Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Overview of sesquiterpenes and chromones of agarwood originating from four main species of the genus Aquilaria
Mei Gaoa,
Xiaomin Hanb,
Ying Suna,
Hongjiang Chenc,
Yun Yangd,
Yangyang Liud,
Hui Mengd,
Zhihui Gaoa,
Yanhong Xua,
Zheng Zhang *a and
Jianping Han*a
*a and
Jianping Han*a
aNational Engineering Laboratory for Breeding of Endangered Medicinal Materials, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College, Malianwabei Road, Beijing 100193, P. R. China. E-mail: zhangzheng@implad.ac.cn; jphan@implad.ac.cn; Tel: +86-10-57833363
bTianjin University of Commerce, No. 409 Guangrong Road, Beichen District, Tianjin 300134, P. R. China
cZhejiang Pharmaceutical College, Ningbo 315100, P. R. China
dHainan Provincial Key Laboratory of Resources Conservation and Development of Southern Medicine, Hainan Branch, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College, Wanning 571533, P. R. China
First published on 30th January 2019
Abstract
The main chemical constituents of agarwood are sesquiterpenes and chromones, which can be divided into different categories depending on their molecular skeletons. Agarwoods are obtained from different plant species: Aquilaria sinensis, A. malaccensis, A. crassna, and A. subintegra. In this review, we systematically summarized the structures of 367 compounds isolated from agarwoods originating from four main species. We structurally classified all the components into 11 different types and summarized the number of compounds in each type. Different and identical components were obtained by enumerating the chemical compositions of the different species. Knowledge regarding the chemical constituents of agarwoods of different species will aid understanding of the chemical compositions of agarwoods and will subsequently identify similar compounds that can serve as standards for quality control to provide a reference for future studies on agarwoods from different species and to increase their usefulness.
1. Introduction
Agarwood is a resinous portion of Aquilaria trees, a genus belonging to the family Thymelaeaceae. Agarwoods have wide uses in traditional medicine, for example, as aphrodisiacs, sedatives, cardiotonics, and carminatives, as well as in the relief of gastric problems, coughs, rheumatism, and high fever.1 In addition, agarwoods are present in important spices and are also used as incense. Agarwood is known as ‘chenxiang’ in Chinese and ‘aloeswood’, ‘agalloch’, ‘eaglewood’, ‘jinkoh’, ‘gaharu’, and ‘kanankoh’ in other parts of the world.2Approximately 15 species of Aquilaria are well known for their production of fragrant heartwood, also known as gaharu, aloeswood or agarwood. Wounding of the tree appears to be essential for the initiation of gaharu production, and fungal infection is likely to enhance the process. According to Eurlings et al.,3 the following nine Aquilaria species produce gaharu: A. beccariana, A. crassna, A. filaria (Oken), A. hirta, A. khasiana, A. malaccensis, A. microcarpa, A. rostrata and A. sinensis; these are mainly sourced from India, Southeast Asia, Papua New Guinea, and China (chiefly in Hainan and Guangdong).3
As stated in reports, sesquiterpenoids and phenylethyl chromone derivatives are the principal compounds in the oleoresin of agarwood, which are mostly found in the species Aquilaria, A. malaccensis, A. agallocha, A. sinensis, and A. crassna.1,4 In 2016, a review of agarwood noted A. agallocha Roxb. (endemic in India), of which the species name is unresolved (Table 1).5 The index of CITES species,6 Missouri Botanical Garden website,7 and the Ayurvedic and Unani Pharmacopoeias all list A. agallocha Roxb. as a synonym of A. malaccensis Lam. Since then, there have been other reports on this species.8–10 Therefore, this article will incorporate all the chemical constituents reviewed in A. agallocha into A. malaccensis. Other genera of the family Thymelaeaceae, such as Aetoxylon, Gyrinops, Phaleria, and Gonystylus, have also been reported to produce agarwoods.3 It has been reported that different countries have endemic species; for example, A. crassna principally grows in Indochina, A. malaccensis is an Indomalesian type found in Malaysia, Thailand and India, and A. sinensis is endemic in China. A. subintegra is principally found in Thailand.8
| Species | Authorship |
|---|---|
| A. apiculata | Merr., 1922 |
| A. baillonii | Pierre ex Lecomte, 1915 |
| A. banaense | P. H. Hô, 1986 |
| A. banaensis | P. H. Hô, 1986 |
| A. beccariana | Tiegh., 1893 |
| A. brachyantha | (Merr.) Hallier L, 1922 |
| A. citrinicarpa | (Elmer) Hallier L, 1922 |
| A. crassna | Pierre ex Lecomte, 1915 |
| A. cumingiana | (Decne.) Ridl., 1901 |
| A. decemcostata | Hallier L, 1922 |
| A. filaria | (Oken) Merr., 1950 |
| A. hirta | Ridl., 1901 |
| A. khasiana | Hallier L, 1922 |
| A. malaccensis | Lam., 1783 |
| A. microcarpa | Baill., 1875 |
| A. parvifolia | (Quisumb) Ding Hou, 1960 |
| A. rostrata | Ridl., 1924 |
| A. rugosa | K. Le-Cong and Kessler, 2005 |
| A. sinensis | (Lour.) Spreng., 1825 |
| A. subintegra | Ding Hou, 1964 |
| A. urdanetensis | (Elmer) Hallier L, 1922 |
| A. yunnanensis | S. C. Huang, 1985 |
All nine of the known Aquilaria species can produce agarwood; however, it is not known which species are most productive. Studies have shown that specific species produce specific chemical components which are quite different from one another.11 Assessing the similarities and differences between these components is a good way to identify species, determine their quality and classify specific species.
Knowledge of the main constituents of agarwoods and their major differences between species would be a tremendous help in identifying the different species of agarwood and improving their quality and efficacy of use in traditional medicine. This review will focus on species that are frequently used and reported, including A. malaccensis (synonymous with A. agallocha), A. sinensis, and A. crassna, and will describe the main chemical constituents of agarwoods from different species. The reference content of this article mainly includes literature abstracts and full-text articles from journals, books, reports and electronic searches, including Google Scholar, Elsevier, PubMed, Springer, Web of Science and other related websites. We have selected nearly one hundred articles from different countries, which have been investigated, analyzed and included in this review. This review discusses compounds that have been isolated since 1963.
2. Sesquiterpenes of agarwoods
Agarwoods contain various types of sesquiterpenes, which can be divided into several categories depending on their molecular skeletons. Some examples of these sesquiterpenes are agarofurans, agarospiranes, eudesmanes, eremophilanes, guaianes, candinanes, and prezizanes (shown in Scheme 1). Other compounds are also present in small amounts (shown in Scheme 1).Almost all types of sesquiterpenes can be found in the following four agarwood species: A. sinensis, A. malaccensis, A. crassna, and A. subintegra; however, there are significant differences among the sesquiterpenes of these species, as described in Table 2.
| No. | Compounds and names | Species | |||
|---|---|---|---|---|---|
| A. s | A. m | A. c | A. su | ||
| a A. s, A. m, A. c, and A. su indicate A. sinensis, A. malaccensis, A. crassna, and A. subintegra, respectively.b The reference was not found.c “*” indicates that the agarwood in this article was artificial agarwood. | |||||
| Agarofurans | |||||
| F1 | α-Agarofuran | 12 and 13 | 14 and 15 | 16 | — |
| F2 | β-Agarofuran | 12, 13 and 17 | 15 and 18 | 16 | 16 |
| F3 | Dihydro-β-agarofuran | 13 | 15 and 16 | 16 | 16 |
| F4 | Epoxy-β-agarofuran | — | 19 | — | — |
| F5 | 4-Hydroxy-dihydro-agarofuran | 13 | 20 | — | — |
| F6 | 3,4-Dihydroxydihydroagarofuran | — | 20 | — | — |
| F7 | Baimuxinol | 13 and 21 | — | — | — |
| F8 | Isobaimuxinol | 12 and 13 | — | — | — |
| F9 | Dehydrobaimuxinol | 21 | — | — | — |
| F10 | (1S,2S,6S,9R)-6,10,10-Trimethyl-11-oxatricyclo[7.2.1.01,6]dodecane-2-carbaldehyde | — | 19 | — | — |
| F11 | Baimuxifuranic acid | 13, 22 and 23 | — | — | — |
| F12 | (1R,6S,9R)-6,10,10-Trimethyl-11-oxatricyclo[7.2.1.01,6]dodecane | — | 24 | — | — |
| F13 | (1R,2R,6S,9R)-6,10,10-Trimethyl-11-oxatricyclo[7.2.1.01,6]dodecan-2-ol | — | 24 | — | — |
| F14 | Nor-keto-agarofuran | — | 20 and 25 | 16 | 16 |
| F15 | 4-Hydroxyl-baimuxinol | 26 | — | — | — |
| Agarospiranes | |||||
| S1 | (2R,5R,10R)-a,a,6,10-tetramethyl-spiro[4,5]dec-6-ene-2-methanol (agarospirol) | 13, 27 and 28* | 16 and 29–31 | 16 | 16 |
| S2 | Isoagarospirol | — | 18 | — | — |
| S3 | Oxo-agarospirol (baimuxinal) | 13, 17, 23, 27, 28* and 32 | 14, 18, 33 and 34 | 16 | 16 |
| S4 | Baimuxinic acid (Bai Mu Xiang acid) | 17 and 27 | — | — | — |
