. Agric. Food Chem., 1994, 42, 1993–1997 CrossRef CAS.
- H. Takahashi, K. Ohba and K. Kikuchi, Biochem. Eng. J., 2003, 16, 311–315 CrossRef CAS.
- J. S. J. Ferrer, S. Laborie, G. Durand and M. Rakib, J. Membr. Sci., 2006, 280, 509–516 CrossRef CAS.
- R. Datta and M. Henry, J. Chem. Technol. Biotechnol., 2006, 81, 1119–1129 CrossRef CAS.
- C. Huang, T. Xu, Y. Zhang, Y. Xue and G. Chen, J. Membr. Sci., 2007, 288, 1–12 CrossRef CAS.
- I. Bechthold, K. Bretz, S. Kabasci, R. Kopitzky and A. Springer, Chem. Eng. Technol., 2008, 31, 647–654 CrossRef CAS.
- Q. Z. Li, X. L. Jiang, X. J. Feng, J. M. Wang, C. Sun, H. B. Zhang, M. Xian and H. Z. Liu, J. Microbiol. Biotechnol., 2016, 26, 1–8 CrossRef CAS PubMed.
- I. H. Aljundi, J. M. Belovich and O. Talu, Chem. Eng. Sci., 2005, 60, 5004–5009 CrossRef CAS.
- W. Boonkong, P. Sangvanich, A. Petsom and N. Thongchul, Chem. Eng. Technol., 2009, 32, 1542–1549 CrossRef CAS.
- X. Cao, H. S. Yun and Y. M. Koo, Biochem. Eng. J., 2002, 11, 189–196 CrossRef CAS.
- A. H. da Silva and E. A. Miranda, J. Chem. Eng. Data, 2013, 58, 1454–1463 CrossRef.
- K. Zhang, L. Zhang and S. T. Yang, Ind. Eng. Chem. Res., 2014, 53, 12802–12808 CrossRef CAS.
- M. T. Gao, T. Shimamura, N. Ishida, E. Nagamori, H. Takahashi, S. Umemoto, T. Omasa and H. Ohtake, Enzyme Microb. Technol., 2009, 44, 350–354 CrossRef CAS.
- A. Keshav, K. L. Wasewar and S. Chand, Chem. Eng. Commun., 2009, 197, 606–626 CrossRef.
- S. J. Li, H. L. Chen, J. Y. Xu and L. Zhang, Sep. Sci. Technol., 2007, 42, 2347–2360 CrossRef CAS.
- M. Marinova, G. Kyuchoukov, J. Albet, J. Molinier and G. Malmary, Sep. Purif. Technol., 2004, 37, 199–207 CrossRef CAS.
- K. K. Cheng, X. B. Zhao, J. Zeng, R. C. Wu, Y. Z. Xu, D. H. Liu and J. A. Zhang, Appl. Microbiol. Biotechnol., 2012, 95, 841–850 CrossRef CAS PubMed.
- L. G. Heding and J. K. Gupta, Biotechnol. Bioeng., 1975, 17, 1363–1364 CrossRef CAS.
- R. Karklin, L. Ramina and R. Raso, Biosint. Oksikilot Ketokislot Mikroorg., 1984, 43–51 Search PubMed.
- D.-J. Min, K. H. Choi, Y. K. Chang and J.-H. Kim, Korean J. Chem. Eng., 2011, 28, 1969 CrossRef CAS.
- K. A. Berglund, P. Elankovan and D. A. Glassner, Carboxylic acid purification and crystallization process, US Pat. no. 5,034,105, 23 Jul. 1991.
- R. Datta, et al., Fermentation and purification process for succinic acid, US Pat. no. 5,168,055, 1 Dec. 1992.
- B. Norddahl, Fermentative production and isolation of lactic acid, US Pat. no. 6,319,382, 20 Nov. 2001.
- A. Demirci, A. L. Pometto and K. R. Harkins, Bioseparation, 1998, 7, 297–308 CrossRef.
- M. Pazouki and T. Panda, Bioprocess Eng., 1998, 19, 435–439 CrossRef CAS.
- T. Harington and M. M. Hossain, Desalination, 2008, 218, 287–296 CrossRef CAS.
- Y. K. Hong and W. H. Hong, Sep. Purif. Technol., 2005, 42, 151–157 CrossRef CAS.
