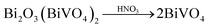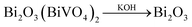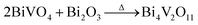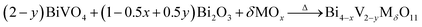Open Access Article
Open Access ArticleGamma-Bi4V2O11 – a layered oxide material for ion exchange in aqueous media
Peiwen Lva and
Feng Huang *ab
*ab
aCAS Key Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: fhuang@fjirsm.ac.cn
bSchool of Materials, Sun Yat-sen University, Guangzhou 510275, China. E-mail: huangfeng@mail.sysu.edu.cn
First published on 15th March 2019
Abstract
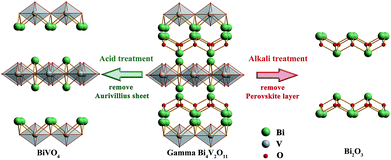
Layered perovskite oxides have attracted considerable attention due to their potential application in photoelectricity and catalysis. The unique character of layered perovskites as ion-exchange materials provides the possibility of creating structural diversity. A new ion-exchange reaction in aqueous solution was observed in layered oxide gamma-Bi4V2O11. When employed in ion exchange, gamma-Bi4V2O11 is converted into the scheelite-type phase (ABO4) by selectively discarding Aurivillius-type sheets, and is also converted into the A2X3 phase by selectively dissolving perovskite-like layers. Metal-doped BiVO4 and Bi2O3 were obtained using such an ion-exchange reaction.
Recently, perovskite oxides and layered perovskite oxides have been widely and thoroughly studied because of their structural simplicity and flexibility, good stability, and their promising applications in solar cells, photocatalysts, and fuel cells, for example.1–3 Perovskite oxides are a type of oxide with the chemistry formula ABO3, where A is a large cation and B is a smaller cation. The skeleton of perovskite oxides comprises corner-sharing BO6 octahedra, where the B-site cation is located in the center of an octahedron of oxygen anions with a coordination number of 6, and the A-site cation is located at the center of eight corner-sharing BO6 octahedra. Layered perovskite oxides are stacks of perovskite oxide layers interspersed with other metal oxide layers. The layered perovskite oxides are well known as The Dion–Jacobson phases, A′[An−1BnO3n+1]; the Ruddlesden–Popper phases, A2′[An−1BnO3n+1]; and the Aurivillius phases, Bi2O2[An−1BnO3n+1].4–6 Unlike the perovskite oxides, the layered perovskite oxides have the unique characteristic of undergoing soft chemical reactions, such as intercalating molecules into their interlayer space, ion-exchange reactions, and exfoliation to nanosheets.6,7 Many soft chemical reactions of layered perovskites have been reported, with the unique characteristic of replacing or modifying the interlayer cations under mild conditions.7–9
BIMEVOX materials are a group of compounds obtained by doping Bi4V2O11 with other metallic ions. These materials exhibit high oxide ion conduction and have potential application at the membrane for oxygen separation.10,11 Among BIMEVOX materials, the gamma phase of Bi4V2O11 consists of Aurivillius-type Bi2O22+ sheets alternating with perovskite-like oxygen-deficient layers (VO3.5–0.5)2−.12 Encouraged by the soft chemical reactions, and using the layered structure of Bi4V2O11 as templates, it is feasible to design perovskites that retain the structural features of the precursor layered phases. Interestingly, we found that it is possible for Bi4V2O11 to be converted by soft chemical reactions. Moreover, the compound is converted in alkaline solution, and the high crystal symmetry is inherited from the parent compound. We believed that this approach would provide a rational route to the design of materials with high symmetry at high temperatures, using soft chemical reactions at room temperature.
Gamma-Bi4V2O11 is synthesized by a standard solid-state reaction. Bi4V2O11 possess three polymorphs: alpha, beta, and gamma. The phase-transition temperature of gamma-Bi4V2O11 is 840 K (ref. 13). Gamma-Bi4V2O11 can be synthesized by quenching in liquid nitrogen after sintering at 1073 K for 48 h or doping with a transition metal to stabilize the high-temperature phase. Gamma-Bi4V2O11 constituted with Aurivillius-type Bi2O22+ sheets alternating with perovskite-like oxygen deficient layers (VO3.5–0.5)2−. The X-ray diffraction (XRD) pattern of gamma-Bi4V2O11 is shown in Fig. 1.