| S5 | rel-(5R,10R)-2-Isopropylidene-10-methyl-spiro[4.5]dec-6-ene-6-carbaldehyde(vetispira-2(11),6-dien-14-al) | — | 25 | — | — |
| S6 | rel-(1R,2R)-9-Isopropyl-2-methyl-8-oxatricyclo[7.2.1.01,6]dodec-5-ene(2,14-epoxy-vetispir-6-ene) | — | 25 | — | — |
| S7 | rel-(1R,2R)-9-Isopropyl-2-methyl-8-oxatricyclo[7.2.1.01,6]dodeca-4,6-dien(2,14-epoxy-vetispira-6(14),7-diene) | — | 25 | — | — |
| S8 | rel-(5R,7S,10R)-2-Isopropylidene-10-methyl-6-methylene-spiro[4.5]decan-7-ol(vetispira-2(11),6(14)-dien-7-ol) | — | 25 | — | — |
| S9 | (4R,5R,7R)-1(10)-Spirovetiven-11-ol-2-one | 23 | 35 | — | — |
| S10 | Hinesol | 2, 13 and 36 | — | — | — |
| S11 | Acorenone B | — | 16 | 16 | 16 |
| S12 | 4-epi-15-Hydroxyacorenone | 37 and 38* | — | — | — |
| S13 | 4-epi-10-Hydroxyacoronene | 37 | — | — | — |
| S14 | 15-Hydroxyacorenone | 23 | |||
| Eudesmanes | |||||
| E1 | 10-epi-γ-Eudesmol | 13 | 14, 18 and 25 | 16 | 16 |
| E2 | (5S,7S,10S)-(−)-Selina-3,11-dien-9-one | — | 16 and 34 | 16 | 16 |
| E3 | (5S,7S,9S,10S)-(+)-Selina-3,11-dien-9-ol | — | 16 and 34 | 16 | 16 |
| E4 | Selina-3,11-dien-14-al | 34 and 39 | 16 | 16 | |
| E5 | Selina-3,11-dien-14-oic acid (as methyl ester) | — | 39 | — | — |
| E6 | Selina-4,11-dien-14-al | — | 16 and 39 | 16 | 16 |
| E7 | Selina-4,11-dien-14-oic acid (as methyl ester) | — | 39 | — | — |
| E8 | 9-Hydroxy-selina-4,11-dien-14-oic acid (as methylester) | — | 39 | — | — |
| E9 | (S)-4a-Methyl-2-(1-methylethylidene)-1,2,3,4,4a,5,6,7-octahydronaphthalene | 13 | 24 | — | — |
| E10 | (S)-4a-Methyl-2-(1-methylethyl)-3,4,4a,5,6,7-hexahydronaphthalene | 13 | 24 | — | — |
| E11 | (2R,4aS)-2-(4a-Methyl-1,2,3,4,4a,5,6,7-octahydronaphthyl)-propan-2-ol(4-nor-epi-γ-eudesmol) | 13 | 24 | — | — |
| E12 | (2R,4aS)-4a-Methyl-2-(1-methylethenyl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | — | 24 | — | — |
| E13 | Agarol (11(13)-eudesmen-12-ol) | — | 40 and 41 | — | — |
| E14 | Selina-3,11-dien-14-ol | — | — | 16 | 16 |
| E15 | Isolongifolene | 36 | — | — | — |
| E16 | α-Eudesmol | 42 | — | — | — |
| E17 | α-Copaen-11-ol | 2 | — | — | — |
| E18 | β-Eudesmol | 13 and 42 | 16 | 16 | 16 |
| E19 | γ-Selinene | 36, 42 and 43 | — | — | — |
| E20 | δ-Selinene | 36 | — | — | — |
| E21 | α-Copaene-8-ol | 43 | — | — | — |
| E22 | β-Maaliene | 36 | — | — | — |
| E23 | β-Eudesmol acetate | — | — | 16 | 16 |
| E24 | α-Selinene | 2 | — | 44 | — |
| E25 | Eudesm-7(11)-en-4a-ol | 2 | — | — | — |
| E26 | Naphthalene, decahydro-7-isopropenyl-4a-methyl-1-methylene- | — | 45- | — | — |
| E27 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | 2 | — | — | — |
| E28 | Acetic acid, 3-hydroxy-6-isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-yl ester | 2 | — | — | — |
| E29 | 5-Desoxylongilobol | 23 and 46 | — | 46 | — |
| E30 | Eudesma-4-en-8,11-diol | — | — | 47 | — |
| E31 | Eudesma-4-en-11,15-diol | 23 | — | 47 | — |
| E32 | Methyl-15-oxo-eudesmane-4,11(13)-dien-12-oate | — | — | 47 | — |
| E33 | Selina-3,11-dien-9,15-diol | 48* | — | — | — |
| E34 | (7S,8R,10S)-(+)-8,12-Dihydroxy-selina-4,11-dien-14-al | 49* | — | — | — |
| E35 | (7S,9S,10S)-(+)-9-Hydroxy-selina-4,11-dien-14-al | 23 and 49* | — | — | — |
| E36 | (5S,7S,9S,10S)-(−)-9-Hydroxy-selina-3,11-dien-14-al | 49* | — | — | — |
| E37 | (5S,7S,9S,10S)-(+)-9-Hydroxy-selina-3,11-dien-12-al | 23 and 49* | — | — | — |
| E38 | (5S,7S,9S,10S)-(+)-9-Hydroxy-eudesma-3,11(13)-dien-12-methylester | 23 and 49* | — | — | — |
| E39 | Selina-3,11-diene-12,15-dial (=12,15-dioxo-α-selinen) | 32 and 49* | — | — | — |
| E40 | (4αβ,7β,8αβ)-3,4,4α,5,6,7,8,8α-Octahydro-7-[1-(hydroxymethyl)ethenyl]-4α-methylnaphthalene-1-carboxaldehyde | 23 and 49* | 50 | — | — |
| E41 | Eudesmane-1β,5α,11-triol | 49* | — | — | — |
| E42 | (−)-7βH-Eudesmane-4α,11-diol | 49* | — | — | — |
| E43 | ent-4(15)-Eudesmen-11-ol-1-one | 49* | — | — | — |
| E44 | 15-Hydroxyl-12-oxo-α-selinen | 49* | — | — | — |
| E45 | Selina-4,11-diene-12,15-dial | 32 | 50 | — | — |
| E46 | (+)-Eudesma-4(14),11(13)-dien-8α,9β-diol | 23 | — | — | — |
| E47 | (+)-9β-Hydroxyeudesma-4,11(13)-dien-12-al | 23 | — | — | — |
| E48 | (+)-Eudesma-4,11(13)-dien-8α,9β-diol | 23 | — | — | — |
| E49 | 12,15-Dioxo-selina-4,11-dine | 23 | — | — | — |
| E50 | 12-Hydroxy-4(5),11(13)-eudesmadien-15-al | 23 | — | — | — |
| E51 | (+)-8α-Hydroxyeudesma-3,11(13)-dien-14-al | 23 | — | — | — |
| E52 | (+)-Eudesma-3,11(13)-dien-8α,9β-diol | 23 | — | — | — |
| E53 | (4R,5R,7S,9S,10S)-(−)-Eudesma-11(13)-en-4,9-diol | 23 | — | — | — |
| E54 | Selin-11-en-4α-ol | 23 | — | — | — |
| E55 | Eudesm-4-ene-11,15-diol | 23 | 50 | — | — |
| Eremophilanes | |||||
| P1 | (+)-(4S,5R)-Dihydrokaranone | 13 and 51 | 18 and 34 | — | — |
| P2 | (+)-(4S,5R)-karanone | — | 18 | 16 | 16 |
| P3 | Eremophila-9,11-dien-8-one (neopetasane) | 2, 13, 26, 38* and 51 | 16, 33 and 39 | 16 | 16 |
| P4 | rel-(2R,8R,8aS)-2-(1,2,3,5,6,7,8,8a-Octahydro-8 | — | 25 | — | — |
| P5 | 8,12-Epoxy-eremophila-9,11(13)-diene | 28* | 25 | — | — |
| P6 | (−)-(4R,5S,7R)-Jinkoh-eremol | 13 | 25, 30 and 39 | 16 | 16 |
| P7 | Dehydro-jinkoh-eremol | — | 16, 25 and 39 | 16 | 16 |
| P8 | (+)-(4R,5S,7R)-Kusunol (=valerianol) | 13, 38* and 52 | 14, 25 and 30 | 16 | 16 |
| P9 | rel-(2R,8S,8aS)-2-(1,2,6,7,8,8a-Hexahydro-8,8a-dimethyl-2-naphthyl)-propan-2-ol(valenca-1(10),8-dien-11-ol) | — | 25 | — | — |
| P10 | Valenc- or eremophil-9-en-12-al(tentative) | — | 25 | — | — |
| P11 | Calarene | — | 53 | — | — |
| P12 | 2,t-3-Dimethyl-r-2-(3-methyl-2-butenyl)-1-cyclohexanone | — | 19 | — | — |
| P13 | Valencene | 42 and 43 | — | 54 | — |
| P14 | Aristolone | — | — | 54 | — |
| P15 | Aristolene | 42 | — | — | — |
| P16 | Nootkatone | 42 | — | — | — |
| P17 | Calarene | — | — | 54 | — |
| P18 | 7b-H-9(10)-ene-11,12-epoxy-8-oxoeremophilane | 26 | — | — | — |
| P19 | 7α-H-9(10)-ene-11,12-epoxy-8-oxoeremophilane | 26, 38*, 46 and 51 | — | 46 | — |
| P20 | 11,13-Dihydroxy-9(10)-ene-8β,12-epoxyemophilane | — | — | 46 | — |
| P21 | (4S,5R,7R)-11,12-Dihydroxy-eremophila-1(10)-ene-2-oxo-11-methyl ester | — | — | 46 | — |
| P22 | 2-[(2β,8β,8aα)-8,8a-Dimethyl-1,2,3,4,6,7,8,8a-octahydronaphthalen-2-yl]-3-hydroxy-2-methoxpropanoic acid | — | — | 47 | — |
| P23 | 2-[(2β,8α,8aα)-8,8a-Dimethyl-1,2,3,4,6,7,8,8a-octahydronaphthalen-2-yl]propane-1,2-diol | — | — | 47 | — |
| P24 | (1β,3α,4aβ,5β,8aα)-4,4a-Dimethyl-6(prop-1-en-2-yl)octahydronaphtha-lene-1,8a(1H)-diol | — | — | 47 | — |
| P25 | (−)-Eremophila-9-en-8β,11-diol | 23 | — | 47 | — |
| P26 | 11-Hydroxy-valenc-1(10)-en-2-one | 23 and 38* | — | — | — |
| P27 | (1β,4αβ,7β,8αβ)-Octahydro-7-[1-(hydroxymethyl)ethenyl]-1,8α-dimethylnaphthalen-4α(2H)-ol | 23 and 38* | 50 | — | — |
| P28 | Ligudicin C | 51 | — | — | — |
| P29 | (+)-9β,10β-Epoxyeremophila-11(13)-en | 23 | — | — | — |
| P30 | (+)-11-Hydroxyvalenc-1(10),8-dien-2-one | 23 | — | — | — |
| P31 | 2-[(2β,4αβ,8β,8αβ)-Decahydro-4α-hydroxy-8,8α-dimethylnaphthalen-2-yl]prop-2-enal | 23 | 50 | — | — |
| P32 | (1αβ,2β,3β,4αβ,5β,8αβ)-Octahydro-4α,5-dimethyl-3-(1-methylethenyl)-3H-naphth[1,8a-b]oxiren-2-ol | — | 50 | — | — |
| Guaianes | |||||
| G1 | α-Guaiene | — | 16 and 34 | 16 | 16 |
| G2 | α-Bulnesene | — | 34 | 16 | 16 |
| G3 | (−)-Epoxyguai-11-ene (epoxybulnesene) | — | 16 and 55 | 16 | 16 |
| G4 | (−)-Guaia-1(10),11-dien-15-ol | — | 16 and 55 | 16 | 16 |
| G5 | (−)-Guaia-1(10),11-dien-15-al | — | 34 | — | — |
| G6 | (−)-Guaia-1(10),11-diene-15-carboxylic acid | — | 55 | — | — |
| G7 | Methyl guaia-1(10),11-diene-15-carboxylate | — | 55 | — | — |
| G8 | (−)-Guaia-1(10),11-dien-15,2-olide | — | 55 | — | — |
| G9 | (−)-2α-Hydroxyguaia-1(10),11-dien-15-oic acid (identified in acidic fraction as Me-ester) | — | 55 and 56 | — | — |
| G10 | (+)-Guaia-1(10),11-dien-9-one | — | 55 | — | — |
| G11 | Rotundone | — | 55 | — | — |
| G12 | (+)-1,5-Epoxy-nor-ketoguaiene | — | 39 | — | — |
| G13 | epi-Ligulyl oxide | 13 | — | — | — |
| G14 | Sinenofuranol | 13, 17, 22 and 57 | — | — | — |
| G15 | Sinenofuranal | 17 | — | — | — |
| G16 | Viridiflorol | 13 and 42 | — | — | — |
| G17 | Ledol | 42 | — | — | — |
| G18 | γ-Gurjunene | 42 | — | — | — |
| G19 | Longifolene | 58 | — | — | — |
| G20 | Aromadendrene | — | — | 54 | — |
| G21 | Guaiol | 36 | — | — | — |
| G22 | δ-Guaiene | — | — | 44 | — |
| G23 | 3,3,7-Trimethyltri-cycloundecan-8-one | 58 | — | — | — |
| G24 | Cyperotundone | — | — | 16 | 16 |
| G25 | Cyclocolorenone | — | 16 | 16 | 16 |
| G26 | α-Cedrol | 36 | — | — | — |
| G27 | 11β-Hydroxy-13-isopropyl-dihydrodehydrocostus lactone | 59 | — | — | — |
| G28 | Jumping | — | — | 54 | — |
| G29 | α-Patchoulene | 28* and 60 | — | — | — |
| G30 | Velleral | 28 and 42 | — | — | — |
| G31 | Isoaromadendrene epoxide | 2 and 42 | — | — | — |
| G32 | Aromadendrene oxide-(1) | 2 | — | — | — |
| G33 | Aromadendrene oxide-(2) | 2 | — | — | — |