- S. P. R. Katikaneni and M. Cheryan, Ind. Eng. Chem. Res., 2002, 41, 2745–2752 CrossRef CAS.
- A. Labbaci, G. Kyuchoukov, J. Albet and J. Molinier, J. Chem. Eng. Data, 2010, 55, 228–233 CrossRef CAS.
- A. M. Baniel and D. Gonen, Production of citric acid, US Pat. no. 4,994,609, 19 Feb. 1991.
- A. F. Tuyun, H. Uslu, S. Gökmen and Y. Yorulmaz, J. Chem. Eng. Data, 2011, 56, 2310–2315 CrossRef CAS.
- Y. K. Hong and W. H. Hong, Bioprocess Eng., 2000, 22, 477–481 CrossRef CAS.
- Y.-S. Jun, E. Z. Lee, Y. S. Huh, Y. K. Hong, W. H. Hong and S. Y. Lee, Biochem. Eng. J., 2007, 36, 8–13 CrossRef CAS.
- H. G. Joglekar, I. Rahman, S. Babu, B. D. Kulkarni and A. Joshi, Sep. Purif. Technol., 2006, 52, 1–17 CrossRef CAS.
- P. von Frieling and K. Schügerl, Process Biochem., 1999, 34, 685–696 CrossRef CAS.
- M. Jarvinen, L. Myllykoski, R. Keiski and J. Sohlo, Bioseparation, 2000, 9, 163–166 CrossRef CAS PubMed.
- H. Honda, Y. Toyama, H. Takahashi, T. Nakazeko and T. Kobayashi, J. Ferment. Bioeng., 1995, 79, 589–593 CrossRef CAS.
- G. Yang, M. S. Jahan, L. Ahsan, L. Zheng and Y. Ni, Bioresour. Technol., 2013, 138, 253–258 CrossRef CAS PubMed.
- L. Ahsan, M. S. Jahan and Y. Ni, Ind. Eng. Chem. Res., 2013, 52, 9270–9275 CrossRef CAS.
- H. Demiral and M. Ercengiz Yildirim, Water Sci. Technol., 2003, 47, 183–188 CrossRef CAS PubMed.
- C. B. Rasrendra, B. Girisuta, H. H. van de Bovenkamp, J. G. M. Winkelman, E. J. Leijenhorst, R. H. Venderbosch, M. Windt, D. Meier and H. J. Heeres, Chem. Eng. J., 2011, 176–177, 244–252 CrossRef CAS.
- S. Kumar and B. Babu, i-Manager's J. Future Eng. Technol., 2008, 3, 21 CrossRef CAS.
- Y. K. Hong, W. H. Hong and D. H. Han, Biotechnol. Bioprocess Eng., 2001, 6, 386 CrossRef CAS.
- Z. Gu, D. A. Rickert, B. A. Glatz and C. E. Glatz, Le Lait, 1999, 79, 137–148 CrossRef CAS.
- A. Keshav, K. L. Wasewar and S. Chand, Desalination, 2009, 244, 12–23 CrossRef CAS.
- I. Inci, S. S. Bayazit and Y. S. Asci, J. Chem. Eng. Data, 2011, 56, 4449–4453 CrossRef CAS.
- C. Efe, M. Pieterse, L. A. M. van der Wielen and A. J. J. Straathof, Chem. Eng. Process., 2011, 50, 1143–1151 CrossRef CAS.
- C. C. Chen and L. K. Ju, Sep. Sci. Technol., 1998, 33, 1423–1437 CrossRef CAS.
- E. Ponnampalam, Purification of organic acids using anion exchange chromatography, US Pat. no. 6,284,904, 4 Sep. 2001.
- Q. Li, J. Xing, W. Li, Q. Liu and Z. Su, Ind. Eng. Chem. Res., 2009, 48, 3595–3599 CrossRef CAS.
- W. Y. Tong, X. Y. Fu, S. M. Lee, J. Yu, J. W. Liu, D. Z. Wei and Y. M. Koo, Biochem. Eng. J., 2004, 18, 89–96 CrossRef CAS.
- Y. R. A. González-Vara, G. Vaccari, E. Dosi, A. Trilli, M. Rossi and D. Matteuzzi, Biotechnol. Bioeng., 2000, 67(2), 147–156 CrossRef.
- A. Srivastava, P. K. Roychoudhury and V. Sahai, Biotechnol. Bioeng., 1992, 39, 607–613 CrossRef CAS PubMed.
- G. Raya Tonetti, P. Córdoba, J. Bruno-bárcena, F. Siñeriz and N. Perotti, Biotechnol. Tech., 1999, 13, 201–205 CrossRef CAS.
- N. Cao, J. Du, C. Gong and G. Tsao, Appl. Environ. Microbiol., 1996, 62, 2926–2931 CAS.
- Y. Zheng, X. Ding, P. Cen, C. W. Yang and G. T. Tsao, Appl. Biochem. Biotechnol., 1996, 57, 627 CrossRef.
- H. J. Lee, Y. Xie, Y. M. Koo and N. H. L. Wang, Biotechnol. Prog., 2004, 20, 179–192 CrossRef CAS PubMed.
- C. Chen and L. K. Ju, Appl. Microbiol. Biotechnol., 2002, 59, 170–174 CrossRef CAS PubMed.
- C. Umpuch, S. Galier, S. Kanchanatawee and H. R.-d. Balmann, Process Biochem., 2010, 45, 1763–1768 CrossRef CAS.
- B. Van der Bruggen, J. Schaep, D. Wilms and C. Vandecasteele, J. Membr. Sci., 1999, 156, 29–41 CrossRef CAS.
- M. I. Gonzalez, S. Alvarez, F. A. Riera and R. Alvarez, Desalination, 2008, 228, 84–96 CrossRef CAS.
- M. S. Kim, J. G. Na, M. K. Lee, H. Ryu, Y. K. Chang, J. M. Triolo, Y. M. Yun and D. H. Kim, Water Res., 2016, 96, 208–216 CrossRef CAS PubMed.
- S. H. Kang, Y. K. Chang and H. N. Chang, Biotechnol. Prog., 2004, 20, 764–770 CrossRef CAS PubMed.
- J. Sikder, S. Chakraborty, P. Pal, E. Drioli and C. Bhattacharjee, Biochem. Eng. J., 2012, 69, 130–137 CrossRef CAS.
- Z. Yao, et al., Method for separation succinic acid from anaerobic fermentation broth, Chinese Patent, CN200610086003.7, 2008.
- H. Wu, et al., Nanofiltration method for separation of succinic acid from its fermented broth, Chinese Patent, CN200910025531.5, 2011.
- Y. Li and A. Shahbazi, in Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals, ed. J. D. McMillan, W. S. Adney, J. R. Mielenz and K. T. Klasson, Humana Press, Totowa, NJ, 2006, pp. 985–996 Search PubMed.
- H. Y. Yu, L. Q. Liu, Z. Q. Tang, M. G. Yan, J. S. Gu and X. W. Wei, J. Membr. Sci., 2008, 311, 216–224 CrossRef CAS.
- A. M. Islam, Master thesis, Chalmers University of Technology, Gothenburg, Sweden, 2004.
- R. Ban, M. Liu, Y. Qin, H. Wang and D. Cui, Trans. Tianjin Univ., 2012, 18, 320–329 CrossRef CAS.
- L. Ahsan, M. S. Jahan, H. Liu and Y. Ni, Bioresour. Technol., 2012, 2, 38–43 Search PubMed.
- A. A. Garcia and C. J. King, Ind. Eng. Chem. Res., 1989, 28, 204–212 CrossRef CAS.
- J. Ecker, T. Raab and M. Harasek, J. Membr. Sci., 2012, 389, 389–398 CrossRef CAS.
- S. Sahin, S. S. Bayazit, M. Bilgin and İ. Inci, J. Chem. Eng. Data, 2010, 55, 1519–1522 CrossRef CAS.
- K. Chawong and P. Rattanaphanee, World Acad. Sci. Eng. Technol., 2011, 80, 239–242 Search PubMed.
- K. Tonova, I. Svinyarov and M. G. Bogdanov, Sep. Purif. Technol., 2014, 125, 239–246 CrossRef CAS.
- M. Boonmee, O. Cotano, S. Amnuaypanich and N. Grisadanurak, Arabian J. Sci. Eng., 2016, 41, 2067–2075 CrossRef CAS.
- D. Pleissner, R. Schneider, J. Venus and T. Koch, J. Chem. Technol. Biotechnol., 2017, 92, 504–511 CrossRef CAS.
- M. D. Waghmare, K. L. Wasewar, S. S. Sonawane and D. Z. Shende, Ind. Eng. Chem. Res., 2011, 50, 13526–13537 CrossRef CAS.
- C. Q. Ma, J. C. Li, J. H. Qiu, M. Wang and P. Xu, Appl. Microbiol. Biotechnol., 2006, 70, 308–314 CrossRef CAS PubMed.
- M. E. Marti and T. Gurkan, Sep. Purif. Technol., 2015, 156, 148–157 CrossRef CAS.
- Y. S. Huh, Y. S. Jun, Y. K. Hong, H. Song, S. Y. Lee and W. H. Hong, Process Biochem., 2006, 41, 1461–1465 CrossRef CAS.
- H. Song, Y. S. Huh, S. Y. Lee, W. H. Hong and Y. K. Hong, J. Biotechnol., 2007, 132, 445–452 CrossRef CAS PubMed.
- E. Z. Lee, Y. S. Huh, Y. S. Jun, H. J. Won, Y. K. Hong and W. H. Hong, Biotechnol. Bioprocess Eng., 2008, 13, 342–346 CrossRef CAS.
- A. Versari, M. Castellari, U. Spinabelli and S. Galassi, J. Chem. Technol. Biotechnol., 2001, 76, 485–488 CrossRef CAS.
- K. N. Kontogiannopoulos, S. I. Patsios and A. J. Karabelas, Sep. Purif. Technol., 2016, 165, 32–41 CrossRef CAS.
- Q. Wang, G. Cheng, X. Sun and B. Jin, Process Biochem., 2006, 41, 152–158 CrossRef CAS.
- Y. H. Cho, H. D. Lee and H. B. Park, Ind. Eng. Chem. Res., 2012, 51, 10207–10219 CrossRef CAS.
- C. Huang and T. Xu, Environ. Sci. Technol., 2006, 40, 5233–5243 CrossRef CAS PubMed.
- J. H. Choi, S. H. Kim and S. H. Moon, Sep. Purif. Technol., 2002, 28, 69–79 CrossRef CAS.
- V. A. Shaposhnik and K. Kesore, J. Membr. Sci., 1997, 136, 35–39 CrossRef CAS.
- P. J. Moon, S. J. Parulekar and S. P. Tsai, J. Membr. Sci., 1998, 141, 75–89 CrossRef CAS.
- T. Xu and C. Huang, AIChE J., 2008, 54, 3147–3159 CrossRef CAS.
- Y. Mei and C. Y. Tang, Desalination, 2018, 425, 156–174 CrossRef CAS.
- L. Bazinet, Crit. Rev. Food Sci. Nutr., 2005, 45, 307–326 CrossRef CAS PubMed.
- L. Alvarado and A. Chen, Electrochim. Acta, 2014, 132, 583–597 CrossRef CAS.
- J. Mulder, Basic principles of membrane technology, Springer Science & Business Media, 2012 Search PubMed.
- M. Moresi and F. Sappino, J. Food Eng., 1998, 35, 75–90 CrossRef.
- N. Boniardi, R. Rota, G. Nano and B. Mazza, J. Appl. Electrochem., 1997, 27, 125–133 CrossRef CAS.
- K. Zhang, M. Wang and C. Gao, J. Membr. Sci., 2011, 366, 266–271 CrossRef CAS.
- Y. Wei, Y. Wang, X. Zhang and T. Xu, Sep. Purif. Technol., 2013, 118, 1–5 CrossRef CAS.
- S. Cattoir, D. Smets and A. Rahier, Desalination, 1999, 121, 123–130 CrossRef CAS.
- E. Güler Akgemci, M. Ersöz and T. Atalay, Sep. Sci. Technol., 2005, 39, 165–184 CrossRef.
- G. S. Luo and F. Y. Wu, Sep. Sci. Technol., 2000, 35, 2485–2496 CrossRef CAS.
- S. S. Yi, Y. C. Lu and G. S. Luo, Sep. Purif. Technol., 2008, 60, 308–314 CrossRef CAS.
- S. Koter, Sep. Purif. Technol., 2008, 60, 251–258 CrossRef CAS.
- M. Kumar, B. P. Tripathi and V. K. Shahi, Ind. Eng. Chem. Res., 2009, 48, 923–930 CrossRef CAS.
- M. Kumar, B. P. Tripathi and V. K. Shahi, Electrochim. Acta, 2009, 54, 4880–4887 CrossRef CAS.
- P. Boyaval, J. Seta and C. Gavach, Enzyme Microb. Technol., 1993, 15, 683–686 CrossRef CAS.
- G. S. Luo, X. Y. Shan, X. Qi and Y. C. Lu, Sep. Purif. Technol., 2004, 38, 265–271 CrossRef CAS.
- S. S. Yi, Y. C. Lu and G. S. Luo, J. Membr. Sci., 2005, 255, 57–65 CrossRef CAS.
- F. Hanada, et al., Bipolar membrane and method for its production, US Pat. no. 5,221,455, 22 Jun. 1993.
- Y. Wang, C. Huang and T. Xu, J. Membr. Sci., 2011, 374, 150–156 CrossRef CAS.
- F. Alvarez, R. Alvarez, J. Coca, J. Sandeaux, R. Sandeaux and C. Gavach, J. Membr. Sci., 1997, 123, 61–69 CrossRef CAS.
- S. Datta, Y. J. Lin, D. J. Schell, C. S. Millard, S. F. Ahmad, M. P. Henry, P. Gillenwater, A. T. Fracaro, A. Moradia, Z. P. Gwarnicki and S. W. Snyder, Ind. Eng. Chem. Res., 2013, 52, 13777–13784 CrossRef CAS.
- P. Boontawan, S. Kanchanathawee and A. Boontawan, Biochem. Eng. J., 2011, 54, 192–199 CrossRef CAS.
- A. K. Wardani, A. N. Hakim, Khoiruddin and I. G. Wenten, Water Sci. Technol., 2017, 75, 2891–2899 CrossRef CAS PubMed.
- J. Wood, J. Gifford, J. Arba and M. Shaw, Desalination, 2010, 250, 973–976 CrossRef CAS.
- V. I. Fedorenko, Pharm. Chem. J., 2003, 37, 157–160 CrossRef CAS.
- F. Yuan, Q. Wang, P. Yang, Y. Tian and W. Cong, Sep. Purif. Technol., 2015, 153, 51–59 CrossRef CAS.
- H. Habe, N. Yamano, S. Takeda, S. Kataoka and A. Nakayama, Desalination, 2010, 253, 101–105 CrossRef CAS.
- J. Du, N. Lorenz, R. R. Beitle and J. A. Hestekin, Sep. Sci. Technol., 2012, 47, 43–51 CrossRef CAS.
- J. Vijayakumar, R. Aravindan and T. Viruthagiri, Chem. Biochem. Eng. Q., 2008, 22, 245–264 CAS.
- S. Suwal, J. Li, A. S. Engelberth and J.-Y. Huang, Food Bioprod. Process., 2018, 109, 41–51 CrossRef CAS.
- L. Yu, Q. Guo, J. Hao and W. Jiang, Desalination, 2000, 129, 283–288 CrossRef CAS.
- O. M. K. Readi, M. Gironès, W. Wiratha and K. Nijmeijer, Ind. Eng. Chem. Res., 2013, 52, 1069–1078 CrossRef CAS.
- T. V. Eliseev, E. V. Krisilova, V. A. Shaposhnik and A. E. Bukhovets, Desalin. Water Treat., 2010, 14, 196–200 CrossRef.
- X. Lin, J. Pan, M. Zhou, Y. Xu, J. Lin, J. Shen, C. Gao and B. Van der Bruggen, Ind. Eng. Chem. Res., 2016, 55, 2813–2820 CrossRef CAS.
- R. Nikbakht, M. Sadrzadeh and T. Mohammadi, J. Food Eng., 2007, 83, 596–604 CrossRef CAS.
- B. Iglinski, S. Koter, R. Buczkowski and M. Lis, Pol. J. Environ. Stud., 2006, 15, 411–417 CAS.
- X. Sun, H. Lu and J. Wang, J. Cleaner Prod., 2017, 143, 250–256 CrossRef CAS.
- K. Prochaska and M. J. Woźniak Budych, J. Membr. Sci., 2014, 469, 428–435 CrossRef CAS.
- H. Habe, Y. Shimada, T. Fukuoka, D. Kitamoto, M. Itagaki, K. Watanabe, H. Yanagishita and K. Sakaki, J. Biosci. Bioeng., 2010, 110, 690–695 CrossRef CAS PubMed.
- V. Habova, K. Melzoch, M. Rychtera, L. Pribyl and V. Mejta, Czech J. Food Sci., 2001, 19, 73–80 CrossRef CAS.
- V. Habova, K. Melzoch, M. Rychtera and B. Sekavova, Desalination, 2004, 162, 361–372 CrossRef CAS.
- H. W. Ryu, Y. M. Kim and Y. J. Wee, Biotechnol. Bioprocess Eng., 2012, 17, 1261–1269 CrossRef CAS.
- G. Q. Chen, F. I. I. Eschbach, M. Weeks, S. L. Gras and S. E. Kentish, Sep. Purif. Technol., 2016, 158, 230–237 CrossRef CAS.
- M. L. Lameloise and R. Lewandowski, J. Membr. Sci., 2012, 403–404, 196–202 CrossRef CAS.
- X. Liu, Q. Li, C. Jiang, X. Lin and T. Xu, J. Membr. Sci., 2015, 482, 76–82 CrossRef CAS.
- D. A. Glassner, P. Elankovan, D. R. Beacom and K. A. Berglund, Appl. Biochem. Biotechnol., 1995, 51, 73–82 CrossRef.
- P. A. Sosa, C. Roca and S. Velizarov, J. Membr. Sci., 2016, 501, 236–247 CrossRef CAS.
- L. Fu, X. Gao, Y. Yang, F. Aiyong, H. Hao and C. Gao, Sep. Purif. Technol., 2014, 127, 212–218 CrossRef CAS.
- M. Szczygiełda, J. Antczak and K. Prochaska, Sep. Purif. Technol., 2017, 181, 53–59 CrossRef.
- K. Prochaska, J. Antczak, M. Regel-Rosocka and M. Szczygiełda, Sep. Purif. Technol., 2018, 192, 360–368 CrossRef CAS.
- L. J. Andrés, F. A. Riera and R. Alvarez, J. Chem. Technol. Biotechnol., 1997, 70, 247–252 CrossRef.
- R. Datta, Recovery and purification of lactate salts from whole fermentation broth by electrodialysis, US Pat. no. 4,885,247, 5 Dec. 1989.
- M. Bailly, Desalination, 2002, 144, 157–162 CrossRef CAS.
- K. N. Mani, J. Membr. Sci., 1991, 58, 117–138 CrossRef CAS.
- M. B. Arora, J. A. Hestekin, S. W. Snyder, E. J. St. Martin, Y. J. Lin, M. I. Donnelly and C. S. Millard, Sep. Sci. Technol., 2007, 42, 2519–2538 CrossRef CAS PubMed.
- T. Ho, A. Kurup, T. Davis and J. Hestekin, Sep. Sci. Technol., 2010, 45, 433–446 CrossRef CAS.
- A. M. Lopez and J. A. Hestekin, J. Membr. Sci., 2015, 493, 200–205 CrossRef CAS.
- Y. J. Lin, et al., Single-stage separation and esterification of cation salt carboxylates using electrodeionization, US Pat. no. 7,141,154, 28 Nov. 2006.
- Y. J. Lin, et al., Carbon dioxide capture using resin-wafer electrodeionization, US Pat. no. 8,506,784, 13 Aug. 2013.
- A. M. Lopez and J. A. Hestekin, Sep. Purif. Technol., 2013, 116, 162–169 CrossRef CAS.
- G. J. Lye and J. M. Woodley, Trends Biotechnol., 1999, 17, 395–402 CrossRef CAS PubMed.
- G. Min tian, M. Hirata, M. Koide, H. Takanashi and T. Hano, Process Biochem., 2004, 39, 1903–1907 CrossRef CAS.
- M. Hirata, M. t. Gao, E. Toorisaka, H. Takanashi and T. Hano, Biochem. Eng. J., 2005, 25, 159–163 CrossRef CAS.
- H. Danner, L. Madzingaidzo, C. Thomasser, M. Neureiter and R. Braun, Appl. Microbiol. Biotechnol., 2002, 59, 160–169 CrossRef CAS PubMed.
- M. Kumar, B. P. Tripathi and V. K. Shahi, J. Chem. Technol. Biotechnol., 2010, 85, 648–657 CrossRef CAS.
- G. Liu, H. Luo, H. Wang, B. Wang, R. Zhang and S. Chen, J. Membr. Sci., 2014, 471, 179–184 CrossRef CAS.
- X. Wang, Y. Wang, X. Zhang and T. Xu, Bioresour. Technol., 2012, 125, 165–171 CrossRef CAS PubMed.
- X. Wang, Y. Wang, X. Zhang, H. Feng and T. Xu, Bioresour. Technol., 2013, 147, 442–448 CrossRef CAS PubMed.
- H. Luo, X. Cheng, G. Liu, Y. Zhou, Y. Lu, R. Zhang, X. Li and W. Teng, J. Membr. Sci., 2017, 523, 122–128 CrossRef CAS.
- Y. Lu, H. Luo, K. Yang, G. Liu, R. Zhang, X. Li and B. Ye, Bioresour. Technol., 2017, 243, 118–125 CrossRef CAS PubMed.
- X. Zhu, M. C. Hatzell, R. D. Cusick and B. E. Logan, Electrochem. Commun., 2013, 31, 52–55 CrossRef CAS.
- H. Danner, L. Madzingaidzo, M. Holzer, L. Mayrhuber and R. Braun, Bioresour. Technol., 2000, 75, 181–187 CrossRef CAS.
- L. Madzingaidzo, H. Danner and R. Braun, J. Biotechnol., 2002, 96, 223–239 CrossRef CAS PubMed.
- C. Fargues, B. Broyart, M. Benmami and M. L. Lameloise, Sep. Sci. Technol., 2006, 41, 359–377 CrossRef CAS.
- M. Bailly, H. Roux de Balmann, P. Aimar, F. Lutin and M. Cheryan, J. Membr. Sci., 2001, 191, 129–142 CrossRef CAS.
- P. Boyaval, C. Corre and S. Terre, Biotechnol. Lett., 1987, 9, 207–212 CrossRef CAS.
- B. Norddahl, Fermentative production and isolation of lactic acid, US Pat. no. 6,319,382, 20 Nov. 2001.
- B. Norddahl, S. Eriksen and F. Pedersen, Method for producing lactic acid, US Pat. application no. 10/297,090, 2004.
- S. Tejayadi and M. Cheryan, Appl. Biochem. Biotechnol., 1988, 19, 61–70 CrossRef CAS.
- S. Pappa, Direction in food science and technology and human nutrition, MSc thesis, Agricultural University of Athens, 2017.
- S. Hong and M. Elimelech, J. Membr. Sci., 1997, 132, 159–181 CrossRef CAS.
- A. W. Zularisam, A. F. Ismail and R. Salim, Desalination, 2006, 194, 211–231 CrossRef CAS.
- H. Choi, K. Zhang, D. D. Dionysiou, D. B. Oerther and G. A. Sorial, Sep. Purif. Technol., 2005, 45, 68–78 CrossRef CAS.
- A. R. Costa, M. N. de Pinho and M. Elimelech, J. Membr. Sci., 2006, 281, 716–725 CrossRef CAS.
- J. R. Du, S. Peldszus, P. M. Huck and X. Feng, Water Res., 2009, 43, 4559–4568 CrossRef CAS PubMed.
- S. Mikhaylin and L. Bazinet, Adv. Colloid Interface Sci., 2016, 229, 34–56 CrossRef CAS PubMed.
- A. H. Braghetta, Master thesis, University of North Carolina, 1995.
- Y.-F. Yang, L.-S. Wan and Z.-K. Xu, J. Adhes. Sci. Technol., 2011, 25, 245–260 CrossRef CAS.
- V. M. Kochkodan and V. K. Sharma, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2012, 47, 1713–1727 CrossRef CAS PubMed.
- J. Drelich, E. Chibowski, D. D. Meng and K. Terpilowski, Soft Matter, 2011, 7(21), 9804–9828 RSC.
- N. F. Himma, S. Anisah, N. Prasetya and I. G. Wenten, J. Polym. Eng., 2016, 36, 329–362 Search PubMed.
- D. Ariono and A. K. Wardani, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 214, 012014 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |