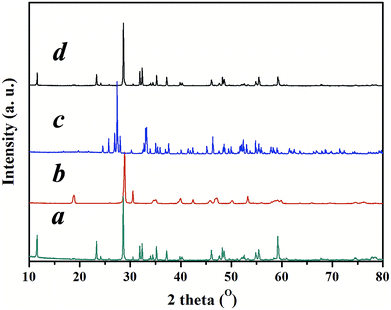 | ||
| Fig. 1 XRD pattern of (a) gamma-Bi4V2O11, (b) BiVO4, and (c) Bi2O3 converted from gamma-Bi4V2O11, (d) the product of a solid-state reaction between BiVO4 and Bi2O3 converted from gamma-Bi4V2O11. | ||
Inspired by the ion-exchange mechanism in layered perovskites, in which the Ruddlesden–Popper phases, Dion–Jacobson phases, and Aurivillius phases can be inter-converted,6 we conjectured that the interlayer cations in the layered structure of gamma-Bi4V2O11 have the potential to be replaced. After ion exchange with protons (0.6 M HNO3), gamma-Bi4V2O11 is converted into a red powder. The red powder is indexed as the space group I21/a, with cell parameters of a = 5.1950, b = 11.701, c = 5.0920, and beta = 90.3800. These cell parameters are highly consistent with BiVO4.14 However, according to the structure of gamma-Bi4V2O11, which consists of Bi2O22+ sheets and (VO3.5–0.5)2− layers, the sheets of Bi2O22+ are thought to be removed in the ion exchange. Thus, the overall reaction could be represented as follows:
| (Bi2O2)2(VO3.5)2 + 2HNO3 → V2O5 + 2H2O |
When examining the structure of gamma-Bi4V2O11, we find that there are two Wyckoff positions for the Bi-atom: the 4e and 16m positions, respectively. Among the Bi2O22+ sheets, the Bi-atom at the 4e position is connected to the oxygen atom in the sheets with a bond length of 2.3149 Å.15 The distance between the Bi-atom (4e) and the oxygen atoms in the (VO3.5–0.5)2− layers is as far as 2.7699 Å, which deviates from the typical Bi–O bond length.16 Thus, the Bi-atom at the 4e position and the oxygen atom in the sheets can be regarded as an integral part of the Bi2O22+ sheets. For the Bi-atom at the 16m position, the distance between the bismuth and the oxygen atom in the Bi2O22+ sheets is 2.6005 Å. Meanwhile, the Bi-atom (16m) connects to the oxygen atoms in the (VO3.5–0.5)2− layers with a bond length of 2.4193 Å. Compared with Bi-atoms at the 4e position, the Bi-atom at the 16m position prefers connecting (VO3.5–0.5)2− layers to Bi2O22+ sheets. We believe the different Wyckoff positions for the Bi-atom leads to different behaviors when extracting Bi2O22+ sheets in acidic solution. Consequently, BiVO4 nanosheets, shown in Fig. 2, are obtained after ion exchange in acid solution.
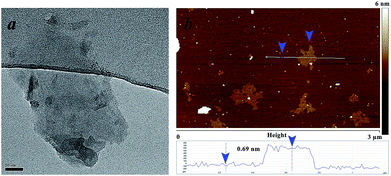 | ||
| Fig. 2 Transmission electron microscopy (TEM) image (a) and atomic force microscopy (AFM) image (b) of BiVO4 nanosheets. | ||
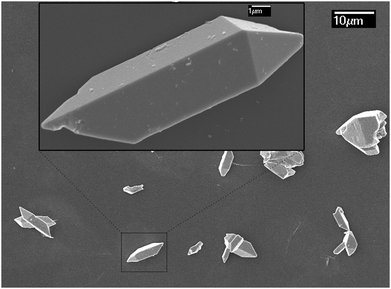
Traditional ion exchange often occurs under acidic conditions or through solid-state reactions with alkali or BiOx at elevated temperatures. Interestingly, we find that the layered structure of gamma-Bi4V2O11 is not only convertible under acidic conditions, but also in alkali solution. After reaction with 20 M KOH solution at room temperature, gamma-Bi4V2O11 was converted to a light green powder. The powder was identified with XRD, and was composed of the alpha phase of Bi2O3 and a small fraction of the gamma phase of Bi2O3. Consistent with BiVO4 leached from the acid treatment of gamma-Bi4V2O11, Bi2O3, which can be regarded as perovskite-like layers (VO3.5–0.5)2−, along with partial bismuth was removed from the layer structure. We believe that the Bi2O22− layers which lacked MOx octahedra would keep growing under a high concentration of alkaline solution, which is regarded as a minimizer, and boost the crystal growth of Bi2O3. In this respect, most of the Bi2O22+ layers leached from gamma-Bi4V2O11 kept growing in the alkaline solution and formed the thermodynamically stable phase – alpha Bi2O3. As a result of crystal growth, the scanning electron microscope (SEM) image of Bi2O3 in Fig. 3 shows the micro-morphology of a single crystal.
The structural evolution is demonstrated in Fig. 4, and the overall reaction is:
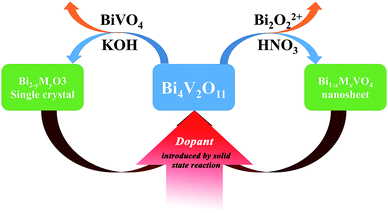
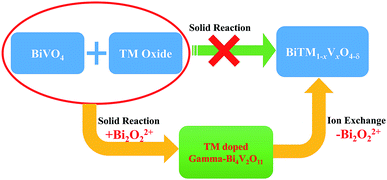
Considering the structural evolution and the overall reaction, the reaction could form a closed loop, as shown in Fig. 5. The Bi2O3 and BiVO4 are obtained by ion exchange from gamma-Bi4V2O11, introducing further dopant, and form gamma-Bi4V2O11, again by solid-state reaction:
Regarding the large solid solution regions of metal-doped Bi4V2O11 and the availability of most cations in the periodic table,17,18 such a toolbox could construct a diverse set of structural architectures, with certain specific morphologies.
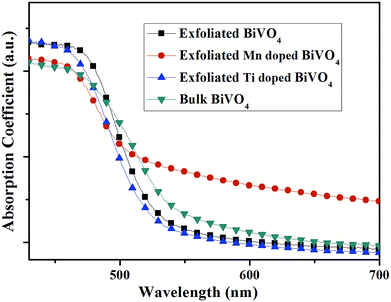
Moreover, the toolbox provides the possibility to design BiVO4 and Bi2O3 with dopants exceeding the equilibrium solid solubility. We first doped Mn into BiVO4 and tuned the doping level at a wide range from 0 to 18% (mole ratio of Mn) by the modified two-step reaction shown in Fig. 7. Moreover, titanium-doped BiVO4 was also realized. Accordingly, many transition metal-doped BiVO4 materials were available by the two-step reaction, which could be employed to insert other elements to trigger various capabilities in the BiVO4 mother compound. Fig. 6 shows the UV-vis absorption spectra of metal-doped BiVO4. The bandgap as well as the band structure could be modified.
Conclusions
In summary, gamma-Bi4V2O11 could be directly converted into the scheelite-type phase (ABO4) by selectively discarding Aurivillius-type sheets. At the same time, gamma-Bi4V2O11 could be converted into the A2X3 phase by selectively dissolving the perovskite-like layers. This ion-exchange reaction, along with a solid-state reaction, formed a closed loop and enabled the formation of a diverse array of structural architectures with certain specific morphologies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U1505252 and 61306075).Notes and references
- J. H. Kim and A. Manthiram, J. Mater. Chem. A, 2015, 3, 24195–24210 RSC.
- I. A. Rodionov and I. A. Zvereva, Russ. Chem. Rev., 2016, 85, 248–279 CrossRef CAS.
- Y. F. Li, W. Q. Zhang, Y. Zheng, J. Chen, B. Yu, Y. Chen and M. L. Liu, Chem. Soc. Rev., 2017, 46, 6345–6378 RSC.
- W. Sugimoto, M. Shirata, Y. Sugahara and K. Kuroda, J. Am. Chem. Soc., 1999, 121, 11601–11602 CrossRef CAS.
- J. Gopalakrishnan, T. Sivakumar, K. Ramesha, V. Thangadurai and G. N. Subbanna, J. Am. Chem. Soc., 2000, 122, 6237–6241 CrossRef CAS.
- R. E. Schaak and T. E. Mallouk, Chem. Mater., 2002, 14, 1455–1471 CrossRef CAS.
- R. Uppuluri, A. Sen Gupta, A. S. Rosas and T. E. Mallouk, Chem. Soc. Rev., 2018, 47, 2401–2430 RSC.
- R. E. Schaak and T. E. Mallouk, Chem. Mater., 2000, 12, 3427–3434 CrossRef CAS.
- Y. S. Han, I. Park and J. H. Choy, J. Mater. Chem., 2001, 11, 1277–1282 RSC.
- R. N. Vannier, E. Pernot, M. Anne, O. Isnard, G. Nowogrocki and G. Mairesse, Solid State Ionics, 2003, 157, 147–153 CrossRef CAS.
- V. M. Zainullina, V. M. Zhukovskii, E. S. Buyanova and Y. V. Emel'yanova, Russ. J. Inorg. Chem., 2007, 52, 225–232 CrossRef.
- E. Capoen, M. C. Steil, N. Tancret, G. Nowogrocki, J. C. Boivin, G. Mairesse, R. N. Vannier, M. Anne and O. Isnard, Solid State Ionics, 2004, 175, 419–424 CrossRef CAS.
- F. Abraham, M. F. Debreuillegresse, G. Mairesse and G. Nowogrocki, Solid State Ionics, 1988, 28, 529–532 CrossRef.
- A. W. Sleight, H. Y. Chen, A. Ferretti and D. E. Cox, Mater. Res. Bull., 1979, 14, 1571–1581 CrossRef CAS.
- G. Mairesse, P. Roussel, R. N. Vannier, M. Anne, C. Pirovano and G. Nowogrocki, Solid State Sci., 2003, 5, 851–859 CrossRef CAS.
- J. B. Boyce, F. G. Bridges, T. Claeson, T. H. Geballe, G. G. Li and A. W. Sleight, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 6961–6972 CrossRef CAS.
- C. K. Lee, G. S. Lim and A. R. West, J. Mater. Chem., 1994, 4, 1441–1444 RSC.
- C. K. Lee, B. H. Bay and A. R. West, J. Mater. Chem., 1996, 6, 331–335 RSC.
| This journal is © The Royal Society of Chemistry 2019 |