| G34 | Diepi-α-cederene epoxide | 2 | — | — | — |
| G35 | 1H-Cycloprop[e]azulen-4-oldecahydro-1,1,4,7-tetramethyl-,[1aR-(1a.alpha.,4.beta.,4a.beta.,7.alpha., 7a.beta., 7b.alpha.)]- | — | 45 | — | — |
| G36 | α-Gurjunene | — | — | 54 | — |
| G37 | Chamaejasmone E | — | 61 | — | — |
| G38 | Chamaejasmone D | — | 61 | — | — |
| G39 | Auranticanol A | — | 61 | — | — |
| G40 | Qinanol A | 57 | — | — | — |
| G41 | Qinanol B | 57 | — | — | — |
| G42 | Qinanol C | 57 | — | — | — |
| G43 | Qinanol D | 57 | — | — | — |
| G44 | Qinanol E | 57 | — | — | — |
| G45 | Sinenofuranol | 57 | — | — | — |
| G46 | 3-Oxo-7-hydroxylholosericin A | 38* | — | — | — |
| G47 | 1,5;8,12-Diepoxyguaia-12-one | 38* | — | — | — |
| G48 | Qinanlactone | 37 | — | — | — |
| G49 | Qinan-guaiane-one | 37 | — | — | — |
| G50 | 7H-Guaia-1(10)-en-12,8-olide | 32 | — | — | — |
| G51 | 1,10-Dioxo-4αH-5αH-7βH-11αH-1,10-secoguaia-2(3)-en-12,8β-olide | 32 | — | — | — |
| G52 | 1α-Hydroxy-4βH-5βH-7βH-11αH-8,9-secoguaia-9(10)-en-8,12-olide | 32 | — | — | — |
| G53 | 1α-Hydroxy-4α,10α-dimethyl-5βH-octahydro-azulen-8-one | 32 | — | — | — |
| Candinanes | |||||
| C1 | 8βH-Dihydrogmelofuran | — | 62 | — | — |
| C2 | Gmelofuran | — | 62 | — | — |
| C3 | (7β,8β,9β)-8,9-Epoxycalamenen-10-one | — | — | 46 | — |
| Prezizanes | |||||
| R1 | Jinkohol | — | 30 and 31 | — | — |
| R2 | Jinkohol II | — | 30 | — | — |
| R3 | Daphnauranol B | — | 61 | — | — |
| R4 | Daphnauranol C | — | 61 | — | — |
| R5 | Daphnauranol D | — | 61 | — | — |
| Others | |||||
| O1 | Patchoulialcohol | 43 | — | — | — |
| O2 | (+)-8β-Hydroxy-longicamphenylone | 59 | — | — | — |
| O3 | Valerenol | — | — | 54 | — |
| O4 | Valerenic acid | 42 | — | 54 | — |
| O5 | Valerenal | 28* | — | 54 | — |
| O6 | Dihydro-neoclovene | — | — | 54 | — |
| O7 | 2,6-Dimethyl-10-methylene-12-oxatricyclo[7.3.1.0(1,6)]tridec-2-ene | 2 | — | — | — |
| O8 | β-Elemene | — | — | 16 | 16 |
| O9 | α-Bisabolol acetate | — | — | — | 16 |
| O10 | β-Caryophyllene | 43 | — | — | — |
| O11 | α-Humulene | 43 | — | — | — |
| O12 | Humulene diepoxide A | 58 | — | — | — |
| O13 | Kobusone | 58 | — | — | — |
| O14 | Santalol | 36, 42 and 58 | — | — | — |
| O15 | (E)-Nerolidol | — | 16 and 18 | 16 | 16 |
| O16 | Caryophyllenol-II | 58 | — | — | — |
| O17 | Caryophylleneoxide | 2, 42 and 43 | 45 | — | — |
| O18 | Baldrinal | 28* | — | — | — |
| O19 | α-Muurolene | 28* and 63 | — | — | — |
| O20 | Elemol | 2 and 13 | — | — | — |
| O21 | cis-Z-α-Bisabolene epoxide | 2 | — | — | — |
| O22 | Cubenol | 2 | — | — | — |
| O23 | 1,2,5,5,8a-Pentamethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-1-ol | 2 | — | — | — |
| O24 | 1,5,9-Trimethyl-1,5,9-cyclododecatriene | 38* | — | — | — |
| O25 | Aquilanol A | — | 61 | — | — |
| O26 | Aquilanol B | — | 61 | — | — |
| O27 | 12-Hydroxyhumula-2Z,6E,9E-triene | — | 61 | — | — |
| O28 | 14-Hydroxy-α-humulene | 23 | — | — | — |
2.1. Sesquiterpenes in A. sinensis
The sesquiterpenoids of agarwood are mainly derived from agarwood oil. Early publications on agarwood essential oils reflect the fact that the agarwood resin components are separated by solvent extraction, followed by column chromatography for purification and structural analysis using spectroscopy, including NMR. For example, Yang et al.12,21,27 and Xu et al.17 isolated sesquiterpenes F1–F2, F7–F9, F11, S1–S3, and G14 from A. sinensis. Yang and coworkers64 isolated G19, G23, O12–O13, and O16 from ethanol and petrol ether extracts of A. sinensis and later found two new sesquiterpenes, G27 and O2.Later articles focused on the use of “combination” techniques to detect and identify compounds. For example, Mei et al.,13,42 Tian et al.,36 Deng et al.,43 Chen et al.,2 and Miao et al.63 detected F3, F5, S10, E1, E9–E11, E15–E22, E24–E28, P1, P3, P6, P8, P13, P15–P16, G13–G14, G16–G18, G21, G26, G30–G34, O1, O5, O7, O10–O11, O17, O20, and O19–O23 from essential oils of A. sinensis by GC/MS. Lin et al. investigated agarwood obtained from fungus-infected Aquilaria at different times by GC-MS and showed the presence of S1, S3, P5, G29–G30, O5, and O18–O19.28 GC-MS combined with multivariate data analysis was used to construct chemical profiles of natural and artificial agarwoods. The chemical composition of agarwood oil was also studied. Agarwood essential oils are produced by steam distillation or the latest supercritical fluid extraction techniques.
With the development of separation technology, increasing numbers of publications are reporting the separation of sesquiterpenoids from extracts of agarwood resin. The purpose of these studies is to isolate and purify compounds from agarwood, to explore the pharmacological activities of these compounds, and to guide the selection of quality indicators and clinical medication. ‘Qi-Nan’ is regarded to have the highest quality and is therefore the most expensive agarwood in the market; Yang and coworkers26,37,57 performed studies on ‘Qi-Nan’ originating from A. sinensis and characterized some new sesquiterpenes, including F15, P18, P19, P27, G40–G44, G14, G48, G49, S13, and S12, from the Et2O extract of agarwood. From the ethanolic (EtOH) extract of agarwood induced by artificial holing, Li et al.38,49 isolated and identified two new guaiane-type sesquiterpenoids (G46, G47) and eleven eudesmane-type sesquiterpenoids (E34–E44) together with some known sesquiterpenoids, S12, O24, P3, P8, P19, P26, and P27. Kuang and coworkers48 were also interested in agarwood induced by artificial holing; they researched the chemical constituents of the n-butanol fraction of an EtOH extract and thereby obtained one new sesquiterpene, E33. Huo et al.23 obtained nine new sesquiterpenes together with seventeen known ones (E29, E31, E35, E37, E38, E40, E47–E54, F11, P24–P27, P29, P30, O28, S3, S9, and S14) from a 95% EtOH extract of resinous wood. Zhao et al.32 isolated sesquiterpenoids G50–G53, S3, E39, and E45 from the 95% EtOH extract of eaglewood of A. sinensis. Additionally, four sesquiterpenes, E31, P1, P19, and P28, were isolated from the resinous wood of A. sinensis in 2018.51
2.2. Sesquiterpenes in A. malaccensis (or A. agallocha)
A. malaccensis is found in Malaysia, Thailand and India and is currently the most widely distributed species.8 The researchers who study this variety are also widely geographically distributed, including Japan, Switzerland, and China. In 1963, Maheshwari and Jain15,20,41 isolated and identified F1–F3, F6–F7, F14, and E13 from A. agallocha. From 1992 to 1995, Näf and coworkers found 21 new sesquiterpenes, including agarofurans (F4, F10, and F12–F14), agarospiranes (S5–S8), five eudesmanes (E1, E9–E12), and seven eremophilanes (P4–P10 and P12).19,24,25 Ishihara, T. Tsuneya and coworkers found seven eudesmane-type sesquiterpenes (E2–E8) and 12 guaiane-type sesquiterpenes (G1–G12, S3, P1, and P3).34,39,55 The first agarospirane-type sesquiterpene, S1,29,45 was found in 1964; later, S9 was obtained from the 70% ethanol extract of Vietnamese agarwood.35 Later, two candinane-type sesquiterpenes, C1 and C2,62 were found. In 2009, Bhuiyan extracted E26 and G35 (among others) from naturally formed agarwood of A. agallocha as well as from agarwood formed through manual stimulation.45 From 1980 to 1983, Nakanishi30,31 discovered three new sesquiterpene alcohols from an agarwood (most likely A. malaccensis), named jinkoh-eremol (P6), jinkohol (R1) and jinkohol II(R2), together with S1 and P8, the major sesquiterpene constituents; their structures have all been established. Wu and coworkers33,50 discovered four new sesquiterpenoids, P27, P31, P32, and E40, together with the four known sesquiterpenoids E55, E45, P3, and S3, all of which were isolated from a 70% MeOH extract of A. malaccensis agarwood chips. Ma et al.61 reported sesquiterpenoids O25–O27, G37–G39, and R3–R5 from the ether extract of agarwood of A. malaccensis.2.3. Sesquiterpenes in A. crassna and A. subintegra
There are currently few studies on A. crassna and A. subintegra.8 In 2001, Pripdeevech and coworkers analyzed the diversity of compounds in A. malaccensis, A. crassna, and A. subintegra by GC-MS and GC-O. The study suggested that these species share sesquiterpenes F3, F15, S3, S11, E2, E3, E6, E18, P3, P7, G1, G3, G4, G25, O14, and O15.16 E24 and G22 were found in the supercritical carbon dioxide extraction of A. crassna.44 Pornpunyapat, Chetpattananondh and Tongurai, assessed the extraction conditions of essential oils obtained from A. crassna and detected P13–P14, P17, G20, G28, G36, and O3–O6.54Wang et al.46 isolated and identified the sesquiterpenoids C3, P19, E2 and E29 from the ethyl acetate (EtOAc) extract of agarwood originating from A. crassna. The following year, Kang and Dai et al.47 separated the sesquiterpenes P22–P25 and E30–E32 from a 95% EtOH extract of agarwood from A. crassna.
3. Chromone derivatives in agarwoods
Chromone derivatives are other major constituents of agarwoods. They have been obtained from only a few plant species, including Eremophila georgei, Bothriochloa ischaemum (Gramineae), and agarwoods originating from Aquilaria spp. (Thymelaeaceae). 2-(2-Phenylethyl)chromone derivatives are the characteristic components of agarwoods, and more than 40 such derivatives have been found in agarwoods belonging to different species. Depending on the molecular skeleton, chromones can be divided into 2-(2-phenylethyl)chromones, 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones, diepoxy-tetrahydro-2-(2-phenylethyl)chromones, and associated chromones (shown in Scheme 2). As shown in Table 3, the variation of chromones in different species is striking.| No. | Compounds and names | Species | ||
|---|---|---|---|---|
| A. s | A. m | A. c | ||
| a A. s, A. m, and A. c indicate A. sinensis, A. malaccensis, and A. crassna, respectively.b The reference was not found.c “*” indicates that the agarwood in this article was artificial agarwood. | ||||
| 2-(2-Phenylethyl)chromones | ||||
| 1 | 2-(2-Phenylethyl)chromone (flindersiachromone) | 51, 65, 76, 84 and 102 | 33, 63 and 99 | — |
| 2 | 6-Hydroxy-2-(2-phenylethyl)chromone (AH3) | 65, 76, 79* and 102 | 33 and 86 | 103 |
| 3 | 6-Methoxy-2-(2-phenylethyl)chromone (AH4) | 65 and 102 | 33 and 86 | — |
| 4 | 6-Hydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone | 65, 76 and 79* | — | — |
| 5 | 6-Methoxy-2-[2-(3-methoxyphenyl)ethyl]chromone (AH5) | 65 and 79* | 50 and 86 | — |
| 6 | 6,7-Dimethoxy-2-(2-phenylethyl)chromone (AH6) | 51, 65, 79* and 102 | 33 and 86 | — |
| 7 | 5,8-Dihydroxy-2-(2-phenylethyl)chromone (AH7) | 66 and 84 | 91 | — |
| 8 | 5,8-Dihydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone | 66 | — | |
| 9 | 6,7-Dimethoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone (AH8) | 48*, 51 and 66 | 33 and 91 | — |
| 10 | 6-Methoxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone | 67 and 79* | 80 and 99* | — |
| 11 | 6,8-Dihydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone | 67 | — | — |
| 12 | 6-Hydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone | 68 and 80* | — | — |
| 13 | 6-Hydroxy-2-(2′-hydroxy-2-phenylethyl)chromone | 76 and 104 | — | — |
| 14 | 5-Hydroxy-6-methoxy-2-(2-phenylethyl)chromone | 104 | — | — |
| 15 | 6-Hydroxy-7-methoxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone | 73, 75 and 80* | — | — |
| 16 | 6,7-Dimethoxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone | 73 and 80* | — | — |
| 17 | 7-Hydroxy-6-methoxy-2-[2-(3′-hydroxy-4′-methoxy-phenyl)ethyl]chromone | 73 | — | — |
| 18 | 6,7-Dimethoxy-2-[2-(4′-hydroxy-3′-methoxyphenyl)ethyl]chromone | 73 | — | — |
| 19 | 6,7-Dihydroxy-2-[2-(4′-methoxyphenyl)ethyl]chromone | 73 and 80* | — | — |
| 20 | 6-Hydroxy-7-methoxy-2-[2-(4′-hydroxyphenyl)ethyl]chromone | 73 | — | — |
| 21 | 6,8-Dihydroxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone | 73 and 75 | — | — |
| 22 | 6-Hydroxy-2-[2-(4′-hydroxy-3′-methoxyphenyl)ethenyl]chromone | 73 and 76 | — | — |
| 23 | 2-[2-(4′-Methoxyphenyl)ethyl]chromone | 76, 84 and 105 | 50 and 90 | — |
| 24 | 6-Methoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone | 79* | 50 and 90 | — |
| 25 | 7,8-Dimethoxy-2-[2-(3′-acetoxyphenyl)ethyl]chromone | — | 98 | — |
| 26 | 7-Hydroxy-2-(2-phenylethyl)chromone | — | 99 | — |
| 27 | 6-Hydroxy-2-[2-(4-hydroxyphenyl)ethyl]chromone | — | 99 | — |
| 28 | 6,8-Dihydroxy-2-(2-phenylethyl)chromone | 79* | 99 | — |
| 29 | 6-Hydroxy-7-methoxy-2-(2-phenylethyl)chromone | 51 | 99 | — |
| 30 | 5-Hydroxy-6-methoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone | 79* | — | — |
| 31 | 6-Methoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone | 79* and 80* | — | — |
| 32 | 5-Hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl]chromone | 79* | — | — |
| 33 | 6-Methoxy-2-[2-(4′-hydroxyphenyl)ethyl]chromone | 79* | — | — |
| 34 | (R)-2-(2-Hydroxy-2-phenylethyl)chromone | — | — | 103 |
| 35 | (S)-2-(2-Hydroxy-2-phenylethyl)chromone | — | — | 103 |
| 36 | 2-[2-(3-Methoxy-4-hydroxyphenyl)ethyl]chromone (qinanones B) | 76 | — | 103 |
| 37 | 2-[2-(3-Hydroxy-4-methoxyphenyl)ethyl]chromone (qinanones A) | 76 | — | — |
| 38 | 2-[2-(2-Hydroxy-4-methoxyphenyl)ethyl]chromone (qinanones C) | 76 | — | 103 |
| 39 | 2-[2-(4-Hydroxyphenyl)ethyl]chromone (qinanones D) | 76 | — | — |
| 40 | 2-[2-(3-Hydroxyphenyl)ethyl]chromone (qinanones E) | 76 | — | — |
| 41 | Qinanone F | 76 | — | — |
| 42 | 6-Hydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]chromone | 76 and 80* | — | — |
| 43 | 5-Hydroxy-6,7-dimethoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone | 84 | — | — |
| 44 | 5-Hydroxyl-7-methoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone | 83* | — | — |
| 45 | 5,8-Dihydroxy-6-methoxy-2-(2-phenylethyl)chromone | 83* | — | — |
| 46 | 6-Methoxy-2-[2-(2′,3′,4′-trihydroxy)phenylethyl]chromone | 83* | — | — |
| 47 | 6-Hydroxy-7-methoxy-2-[2-(4-methoxyphenyl)ethyl]chromone | 80* | — | — |
| 48 | 6-Hydroxy-2-[2-(3,4-dimethoxyphenyl)ethyl]chromone | 80* | — | — |
| 49 | 6,8-Dihydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone | 80* | — | — |
| 50 | 8-Chloro-6-hydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone | 80* | — | — |
| 51 | 5-Methoxy-6-hydroxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone | 80* | — | — |
| 52 | (R)-6,7-Dimethoxy-2-(2-hydroxy-2-phenylethyl)chromone | 80* | — | — |
| 53 | (S)-6,7-Dimethoxy-2-(2-hydroxy-2-phenylethyl)chromone | 80* | — | — |
| 54 | 7-Methoxy-2-[2-(4′-hydroxy-phenyl)ethyl]chromone | 51 | — | — |
| 55 | 7-Hydroxy-2-[2-(4′-methoxyphenyl)ethyl]chromone | 51 | — | — |
| 56 | 5,6-Dihydroxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone | 51 | — | — |
| 57 | 6-Hydroxy-5-methoxy-2-(2-phenyl-ethyl)chromone | 51 | — | — |
| 58 | 7-Methoxy-2-(2-phenylethyl)chromone | 51 and 84 | 50 | — |
| 59 | 8-Chloro-6-hydroxy-2-(2-phenylethyl)chromone | 74 and 84 | — | — |
| 60 | 5-Hydroxy-2-(2-phenylethyl)chromone | 84 | — | — |
| 61 | 6,7-Dimethoxy-2-[2-(4-hydroxyphenyl)ethyl]chromone | 79* and 80* | — | — |
| 62 | 6,7-Dimethoxy-2-[2-(3-methoxy-4-hydroxyphenyl)ethyl]chromone | 80* | — | — |
| 63 | 6-Methoxy-7-hydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone | 80* | — | — |
| 64 | 4′,6-Dihydroxy-3′,7-dimethoxy-2-(2-phenyl)ethylchromone (aquilarone G) | 75 | — | — |
| 65 | 4′-Hydroxy-6-methoxy-2-(2-phenylethyl)chromone (aquilarone H) | 75 | — | — |
| 66 | 3′,6-Dihydroxy-4′-methoxy-2-(2-phenylethyl)chromone (aquilarone I) | 75 | — | — |
| 67 | 5-Hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one | — | 50 | — |
| 68 | 7-Hydroxy-6-methoxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one | — | 33 | — |
| 69 | 8-Chloro-6-hydroxy-2-[2-(4-methoxyphenyl)ethyl]chromone | 74 | — | — |
| 5,6,7,8-Tetrahydro-2-(2-phenylethyl)chromones | ||||
| 70 | 6,7-Dihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone | 104 | — | — |
| 71 | 8-Chloro-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydrochromone | 84, 102 and 104 | — | — |
| 72 | 8-Chloro-5,6,7-trihydroxy-2-(3-hydroxy-4-methoxyphenethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one | 69 | — | — |
| 73 | 5,6,7,8-Tetrahydroxy-2-(3-hydroxy-4-methoxyphenethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one | 70 | — | — |
| 74 | (5S,6R,7S,8R)-2-[2-(3′-Hydroxy-4′-methoxyphenyl)ethyl]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone (aquilarone D) | 48 and 75 | — | — |
| 75 | (5S,6R,7S)-5,6,7-Trihydroxy-2-(3-hydroxy-4-methoxyphenethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one | 71 | 87 | — |
| 76 | (5S,6R,7R)-5,6,7-Trihydroxy-2-(3-hydroxy-4-methoxyphenethyl)-5,6,7,8-tetrahydro-4H-chromen-4-one | 71 and 84 | 87 | — |
| 77 | Agarotetrol (AH1) | 77 and 81* | 88 | — |
| 78 | (5S,6R,7S,8R)-2-(2-Phenylethyl)-5e′,6e,7e,8e-tetrahydroxy-5,6,7,8-tetrahydrochromone(isoagarotetrol) (AH2) | — | 88 | — |
| 79 | (5R,6R,7S,8R)-2-(2-Phenylethyl)-5e′,6a,7e,8e-tetrahydroxy-5,6,7,8-tetrahydrochromone (AH16) | 77 | 94 | — |
| 80 | 5α,6β,7β,8α-Tetrahydroxy-2-[2-(2-hydroxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone (AH23) | — | 95 | — |
| 81 | 5α,6β,7β-Trihydroxy-8α-methoxy-2-(2-phenylethy)chromone (AH17) | 81* | 95 | — |
| 82 | 5α,6β,7α,8β-Tetrahydroxy-2-[2-(2-hydroxy-phenyl)ethyl]-5,6,7,8-tetrahydrochromone (AH2b) | 48* | 89 | — |
| 83 | 5α,6β,7α,8β-Tetrahydroxy-2-[2-(4-methoxy-phenyl)ethyl]-5,6,7,8-tetrahydrochromone (AH2a) | — | 89 | — |
| 84 | 5α,6β,7β,8α-Tetraacetoxy-2-[2-(4-methoxy-phenyl)ethyl)]-5,6,7,8-tetrahydrochromone (AH1A) | — | 89 | — |
| 85 | (5S,6S,7R)-2-[2-(2-Acetoxyphenyl)ethyl]-5a′,6a,7a-tri-acetoxy-5,6,7,8,8-pentahydrochromone (AH9) | — | 91 | — |
| 86 | (5S,6S,7R,8S)-2-[2-(4-Methoxyphenyl)ethyl]-6,7,8-trihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone A) | 81* | — | — |
| 87 | (5R,6R,7S,8R)-2-(2-Phenylethyl)-6,7,8-trihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone B) | 81* | — | — |
| 88 | (5S,6S,7R,8S)-2-[2-(3′-Hydroxy-4′-methoxyphenyl)ethyl]-6,7,8-trihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone C) | 81* | — | — |
| 89 | (5S,6S,7R,8S)-2-[2-(4′-Methoxyphenyl)ethyl]-8-chloro-6,7-dihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone D) | 81* | — | — |
| 90 | (5S,6R,7R,8S)-2-[2-(4′-Methoxyphenyl)ethyl]-5,6,7-trihydroxy-8-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone E) | 81* | — | — |
| 91 | (5S,6R,7S,8R)-2-[2-(4-Methoxyphenyl)ethyl]-6,7,8-trihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone F) | 81* | — | — |
| 92 | (5R,6S,7R,8S)-2-[2-(4-Methoxyphenyl)ethyl]-6,7,8-trihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone G) | 81* | — | — |
| 93 | (5S,6R,7S,8R)-2-[2-(3′-Hydroxy-4′-methoxyphenyl)ethyl]-6,7,8-trihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone H) | 81* | — | — |
| 94 | (5S,6R,7S,8R)-2-[2-(4-Methoxyphenyl)ethyl]-8-chloro-6,7-dihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone I) | 81* | — | — |
| 95 | (5S,6R,7S,8R)-2-[2-(3′-Hydroxy-4′-methoxyphenyl)ethyl]-8-chloro-6,7-dihydroxy-5-methoxy-5,6,7,8-tetrahydrochromone (tetrahydrochromone J) | 81* | — | — |
| 96 | (5S,6R,7R,8S)-2-[2-(4′-Methoxyphenylethyl)]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone | 81* | — | — |
| 97 | rel-(5R,6S,7S,8R)-8-Chloro-5,6,7,8-tetrahydro-5,6,7-trihydroxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one | 81* | 33 | — |
| 98 | (5S,6S,7S,8R)-2-[2-(3′-Hydroxy-4′-methoxyphenyl)ethyl]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone (aquilarone A) | 75 and 81* | — | — |
| 99 | (5S,6S,7S,8R)-2-(2-Phenylethyl)-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone (aquilarone B) | 75, 81* and 84 | — | — |
| 100 | (5S,6S,7S,8R)-2-[2-(4′-Methoxyphenyl)ethyl]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone (aquilarone C) | 75, 81* and 84 | — | — |
| 101 | (5S,6R,7R,8S)-2-[2-(3′-Hydroxy-4′-methoxyphenyl)ethyl]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone (aquilarone E) | 75 and 81* | — | — |
| 102 | (5R,6R,7R,8S)-8-Chloro-5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone | 84 | — | — |
| 103 | (5S,6S,7S,8S)-8-Chloro-5,6,7-trihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone | 84 | — | — |
| 104 | (5R,6R,7R,8R)-8-Chloro-5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone | 84 | — | — |
| 105 | (5R,6S,7S)-5,6,7-Trihydroxy-2-(4-hydroxy-3-methoxyphenethyl)-5,6,7,8-tetrahydrochromone | 84 | — | — |
| 106 | (5S,6R,7R,8S)-2-[2-(4′-Hydroxyphenyl)ethyl]-5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone (aquilarone F) | 75 | — | — |
| 107 | rel-(5R,6S,7S,8R)-8-Chloro-5,6,7,8-tetrahydro-5,6,7-trihydroxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one | — | 33 | — |
| 108 | rel-(5R,6S,7R)-5,6,7,8-Tetrahydro-5,6,7-trihydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one | — | 33 | — |
| 109 | rel-(5R,6S,7R)-5,6,7,8-Tetrahydro-5,6,7-trihydroxy-2-[2-(4-methoxyphenyl)ethyl]-4H-1-benzopyran-4-one | — | 33 | — |
| Diepoxy-tetrahydro-2-(2-phenylethyl)chromones | ||||
| 110 | 5,6:7,8-Diepoxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone (oxidoagarochromone A) | 72*, 79* and 81* | 33 | 72* |
| 111 | 5,6:7,8-Diepoxy-2-[2-(4-methoxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone (oxidoagarochromone B) | 72* and 79* | 33 | 72* |
| 112 | 5,6:7,8-Diepoxy-2-[2-(3-hydroxy-4-methoxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone (oxidoagarochromone C) | 72* | 33 | 72* |
| 113 | 5,6-Epoxy-7β-hydroxy-8β-methoxy-2-(2-phenylethyl)chromone | 79* | — | — |
| 114 | (5S,6R,7R,8R)-2-(2-Phenylethyl)-7,8-epoxy-5,6-dihydroxy-5,6,7,8-tetrahydrochrome (tetrahydrochromone K) | 81* | — | — |
| 115 | (5R,6S,7S,8S)-2-[2-(4′-Methoxyphenyl)ethyl]-7,8-epoxy-5,6-dihydroxy-5,6,7,8-tetrahydrochrome (tetrahydrochromone L) | 81* | — | — |
| 116 | (5R,6S,7S,8S)-2-[2-(3′-Hydroxy-4′-methoxyphenyl)ethyl]-7,8-epoxy-5,6-dihydroxy-5,6,7,8-tetrahydrochrome (tetrahydrochromone M) | 81* | — | — |
| 117 | 5α,6α-Epoxy-7β,8α,3′-trihydroxy-4′-methoxy-2-(2-phenylethyl)chromone | 83* | — | — |
| 118 | rel-(1aR,2R,3R,7bS)-1a,2,3,7b-Tetrahydro-2,3-dihydroxy-5-[2-(4-methoxyphenyl)ethyl]-7H-oxireno[f][1]benzopyran-7-one | 84 | 33 | — |
| 119 | rel-(1aR,2R,3R,7bS)-1a,2,3,7b-Tetrahydro-2,3-dihydroxy-5-(2-phenylethyl)-7H-oxireno[f][1]benzopyran-7-one | 79* | 33 | — |
| 120 | Qinanmer | 77 | — | — |
| 121 | 2-[2-(4-Glucosyloxy-3-methoxyphenyl)ethyl]chromone | 78 | — | — |
| 122 | (5S,6S,7R,8S)-2-(2-Phenylethyl)-6,7,8-trihydroxy-5,6,7,8-tetrahydro-5-[2-(2-phenylethyl)chromonyl-6-oxy]chromone (AH10) | — | 92 | — |
| 123 | (5S,6S,7R,8S)-2-(2-Phenylethyl)-6,7,8-trihydroxy-5,6,7,8-tetrahydro-5-[2-(2-phenylethyl)-7-hydroxy-chromonyl-6-oxy]chromone (AH15) | — | 92 | — |
| 124 | 2,2′-Di-(2-phenylethyl)-8,6′-dihydroxy-5,5′-bichromone (AH11) | — | 92 | — |
| 125 | (5S,6R,7R,8S)-2-(2-Phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)-7-methoxychromonyl-6-oxy]chromone (AH12) | — | 92 | — |
| 126 | (5S,6R,7R,8S)-2-(2-Phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone (AH13) | — | 92 | — |
| 127 | (5S,6S,7S,8R)-2-(2-Phenylethyl)-6,7,8-trihydroxy-5,6,7,8-tetrahydro-5-[2-(2-phenylethyl)-chromonyl-6-oxy]chromone (AH14) | — | 92 | — |
| 128 | Dioxin-linked bi-2-(2-phenylethyl)chromone (AH21) | — | 97 | — |
| 129 | Bi-(5S,6S,7R,8S)-2-(2-phenylethyl)-6,7,8-trihydroxy-5,6,7,8-tetrahydro-5-[2-(2-phenylethyl)chromonyl-6,7-dioxy]chromone (AH18) | — | 93 | — |
| 130 | AH19a | — | 96 | — |
| 131 | AH19b | — | 96 | — |
| 132 | AH20 | — | 95 | — |
| 133 | (5S,6R,7S,8R)-2-[2-(4-Methoxyphenyl)ethyl]-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-{6-methoxy-2-[2-(3′′′-methoxy-4′′′-hydroxyphenyl)ethyl]chromonyl-7-oxy}chromone | 82* | — | — |
| 134 | (5S,6R,7S,8R)-2-[2-(4-Methoxyphenyl)ethyl]-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-{2-[2-(4′′′-methoxyphenyl)ethyl]chromonyl-6-oxy}chromone | 82* | — | — |
| 135 | (5S,6R,7S,8R)-2-(2-Phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone | 82* | — | — |
| 136 | (5R,6R,7R,8S)-2-(2-Phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone | 82* | — | — |
| 137 | Crassin A | — | — | 100 |
| 138 | (5R,6S,7R,8S)-Configuration (crassin B) | — | — | 100 |
| 139 | (5S,6R,7S,8R)-Configuration (crassin C) | — | — | 100 |
| 140 | Crassin D | — | — | 100 |
| 141 | Aquilacrassnin A | — | — | 101 |
| 142 | Aquilacrassnin B | — | — | 101 |
| 143 | Aquilacrassnin C | — | — | 101 |
| 144 | Aquilacrassnin D | — | — | 101 |
| 145 | Aquilacrassnin E | — | — | 101 |
| 146 | Aquilacrassnin F | — | — | 101 |
| 147 | (5S,6R,7S,8R)-(+)-Aquisinenone A | 85 | — | — |
| 148 | (5R,6S,7R,8S)-(−)-Aquisinenone A | 85 | — | — |
| 149 | (5R,6S,7R,8S)-(−)-4′-Methoxyaquisinenone A | 85 | — | — |
| 150 | (5R,6S,7R,8S)-(+)-Aquisinenones B | 85 | — | — |
| 151 | (5S,6R,7S,8R)-(−)-Aquisinenones B | 85 | — | — |
| 152 | (5S,6R,7S,8R)-(−)-6′′-Hydroxyaquisinenone B | 85 | — | — |
| 153 | (5R,6S,7R,8S)-(+)-6′′-Hydroxy-4′,4′′′-dimethoxyaquisinenone B | 85 | — | — |
| 154 | (5R,6S,7R,8S)-(+)-Aquisinenones C | 85 | — | — |
| 155 | (5S,6R,7S,8R)-(−)-Aquisinenones C | 85 | — | — |
| 156 | (5S,6R,7S,8R)-(−)-Aquisinenone D | 85 | — | — |
| 157 | (5R,6S,7R,8S)-4′-Demethoxyaquisinenone D | 85 | — | — |
| 158 | (5S,6R,7S,8R)-4′-Demethoxyaquisinenone D | 85 | — | — |
| 159 | (5S,6R,7S,8R)-(+)-Aquisinenone E | 85 | — | — |
| 160 | (5S,6R,7S,8R)-(−)-Aquisinenone F | 85 | — | — |
| 161 | (5S,6R,7S,8R)-(−)-Aquisinenone G | 85 | — | — |
| 162 | (+)-4′-Methoxyaquisinenone G | 85 | — | — |
Regarding the study of chromones, most researchers use agarwood extracts, usually ethanol (EtOH) extracts, to extract and separate the monomers. The structures of the compounds are determined by a series of assays, including LC/MS, and nuclear magnetic resonance.
3.1. Chromones in A. sinensis
Approximately 130 chromone derivatives have been obtained from A. sinensis, comprising 22 forms of 2-(2-phenylethyl)chromones, six 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones, and three diepoxy-tetrahydro-2-(2-phenylethyl)chromones.Yang et al. obtained 1–6 from an EtOH extract of A. sinensis, which belong to the group of 2-(2-phenylethyl)chromones;65 they later extracted 7–9 from an EtOAc–EtOH extract.66 In addition, 10–12 were isolated by Liu et al.67,68 Dai et al. extracted 165–168 from the same species.69–71 Yagura and coworkers obtained four chromones, 13, 14, 70 and 71, in 2003 and later extracted 110–112;72 these are all diepoxy tetrahydrochromones. In 2012, Yang and coworkers isolated eight new chromone derivatives, 15–22.73 Gao et al.74 and Chen et al.75 isolated 59, 69, and aquilarones A–I (64–66, 74, 98–101, 106), with two known chromones, 15 and 21, from an EtOH extract of resinous wood of A. sinensis. Yang76 obtained 2-(2-phenylethyl)chromone derivatives 1, 2, 4, 13, 23, 22, and 36–42 from a Et2O extract of “Qi-Nan”. Later, this research team77,78 found a new compound, 120, comprising 2-(2-phenylethyl)chromone and sesquiterpene moieties, named “Qinanmer”; a 2-(2-phenylethyl)chromone glycoside, 121, together with two 2-(2-phenylethyl)chromone derivatives, 77 and 79, were obtained from a EtOH extract of “Qi-Nan”.
Since 2014, researchers have been engaged in the study of artificial agarwood induced by the holing method. Li et al.79 isolated three previously undescribed 2-(2-phenylethyl)chromone derivatives, 30, 31, and 113, and thirteen 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones, named tetrahydrochromones A–M (86–95, 114–116), together with thirteen known ones (2, 4–6, 10, 24, 28, 32, 33, 110, 111, 118, and 119) from an EtOAC extract. Liao et al.80,81 used the same method and found 2-(2-phenylethyl)chromone derivatives 12, 15, 16, 19, 42, 47–53, 61–63, 77, 81, 96–101, and 110. The EtOAc fraction also contained four new bi-phenylethylchromones, 133–136.82 Kuang et al.48 were also interested in agarwood induced by artificial holing; they researched the chemical constituents of the n-butanol fraction of an EtOH extract and obtained 9, 74, and 82.
Liu et al.83 separated and identified 44–46 and 117 from an EtOH extract of agarwood produced via the whole-tree agarwood-inducing technique.
Huo and coworkers84 isolated 2-(2-phenylethyl)chromone derivatives 1, 7, 23, 43, 58–60, 71, 76, 99, 100, 102–105, and 118 from a 95% EtOH–EtOAc extract of resinous wood of A. sinensis. Subsequently, through LC-MS-guided separation and purification, they obtained sixteen new 2-(2-phenylethyl)chromone dimers, including four pairs of enantiomers, along with eight optically pure analogues (151–162).85 Wang et al.51 isolated compounds 54–57, which belong to the group of 2-(2-phenylethyl)chromone derivatives, from resinous wood, together with five known compounds, 1, 6, 9, 29, and 58, from a MeOH extract.
3.2. Chromones in A. malaccensis (or A. agallocha)
More than 30 chromones have been reported from A. agallocha, of which nine are the same as in A. sinensis, namely 1,63 2, 3,86 5, 6,86 9,87 and 71 and 72.87 Since 1982, Shimada and coworkers have been engaged in the isolation of chromones 2–3, 5–6, 77–78,88 82,89 and 83.89 In 1986, Nakanishi isolated a known chromone, 23, and a new chromone, 24.90 Then, 7, 9, 85,91 122–127,92 and 129 (ref. 93) were isolated and identified. Konishi devoted himself to this work, also aiding other researchers in the field; from 1989 to 1992, he found 79,94 80, 81, 132,95 130–131,96 128,97 and 71–72.87 Iwagoe obtained 123 and 129,93 and in 2005, Alkhathlan isolated 3, 6, and 25 from A. agallocha.98The chromones isolated from A. malaccensis were mainly reported by T. Konishi in 2002, namely 1, 26–29, and 10.99 Wu et al.33,50 reported the 2-(2-phenylethyl)-4H-chromone derivatives 1–3, 5, 6, 9, 14, 23, 24, 58, 67, 68, 97, 107–112, 118, and 119 from a 70% MeOH extract of A. malaccensis agarwood.
3.3. Chromones in A. crassna
There are few reports on chromones in A. crassna. Diepoxy-tetrahydro-2-(2-phenylethyl) chromones 110–112 were obtained from A. crassna.72 Yang et al.100,101 obtained four new bi-2-(2-phenylethyl)chromone derivatives, crassins A–D (137–140), and six previously undescribed uncommon ester-bonded dimeric compound aquilacrassnins A–F (141–146) from the EtOAc extract of agarwood originating from A. crassna.4. Discussion
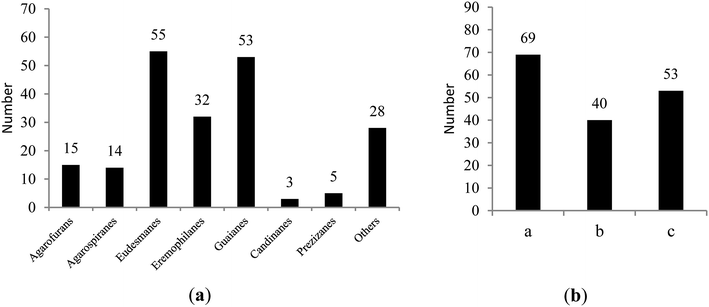
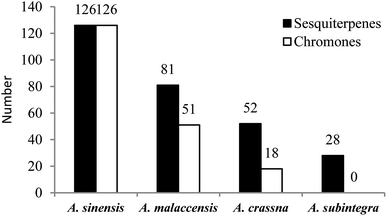
Among the 367 new main chemical constituents from agarwoods that were statistically assessed in this paper, chromone derivatives and sesquiterpenes accounted for 44.14% and 55.86%, respectively, of the total constituents. It can be seen in Fig. 1(a) that the largest numbers of sesquiterpenes in agarwood are eudesmanes, guaianes and eremophilanes. Fig. 1(b) reflects the number of different chromones in agarwood, where 2-(2-phenylethyl)chromones are currently the most commonly isolated types.From the statistical results (shown in Fig. 2), researchers are currently mainly engaged in the study of the chemical constituents of agarwood originating from A. sinensis, A. malaccensis, and A. crassna, respectively, of which most of the new compounds were isolated from A. sinensis. It can be seen that resources are important prerequisites for the study of agarwood; thus, there are many studies on species with relatively abundant resources, such as A. sinensis, A. malaccensis, and A. crassna. Of course, this is also closely related to geographical distribution. Agarwoods originating from different Aquilaria plants contain some common compounds as well as some different compounds. Among different species of agarwood, the chemical compositions are quite different. Therefore, it is necessary to indicate the species from which the used agarwood is derived. However, during the writing process, we found that many articles on the separation of compounds from agarwood did not indicate which species of the genus Aquilaria the agarwood was derived from. Therefore, we encourage researchers studying agarwood to indicate more information about the origin and tree species to clarify the source of the material.
According to the data, the number of sesquiterpenes isolated from agarwood has thus far been higher than the number of chromones, and the proportion of articles is also the same. In the past 10 years, the number of articles on chromones has increased rapidly. The study of the chemical constituents of agarwood from sesquiterpenes to chromone derivatives shows that increasing numbers of researchers are beginning to focus on revealing the main components of agaric pharmacologically active substances rather than only fragrance components. Therefore, we can see that research on the separation and activity of chromone derivatives still has broad research prospects.
By summarizing and comparing the chemical compositions of different tree species, we can provide more research ideas. The same components can be used as standards for quality assessment, with reliable and stable characteristics, and different components can guide the selection of high quality agarwood species. By reviewing the chemical compositions of agarwoods from the four species, we believe that the following characteristics should be considered when selecting quality control standards. Due to the complex composition of agarwood, sesquiterpenes and chromone derivatives should be considered first, especially chromones, mainly because they are characteristic components of agarwood, and chromone derivatives are easier to separate and preserve. Due to the extremely complex sources and types of agarwood, researchers in different countries should fully consider the common chemical composition when selecting control indicators and formulating quality testing methods to improve the scope and scientificity of the testing methods, such as F2 and F3. Of course, even with the limited amounts of research on individual species, it is possible to flexibly select components, such as chromone 1.
5. Conclusion
Agarwood, which is expensive and widely used, is derived from the resin-containing wood of Aquilaria species trees. The chemical components of agarwood are diverse and complex; 367 new chemical constituents from agarwood were statistically assessed in this paper. This review summarizes the main molecular skeletons of agarwood compounds, revealing the differences in the chemical compositions of agarwood originating from different Aquilaria species. This will help researchers to better understand research on agarwood and select more suitable detection indicators.With the continuous exploration and efforts made by scientists in recent years, the understanding of the chemical compositions of agarwood from different sources is continuously improving, and some specific chemical compositions may become identification indices and judgement standards of agarwood samples from different sources. In the future, we expect to see more research on the chemical components of agarwood from different species in order to help identify characteristic compounds of agarwood, establish a stable, effective, comprehensive, and reliable quality evaluation system, and consequently elucidate which species best produce agarwood.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (81773844, 31000136), Beijing Municipal Natural Science Foundation (6102024).References
- J. S. Yang, Nat. Prod. Res. Dev., 1998, 10, 99–103 CAS.
- H. Q. Chen, Y. Yang, J. Xue, J. H. Wei, Z. Zhang and H. J. Chen, Molecules, 2011, 16, 4884–4896 CrossRef CAS PubMed.
- M. C. M. Eurlings and B. Gravendeel, Plant Syst. Evol., 2005, 254, 1–12 CrossRef CAS.
- R. Naef, Flavour Fragrance J., 2011, 26, 73–87 CrossRef CAS.
- The Plant List, http://www.theplantlist.org/tpl1.1/search?q=Aquilaria, accessed December 6, 2018.
- Checklist of CITES species, http://checklist.cites.org/#/en, accessed December 6, 2018.
- CITES Secretariat and Missouri Botanical Garden, http://www.tropicos.org/, accessed December 6, 2018.
- Y. Z. H. Y. Hashim, P. G. Kerr, P. Abbas and H. M. Salleh, J. Ethnopharmacol., 2016, 189, 331–360 CrossRef CAS PubMed.
- K. Jayachandran, I. Sekar, K. T. Parthiban, D. Amirtham and K. K. Suresh, Indian J. Nat. Prod. Resour., 2014, 1, 44–47 Search PubMed.
- M. Saikia, K. Shrivastava and S. S. Singh, Int. J. Plant Sci., 2012, 2, 188–194 Search PubMed.
- Y. B. Li, Doctor thesis, Guangzhou University of Chinese Medicine, 2017.
- J. Yang, Y. L. Wang, Y. L. Su, C. H. He, Q. T. Zheng and J. Yang, Acta Pharmacol. Sin., 1989, 264–268 CAS.
- W. L. Mei, Y. B. Zeng, J. Wu, H. B. Cui and H. F. Dai, J. Chin. Pharm. Sci., 2008, 17, 225–229 CAS.
- T. Nakanishi, E. Yamagata, K. Yoneda, T. Nagashima, I. Kawasaki, T. Yoshida, H. Mori and I. Miura, Phytochemistry, 1984, 23, 2066–2067 CrossRef CAS.
- M. L. Maheshwari, T. C. Jain, R. B. Bates and S. C. Bhattacharyya, Tetrahedron, 1963, 19, 1079–1090 CrossRef CAS.
- P. Pripdeevech, W. Khummueng and S. K. Park, J. Essent. Oil Res., 2011, 23, 46–53 CrossRef CAS.
- J. F. Xu, L. F. Zhu, B. Y. Lu and C. T. Liu, Acta Bot. Sin., 1988, 6, 638 Search PubMed.
- T. Nagashima, I. Kawasaki, T. Yoshida, T. Nakanishi and K. Yoneda, New sesquiterpenoids from agarwood, Singapore, 1983, vol. 3, pp. 12–16 Search PubMed.
- R. Näf, A. Velluz, W. Thommen, R. Brauchli, C. Sigwart and J. M. Gaudin, Flavour Fragrance J., 1993, 8, 307–313 CrossRef.
- M. L. Maheshwari, K. R. Varma and S. C. Bhattacharyya, Tetrahedron, 1963, 19, 1519–1525 CrossRef CAS.
- J. S. Yang and Y. W. Chen, Acta Pharmacol. Sin., 1986, 516–520 CAS.
- J. S. Yang, Y. L. Wang and Y. L. Su, Chin. Chem. Lett., 1992, 983–984 CAS.
- H. X. Huo, Z. X. Zhu, D. R. Pang, Y. T. Li, Z. Huang, S. P. Shi, J. Zheng, Q. Zhang, Y. F. Zhao and P. F. Tu, Fitoterapia, 2015, 106, 115–121 CrossRef CAS PubMed.
- R. Näf, A. Velluz, N. Busset and J. M. Gaudin, Flavour Fragrance J., 1992, 7, 295–298 CrossRef.
- R. Näf, A. Velluz, R. Brauchli and W. Thommen, Flavour Fragrance J., 1995, 10, 147–152 CrossRef.
- D. L. Yang, H. Wang, Z. K. Guo, W. Li, W. L. Mei and H. F. Dai, Phytochem. Lett., 2014, 8, 121–125 CrossRef CAS.
- J. S. Yang and Y. W. Chen, Acta Pharmacol. Sin., 1983, 18, 191–198 CAS.
- F. Lin, H. F. Dai, H. Wang and W. L. Mei, Lishizhen Med. Mater. Med. Res., 2010, 1901–1902 CAS.
- M. L. Maheshwari, S. C. Bhattacharyya and K. R. Varma, Tetrahedron, 1965, 21, 115–138 CrossRef.
- T. Nakanishi, E. Yamagata, K. Yoneda, I. Miura and H. Mori, J. Chem. Soc., Perkin Trans. 1, 1983, 14, 601–604 RSC.
- T. Nakanishi, E. Yamagata, K. Yoneda and I. Miura, Phytochemistry, 1981, 20, 1597–1599 CrossRef CAS.
- H. Zhao, Q. Peng, Z. Han, L. Yang and Z. Wang, Molecules, 2016, 21, 281 CrossRef PubMed.
- B. Wu, S. W. Kwon, G. S. Hwang and J. H. Park, Helv. Chim. Acta, 2012, 95, 1657–1665 CrossRef CAS.
- M. Ishihara, T. Tsuneya, M. Shiga and K. Uneyama, Phytochemistry, 1991, 30, 563–566 CrossRef CAS.
- J. ya Ueda, L. Imamura, Y. Tezuka, Q. L. Tran, M. Tsuda and S. Kadota, Bioorg. Med. Chem., 2006, 14, 3571–3574 CrossRef PubMed.
- J. J. Tian, X. L. Guo, L. C. Lai, H. B. Zhou, W. M. Zhang and X. X. Gao, Lishizhen Med. Mater. Med. Res,, 2009, 20, 2505–2507 CAS.
- D. L. Yang, J. Wang, W. Li, W. H. Dong, W. L. Mei and H. F. Dai, Phytochem. Lett., 2016, 17, 94–99 CrossRef CAS.
- W. Li, G. Liao, W. H. Dong, F. D. Kong, P. Wang, H. Wang, W. L. Mei and H. F. Dai, Molecules, 2016, 21, 274 CrossRef PubMed.
- M. Ishihara, T. Tsuneya and K. Uneyama, Phytochemistry, 1993, 33, 1147–1155 CrossRef CAS.
- S. P. Gunasekera, A. D. Kinghorn, G. A. Cordell and N. R. Farnsworth, J. Nat. Prod., 1981, 44, 569–572 CrossRef CAS.
- T. C. Jain and S. C. Bhattacharyya, Tetrahedron Lett., 1959, 1, 13–17 CrossRef.
- W. L. Mei, Y. B. Zeng, J. Liu and H. F. Dai, J. Chin. Med. Mater., 2007, 30, 551–555 CAS.
- H. M. Deng, H. Q. Tong and R. J. Zhou, West China J. Pharm. Sci., 2008 Search PubMed.
- P. Wetwitayaklung, N. Thavanapong and J. Charoenteeraboon, Silpakorn Univ. Sci. Technol. J., 2009, 3, 25–33 CAS.
- M. N. I. Bhuiyan, J. Begum and M. N. H. Bhuiyan, Bangladesh J. Pharmacol., 2009, 4, 24–28 Search PubMed.
- H. N. Wang, W. H. Dong, S. Z. Huang, W. Li, F. D. Kong, H. Wang, J. Wang, W. L. Mei and H. F. Dai, Fitoterapia, 2016, 114, 7–11 CrossRef CAS PubMed.
- K. X. Kang, H. F. Dai, P. Wang, F. D. Kong, L. M. Zhou, W. H. Dong, G. P. Zhu and W. L. Mei, Chin. Tradit. Herb. Drugs, 2017, 4601–4607 Search PubMed.
- T. D. Kuang, H. Q. Chen, W. Li, J. L. Yang, L. M. Zhou, C. H. Cai, W. H. Dong, W. L. Mei and H. F. Dai, China J. Chin. Mater. Med., 2017, 4618–4623 Search PubMed.
- W. Li, C. H. Cai, Z. K. Guo, H. Wang, W. J. Zuo, W. H. Dong, W. L. Mei and H. F. Dai, Fitoterapia, 2015, 100, 44–49 CrossRef CAS PubMed.
- B. Wu, J. G. Lee, C. J. Lim, S. D. Jia, S. W. Kwon, G. S. Hwang and J. H. Park, Helv. Chim. Acta, 2012, 4, 636–642 CrossRef.
- S. L. Wang, Y. C. Tsai, S. L. Fu, M. J. Cheng, M. I. Chung and J. J. Chen, Molecules, 2018, 23, 289 CrossRef PubMed.
- L. D. Lin and S. Y. Qi, Chin. Tradit. Herbal Drugs, 2000 Search PubMed.
- H. Takemoto, M. Ito, T. Shiraki, T. Yagura and G. Honda, J. Nat. Med., 2008, 62, 41–46 CrossRef CAS PubMed.
- J. Pornpunyapat, P. Chetpattananondh and C. Tongurai, Bangladesh J. Pharmacol., 2011, 6, 18–24 Search PubMed.
- M. Ishihara, T. Tsuneya and K. Uneyama, Phytochemistry, 1991, 30, 3343–3347 CrossRef CAS.
- M. Ishihara, Y. Masatsugu and K. Uneyama, Tetrahedron, 1992, 48, 10265–10276 CrossRef CAS.
- D. L. Yang, W. Li, W. H. Dong, J. Wang, W. L. Mei and H. F. Dai, Fitoterapia, 2016, 112, 191–196 CrossRef CAS PubMed.
- L. Yang, L. R. Qiao, D. Xie, J. G. Dai and S. X. Guo, China J. Chin. Mater. Med., 2012, 37, 1973 CAS.
- L. Yang, L. R. Qiao, J. J. Zhang, J. G. Dai and S. X. Guo, J. Asian Nat. Prod. Res., 2012, 14, 1054–1058 CrossRef CAS PubMed.
- J. Liu, Master thesis, Hainan University, 2008.
- C. T. Ma, T. Eom, E. Cho, B. Wu, T. R. Kim, K. B. Oh, S. B. Han, S. W. Kwon and J. H. Park, J. Nat. Prod., 2017, 80, 3043–3048 CrossRef CAS PubMed.
- P. Pant and R. P. Rastogi, Phytochemistry, 1980, 19, 1869–1870 CrossRef CAS.
- C. L. Miao, B. T. Sun, L. P. Luo, D. X. Liu and B. Y. Yang, Food Sci., 2010, 30, 215–217 Search PubMed.
- L. Yang, L. R. Qiao, J. J. Zhang, J. G. Dai and S. X. Guo, J. Asian Nat. Prod. Res., 2012, 14, 1054–1058 CrossRef CAS PubMed.
- J. S. Yang, Y. L. Wang and Y. L. Su, Acta Pharmacol. Sin., 1989, 24, 678–683 CAS.
- J. S. Yang, Y. L. Wang and Y. L. Su, Acta Pharmacol. Sin., 1990, 25, 186–190 CAS.
- J. M. Liu, Y. H. Gao, H. H. Xu and H. J. Chen, Chin. Tradit. Herb. Drugs, 2006, 325–327 Search PubMed.
- J. M. Liu, Y. H. Gao, H. H. Xu and Z. Q. Xu, Chin. Tradit. Herb. Drugs, 2007, 1138–1140 Search PubMed.
- J. Liu, J. Wu, Y. X. Zhao, Y. Y. Deng, W. L. Mei and H. F. Dai, Chin. Chem. Lett., 2008, 19, 934–936 CrossRef CAS.
- H. F. Dai, J. Liu, Y. B. Zeng, Z. Han, H. Wang and W. L. Mei, Molecules, 2009, 14, 5165–5168 CrossRef CAS PubMed.
- H. F. Dai, J. Liu, Z. Han, Y. B. Zeng, H. Wang and W. L. Mei, J. Asian Nat. Prod. Res., 2010, 12, 134–137 CrossRef CAS PubMed.
- T. Yagura, N. Shibayama, M. Ito, F. Kiuchi and G. Honda, Tetrahedron Lett., 2005, 46, 4395–4398 CrossRef CAS.
- L. Yang, L. R. Qiao, D. Xie, Y. H. Yuan, N. H. Chen, J. G. Dai and S. X. Guo, Phytochemistry, 2012, 76, 92–97 CrossRef CAS PubMed.
- Y. H. Gao, J. M. Liu, H. X. Lu and Z. X. Wei, Helv. Chim. Acta, 2012, 95, 951–954 CrossRef CAS.
- D. Chen, Z. R. Xu, X. Y. Chai, K. W. Zeng, Y. Jia, D. Bi, Z. Z. Ma and P. F. Tu, Eur. J. Org. Chem., 2012, 2012, 5389–5397 CrossRef CAS.
- D. L. Yang, W. L. Mei, Y. B. Zeng, Z. K. Guo, Y. X. Zhao, H. Wang, W. J. Zuo, W. H. Dong, Q. H. Wang and H. F. Dai, Planta Med., 2013, 79, 1329–1334 CrossRef CAS PubMed.
- H. Shao, F. D. Kong, H. Wang, W. L. Mei and H. F. Dai, J. Asian Nat. Prod. Res., 2017, 19, 935–940 CrossRef CAS PubMed.
- H. Shao, W. L. Mei, F. D. Kong, W. H. Dong, W. Li, G. P. Zhu and H. F. Dai, J. Asian Nat. Prod. Res., 2017, 19, 42–46 CrossRef CAS PubMed.
- W. Li, C. H. Cai, W. H. Dong, Z. K. Guo, H. Wang, W. L. Mei and H. F. Dai, Fitoterapia, 2014, 98, 117–123 CrossRef CAS PubMed.
- G. Liao, W. L. Mei, W. H. Dong, W. Li, P. Wang, F. D. Kong, C. J. Gai, X. Q. Song and H. F. Dai, Fitoterapia, 2016, 110, 38–43 CrossRef CAS PubMed.
- G. Liao, W. L. Mei, F. D. Kong, W. Li, J. Z. Yuan and H. F. Dai, Phytochemistry, 2017, 139, 98–108 CrossRef CAS PubMed.
- P. Xiang, W. L. Mei, H. Q. Chen, F. D. Kong, H. Wang, G. Liao, L. M. Zhou and H. F. Dai, Fitoterapia, 2017, 120, 61–66 CrossRef CAS PubMed.
- Y. Y. Liu, D. L. Chen, J. H. Wei, J. Feng, Z. Zhang, Y. Yang and W. Zheng, Molecules, 2016, 21, 1433 CrossRef PubMed.
- H. X. Huo, Y. F. Gu, H. Sun, Y. F. Zhang, W. J. Liu, Z. X. Zhu, S. P. Shi, Y. L. Song, H. W. Jin, Y. F. Zhao, P. F. Tu and J. Li, Fitoterapia, 2017, 118, 49–55 CrossRef CAS PubMed.
- H. X. Huo, Z. X. Zhu, Y. L. Song, S. P. Shi, J. Sun, H. Sun, Y. F. Zhao, J. Zheng, D. Ferreira, J. K. Zjawiony, P. F. Tu and J. Li, J. Nat. Prod., 2018, 81, 543–553 CrossRef CAS PubMed.
- Y. Shimada, T. Tominaga, T. Konishi and S. Kiyosawa, Chem. Pharm. Bull., 1982, 30, 3791–3795 CrossRef CAS.
- T. Konishi, A. Sugimoto, S. Kiyosawa and Y. Fujiwara, Chem. Pharm. Bull., 1992, 40, 778–779 CrossRef CAS.
- Y. Shimada, T. Konishi, S. Kiyosawa, M. Nishi, K. Miyahara and T. Kawasaki, Chem. Pharm. Bull., 1986, 34, 2766–2773 CrossRef CAS.
- Y. Shimada, T. Konishi and S. Kiyosawa, Chem. Pharm. Bull., 1986, 34, 3033–3037 CrossRef CAS.
- T. Nakanishi, A. Inada, M. Nishi, E. Yamagata and K. Yoneda, J. Nat. Prod., 2004, 49, 1106–1108 CrossRef.
- K. Iwagoe, T. Konishi, S. Kiyosawa, Y. Shimada, K. Miyahara and T. Kawasaki, Chem. Pharm. Bull., 1988, 36, 2417–2422 CrossRef CAS.
- K. Iwagoe, T. Kakae, T. Konishi, S. Kiyosawa, Y. Fujiwara, Y. Shimada, K. Miyahara and T. Kawasaki, Chem. Pharm. Bull., 1989, 37, 124–128 CrossRef CAS.
- K. Iwagoe, S. Kodama, T. Konishi, S. Kiyosawa, Y. Fujiwara and Y. Shimada, Chem. Pharm. Bull., 1987, 35, 4680–4682 CrossRef CAS.
- T. Konishi, S. Kiyosawa, Y. Shimada, K. Miyahara and T. Kawasaki, Chem. Pharm. Bull., 2008, 37, 1428–1430 CrossRef.
- T. Kornshi, K. Iwagoe, A. Sugimoto, S. Kiyosawa, Y. Fujiwara and Y. Shimada, Chem. Pharm. Bull., 1991, 39, 207–209 CrossRef.
- T. Konishi, K. Iwagoe, S. Kiyosawa and Y. Fujiwara, Phytochemistry, 1989, 28, 3548–3550 CrossRef CAS.
- T. Konishi, K. Iwagoe, S. Kiyosawa and Y. Fujiwara, Chem. Pharm. Bull., 1991, 39, 1869–1870 CrossRef CAS.
- H. Z. Alkhathlan, H. M. Al-Hazimi, F. S. Al-Dhalaan and A. A. Mousa, Nat. Prod. Res., 2005, 19, 367–372 CrossRef CAS PubMed.
- T. Konishi, T. Konoshima, Y. Shimada and S. Kiyosawa, Chem. Pharm. Bull., 2002, 50, 419 CrossRef CAS PubMed.
- Y. Yang, W. L. Mei, F. D. Kong, H. Q. Chen, W. Li, Z. B. Chen and H. F. Dai, Fitoterapia, 2017, 119, 20–25 CrossRef CAS PubMed.
- Y. Yang, H. Q. Chen, F. D. Kong, L. M. Zhou, W. Li, W. H. Dong, Z. B. Chen, W. L. Mei and H. F. Dai, Phytochemistry, 2018, 145, 207–213 CrossRef CAS PubMed.
- T. Sugiyama, Y. Narukawa, S. Shibata, R. Masui and F. Kiuchi, J. Nat. Med., 2018, 1–8 Search PubMed.
- H. N. Wang, W. L. Mei, W. H. Dong, F. D. Kong, W. Li, J. Z. Yuan and H. F. Dai, J. Asian Nat. Prod. Res., 2018, 20, 122–127 CrossRef CAS PubMed.
- T. Yagura, M. Ito, F. Kiuchi, G. Honda and Y. Shimada, Chem. Pharm. Bull., 2003, 51, 560–564 CrossRef CAS PubMed.
- K. Hashimoto, S. Nakahara, T. Inoue, Y. Sumida, M. Takahashi and Y. Masada, Chem. Pharm. Bull., 1985, 33, 5088–5091 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |