Open Access Article
Open Access ArticleGreen and facile production of high-quality graphene from graphite by the combination of hydroxyl radicals and electrical exfoliation in different electrolyte systems
Xin Wang ab and
Long Zhang
ab and
Long Zhang *a
*a
aJilin Provincial Engineering Laboratory for the Complex Utilization of Petro-resources and Biomass, School of Chemical Engineering, Changchun University of Technology, Changchun, Jilin 130012, P. R. China. E-mail: zhanglongzhl@163.com; Tel: +86 18686672766
bSchool of Petrochemical Technology, Jilin Institute of Chemical Technology, Jilin 132022, P. R. China
First published on 28th January 2019
Abstract
A novel, simple and efficient method by the combination of hydroxyl radicals and electrical exfoliation of graphite for the green production of high-quality graphene from graphite was first developed in our self-manufactured exfoliation apparatus. In this work, we focused on the investigation of the roles of various electrolyte systems for the exfoliation of graphite. Sodium chloride, sodium hydroxide, poly vinyl pyrrolidone (PVP), dodecyl trimethyl ammonium bromide (DTAB) and sodium dodecyl benzene sulfonate (SDBS) were tested as the electrolyte. The yields of the graphene product in sodium hydroxide, PVP, DTAB, sodium chloride and SDBS electrolyte system were 32.9%, 34.0%, 45.2%, 77.5% and 83.5%, respectively. The experimental result demonstrated that graphite can be successfully exfoliated to graphene in these electrolytes, with SDBS showing the best exfoliation effect. We further investigated the effects of process parameters on the graphite exfoliation in the SDBS system by single factor experiments. The obtained optimal process parameters were as follows: graphite dosage, 5.0 g; SDBS solution concentration, 10.0% (w/v); applied current strength, 10 mA; air flow, 1.0 L h−1; and exfoliation time, 3 h. At these conditions, the yield of the graphene product was 89.7%. TEM results revealed that the graphene product possessed the characteristic features of a thin-layer graphene sheet. XRD results showed that the graphene products still maintained the structures of carbon atoms or molecules. FT-IR and Raman results indicated that the products exhibited the characteristic peaks and the absorption peaks of graphene. AFM test results revealed that the layer number of graphene product obtained was about 2, while the layer numbers of the graphene products obtained from sodium hydroxide, PVP, DTAB and sodium chloride systems were 30, 20, 4 and 3, respectively, at the same experimental conditions. The observed exfoliation effect in the SDBS system was due to its good electrical conductivity, which was favorable for the formation of hydroxyl radicals in exfoliation. Furthermore, SDBS has good hydrophilic properties and can enable even dispersion of graphite in the system. These two effects facilitated the exfoliation of graphite to form good-quality graphene. SDBS as the electrolyte did not corrode the electrode, and it could be recycled; also, it does not pollute the environment and reduces the production cost, which is favorable for mass production.
1. Introduction
Graphene is the latest member of the nano-carbon family with a two-dimensional honeycomb lattice structure of closely stacked carbon atoms in a single layer.1 It exhibits many special physicochemical properties such as strong mechanical properties,2 high electrical conductivity,3 high barrier properties4 and good catalytic properties.5 Due to these properties, graphene shows numerous application potentials in many fields. Since Geim et al.6 obtained a single layer of graphene by mechanical stripping in 2004, many other methods for preparing graphene have also been developed. At present, the most popular methods for the preparation of graphene include micro-mechanical stripping,7–11 ball milling,12–14 chemical vapor deposition,15–18 solvothermal method,19–21 liquid phase exfoliation22,23 and oxidation–dispersion.24–29 The above preparation methods often employ aggressive reagents and are restricted by the relatively high energy consumption, complex operation, environmental pollution, occasional low yields and poor product quality. Electrochemical exfoliation has been widely studied since it can be easily operated and can be employed to obtain a graphene product from graphite.30–32 Sahoo et al.30 reported graphene preparation with concentrated sulfuric acid solution as the electrochemical exfoliation electrolyte to produce 4–6 layered graphene sheets. Chakrabarti et al.32 conducted a similar research with an ionic liquid, eutectic solvent and acetonitrile electrolyte to obtain a graphene sheet of 4–5 layers. However, the use of organic solvents and inorganic strong acids often leads to environmental problems and increases the production expense; also, the product quality is not satisfactory. Therefore, it is necessary to develop a novel greener production method to resolve the problems mentioned above.Hydroxyl radicals have a standard oxidation–reduction potential of 2.8 V, which is only lower than that of F.33 They can react with many organic molecules due to their strong oxidation capacity. Moreover, hydroxyl radicals can initiate and transfer the chain reaction as well as oxidize and decompose organic substances into less toxic or non-toxic small molecules. The most commonly used methods to generate hydroxyl radicals include Fenton reaction,33 Haber–Weiss reaction34 and electrochemical methods.35,36 These hydroxyl radical generation methods have the disadvantages of higher energy consumption and complex production procedures; also, they are not suitable for mass applications. The hydroxyl radicals are mainly used in the fields of sewage treatment, sterilization, food and vegetable preservation and carbon nanotube modification.34–36 G. Feng et al.37 introduced ·OH (produced by UV light irradiation) into the synthetic process of zeolite for the first time, and the experimental results showed that ·OH could significantly accelerate the nucleation of zeolite, thereby accelerating its crystallization process. However, there is no report on the application of hydroxyl radicals used for graphite exfoliation to prepare graphene.
In our previous research,38 for the first time, we successfully used hydroxyl radical exfoliation for the facile and green production of high-quality graphene from graphite with sodium chloride as the electrolyte in our self-manufactured device; also, the graphite was successfully exfoliated to graphene sheets. Considering the key roles of the electrolyte in the exfoliation process, PVP, SDBS, DTAB, etc. were widely39–43 tested, and they exhibited better dispersion and stabilization functions. In this work, we compared the effects of different electrolyte systems on the exfoliation of graphite to find a suitable electrolyte. To achieve this goal, experiments with sodium chloride, sodium hydroxide, PVP, DTAB and SDBS as the electrolytes were carried out. The effects of process parameters (such as exfoliation time, electrolyte concentration, graphite dosage, applied current strength and air flow) on the production of graphene were investigated using SDBS as the suitable electrolyte. The experimental results proved that graphite was successfully exfoliated into the graphene product. The layer number of graphene obtained in the SDBS electrolyte system was about 2, while the layer numbers of graphene products in sodium hydroxide, PVP, DTAB and sodium chloride electrolytes were 30, 20, 4 and 3, respectively, at the same experimental conditions.
2. Experimental
2.1 Materials and instruments
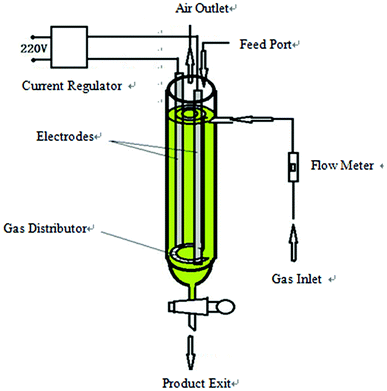
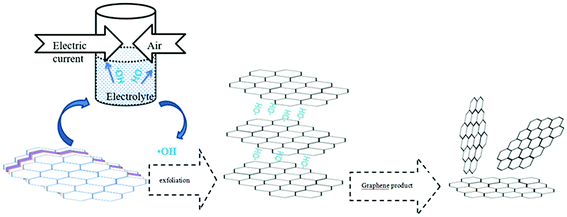
Flake graphite (carbon content of 97%, moisture content of less than 0.5%, density of 1.6 g cm−3, flake size of 0.5 mm) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sodium chloride (AR), sodium hydroxide (AR), poly vinyl pyrrolidone (PVP) (AR), dodecyl trimethyl ammonium bromide (DTAB) (AR), sodium dodecyl benzene sulfonate (SDBS) (AR) and ethanol (AR) were all purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).The hydroxyl radical production and graphite exfoliation apparatus was designed and manufactured by our laboratory, and the detailed device diagram is shown in Fig. 1. An analytical balance (TG328A) was purchased from Balance Instrument Factory (Shanghai, China). The pumping equipment was purchased from Guohua Electric Co. (Shanghai, China). The vacuum drying oven was purchased from Anteing Electronic Instrument Factory (Shanghai, China).
2.2 Experimental devices
This device is a high efficiency hydroxyl radical generation apparatus. The apparatus consists of a current regulator, an electrode system, a gas inlet and a distributor, a flow meter, a feed port, an air outlet and a product exit. The reaction liquid (water and electrolyte) and graphite were added from the feed port. The air flow rate was adjusted by a flow meter. The air and graphite were evenly distributed in the reaction medium under the action of the air distributor, and the intensity of the applied current was adjusted by the current regulator. When an electric current was applied, water was decomposed and oxidized to produce ·OH in the reactor with graphite as the catalyst, and the formed ·OH subsequently reacted with graphite to produce graphene.After the completion of exfoliation, graphene was removed from the product exit, filtered, and washed with distilled water and dried (60 °C for 18 hours) to get the product. The diagram of the apparatus is shown in Fig. 1.
2.3 Preparation procedures
Graphite powders were loaded into the hydroxyl radical apparatus containing a volume of SDBS solution according to the experimental design. The applied current strength in the process was adjusted by a variable resistor having 50–500 Ω. The exfoliation experiments were started when the electric current was applied; the electrode oxidatively decomposed the water to produce ·OH in the reactor with graphite as the catalyst, which subsequently reacted with graphite to produce graphene. The graphite added to the reaction liquid also served as a catalyst, due to which the generation of hydroxyl radicals and the reaction between the hydroxyl radicals and the graphite in the solution occurred simultaneously. After the designed exfoliation time, the solution was filtered and washed and the solid was dried (60 °C for 18 hours) and ground to powder for characterization; the filtrate was reused for the next experiment.To compare the effects of different electrolyte systems on exfoliation, experiments with sodium chloride, sodium hydroxide, PVP, SDBS and DTAB as the electrolytes were carried out. Then, we chose the suitable electrolyte system for further process optimization. Five process factors (exfoliation time, electrolyte concentration, graphite dosage, applied current strength and air flow) were designed and tuned in the exfoliation.
2.4 Characterization
| Yield = C ÷ (4 g/1 L) × 100% | (1) |
| Yield = C ÷ (5 g/1 L) × 100% | (2) |
3. Results and discussion
3.1 Graphite exfoliation in various electrolyte systems
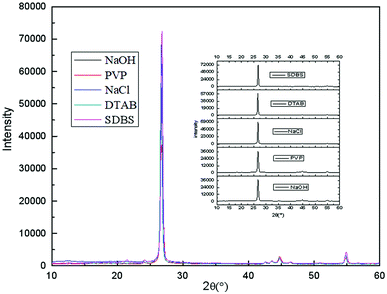
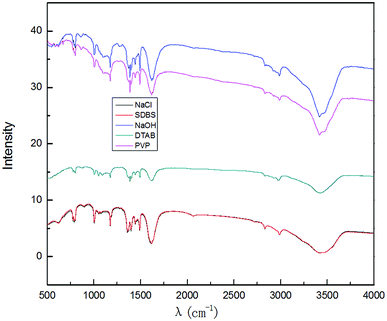
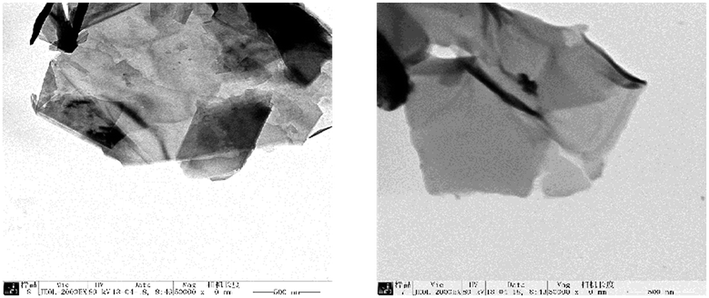
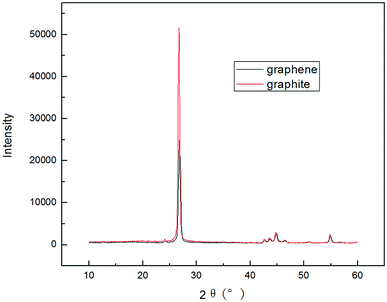
To compare the effects of different electrolyte systems on the exfoliation of graphite to produce graphene, experiments with sodium chloride, sodium hydroxide, PVP, SDBS and DTAB as electrolytes were carried out. The experiments were performed at an electrolyte concentration of 5.0%, air flow of 1.0 L h−1, applied current strength of 10 mA, graphite dosage of 4.0 g and reaction time of 3 h.The XRD results are shown in Fig. 2. Fig. 12 shows that there are two characteristic peaks in the XRD pattern of the graphene product (around 2θ = 27° and 2θ = 54°), indicating that the product has carbon-based material composition and internal carbon atoms or a molecular structure.32,33,44,45 This indicated that the five electrolytes have an effect on exfoliation while forming graphene by our method. Visibly, the positions of the characteristic XRD peaks for the products obtained from different electrolytes did not shift, illustrating that the electrolytes did not change the internal structure of the product. The TEM images in Fig. 3 display that the samples obtained using different electrolytes possess the characteristic features of a thin-layer graphene sheet, which indicates that graphite is successfully exfoliated into a uniform thin layer of graphene. Fig. 4 and 5 show the FT-IR and Raman diagrams, respectively. The key absorption peaks of graphene were observed. The strength of the absorption peaks of graphene products in sodium hydroxide and PVP electrolyte systems were significantly weaker than those observed for the other 3 electrolyte systems. This result was obtained due to the following reasons: the addition of sodium hydroxide can increase the oxygen precipitation side effect in the anode46,47 to hinder the generation of hydroxyl radicals; also, PVP is poorly hydrophilic, which is not conducive to the uniform dispersion of graphite in the system. Meanwhile, the absorption peaks of the products with DTAB as the electrolyte were weaker than those with sodium chloride and SDBS. Although the three electrolytes were strongly hydrophilic, the solubilities at 20 °C were 13 g L−1, 362 g L−1 and 100 g L−1, respectively. As DTAB is relatively less hydrophilic, it was not favorable for the formation of hydroxyl radicals. The absorption peak strength of the product with SDBS as the electrolyte was stronger than that in the sodium chloride system. On one hand, this was because SDBS is an anionic surfactant with good electrical conductivity, which is favorable for the formation of hydroxyl radicals and it can avoid the corrosive effect of the salt electrolyte. On the other hand, SDBS is strongly hydrophilic, which enables even dispersion of graphite in the system. Therefore, these two effects facilitated the exfoliation of graphite to form good-quality graphene. Meanwhile, the anions generated during the ionization in water ensured the charge balance in the solution. In summary, SDBS is the best electrolyte for our method. We further studied the effects of process parameters on exfoliation in the SDBS electrolyte system.
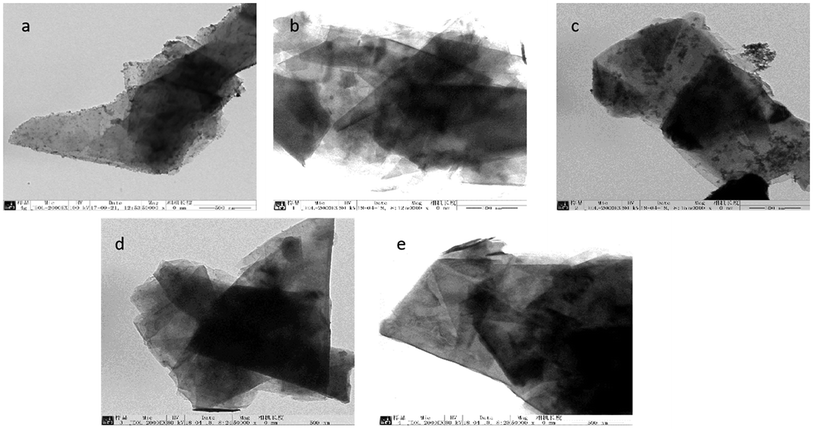 | ||
| Fig. 3 TEM images of the samples produced with (a) sodium chloride; (b) sodium hydroxide; (c) PVP; (d) SDBS and (e) DTAB as the electrolytes. | ||
3.2 Optimization of process parameters
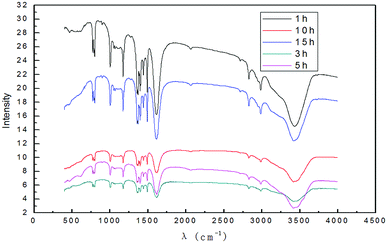
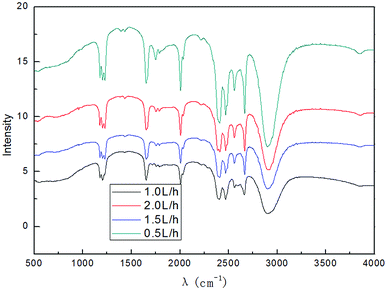
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration peak.53 The broad and strong absorption peak in the fingerprint regions around 3000–3700 cm−1 could be attributed to hydroxyl stretching vibration.48,51 These results further indicated that the preparation method introduced hydroxyl radicals into graphene without changing the carbon-based structure. Fig. 6 shows that when the time was extended, the intensity of the characteristic peak of graphene first increased and then decreased and reached the maximum at 3 h. This is due to the synergistic effect of electrical exfoliation and oxidation of hydroxyl radicals. At the beginning, more hydroxyl radicals were produced with the increase in time, enhancing the synergistic effect. However, when the time was prolonged, graphene restacking was more pronounced. This resulted in decrease in the intensity of the characteristic peaks. Therefore, the appropriate reaction time was 3 h, and the subsequent experiments were carried out for 3 h.
C stretching vibration peak.53 The broad and strong absorption peak in the fingerprint regions around 3000–3700 cm−1 could be attributed to hydroxyl stretching vibration.48,51 These results further indicated that the preparation method introduced hydroxyl radicals into graphene without changing the carbon-based structure. Fig. 6 shows that when the time was extended, the intensity of the characteristic peak of graphene first increased and then decreased and reached the maximum at 3 h. This is due to the synergistic effect of electrical exfoliation and oxidation of hydroxyl radicals. At the beginning, more hydroxyl radicals were produced with the increase in time, enhancing the synergistic effect. However, when the time was prolonged, graphene restacking was more pronounced. This resulted in decrease in the intensity of the characteristic peaks. Therefore, the appropriate reaction time was 3 h, and the subsequent experiments were carried out for 3 h.
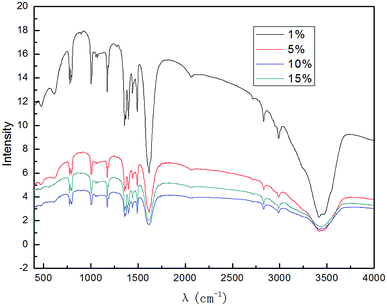
It can be easily seen from Fig. 7 that the intensity of the graphene characteristic peak increased as the electrolyte concentration was increased; it reached the maximum at 10.0% (w/v) and then decreased. This phenomenon can be attributed to the increase in the conductivity of the solution when a higher concentration of electrolyte is used. Good electrical conductivity leads to good ·OH formation and a better synergistic effect with electrical exfoliation. Furthermore, the solubility of SDBS at 20 °C was 100 g L−1, and the conductivity did not continue to increase when the solution was saturated at a higher electrolyte concentration; this indicated that the generation of hydroxyl radicals did not increase, and excess undissolved SDBS was not conducive to the dispersion of graphite in the system, due to which the intensity of the characteristic peak of graphene decreased. Therefore, when the electrolyte concentration was less than 10.0% (w/v), the increase in the conductivity was dominant; when the concentration exceeded 10.0% (w/v), the reduction in hydroxyl radicals contributed more and was predominant. The selection of optimal electrolyte concentration for the generation of hydroxyl radicals in different electrolyte systems by different production methods is also reported in literatures.54–57 Therefore, it is reasonable to expect that an optimal concentration of the electrolyte may exist. Meanwhile, SDBS is a clean anionic surfactant; it has almost no corrosion for the electrodes at higher concentrations. As can be inferred from the results, 10.0% (w/v) electrolyte concentration was found to be suitable for the investigation.
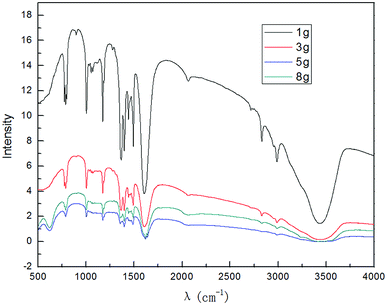
As can be seen from Fig. 8, as the amount of graphite was increased, the peak intensity of graphene increased first and reached the maximum at 5.0 g; then, it decreased. This is because higher dosage of graphite resulted in better catalytic activity, which led to an increase in the intensity of the characteristic peaks of graphene. At the same time, the higher the dosage of graphite, the higher the restacking of graphene and its uneven dispersion, which resulted in decrease in the characteristic peak intensity of graphene. From the results, the suitable dosage of graphite was found to be 5.0 g.
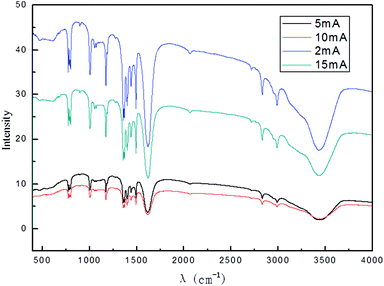
The result in Fig. 9 shows that when the applied current intensity was increased, the intensity of graphene characteristic peak first increased, achieved a maximum at 10 mA and then decreased. This may be due to the combination of hydroxyl radical exfoliation and electrical exfoliation. Within a certain range, a relatively higher applied current intensity, indicating a higher power input, can result in higher hydroxyl radical generation and better electrical exfoliation effects. However, when the current is very high, the cathode and the anode will have hydrogen and oxygen precipitation side reactions.58,59 The reactions are given as (3) and (4). Bipolar side effects can lead to decrease in hydroxyl radical production, current efficiency and the effect of electrical exfoliation.
| 2H2O − 4e → O2↑ + 4H+ | (3) |
| 2H+ + 2e →H2↑ | (4) |
To ensure better exfoliation, 10 mA was applied as the suitable applied current strength.
In summary, the optimal exfoliation conditions of graphene were as follows: SDBS electrolyte concentration, 10.0% (w/v); graphite dosage, 5.0 g; exfoliation time, 3 h; applied current strength, 10 mA; air flow, 1.0 L h−1.
3.3 Mechanism discussion
The results are shown in Fig. 11. As can be seen from Fig. 11, the presence of wrinkles and folds on the sheet is the characteristic feature of thin-layer graphene sheets,60 which indicates that graphite was successfully exfoliated into a uniform thin layer of graphene.
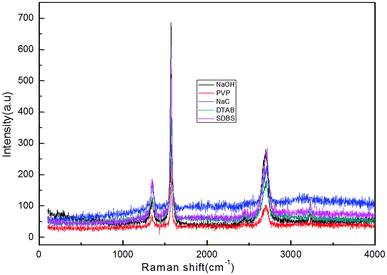
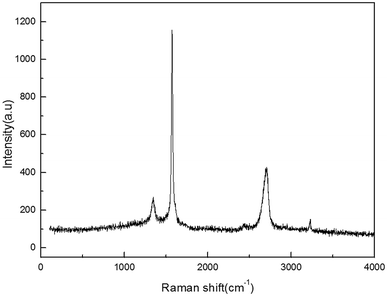
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the graphite layer. In addition, the intensity ratio of the G band to the D band also represents the sp2/sp3 carbon atom ratio.64,65 As can be seen from Fig. 13, the intensity of the G band is much stronger than that of the D band, indicating that the carbon skeleton structure has not changed. This is very consistent with the FT-IR analysis. D′(2 D) and G modes belong to the sum and the frequency of the unordered Raman mode, and Raman allows the presence of intact graphite crystals and defects. Graphene has a low degree of graphitization. Therefore, D′ (2D) and G′ modes are usually very weak and wide. The second-order Raman peak was not considered here.51 The graphene absorption peak at 2720 cm−1 shifted slightly at different layers. Femri et al.66 studied the change in the 2D peak position with the number of layers of graphene and used the double resonance model to explain this phenomenon. To further investigate the number of layers of graphene products, AFM analysis was used.
C double bond in the graphite layer. In addition, the intensity ratio of the G band to the D band also represents the sp2/sp3 carbon atom ratio.64,65 As can be seen from Fig. 13, the intensity of the G band is much stronger than that of the D band, indicating that the carbon skeleton structure has not changed. This is very consistent with the FT-IR analysis. D′(2 D) and G modes belong to the sum and the frequency of the unordered Raman mode, and Raman allows the presence of intact graphite crystals and defects. Graphene has a low degree of graphitization. Therefore, D′ (2D) and G′ modes are usually very weak and wide. The second-order Raman peak was not considered here.51 The graphene absorption peak at 2720 cm−1 shifted slightly at different layers. Femri et al.66 studied the change in the 2D peak position with the number of layers of graphene and used the double resonance model to explain this phenomenon. To further investigate the number of layers of graphene products, AFM analysis was used.
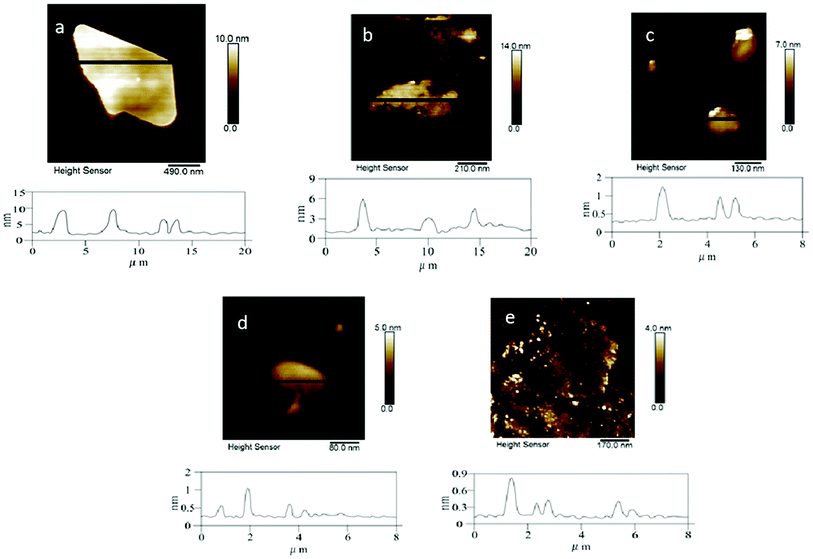 | ||
| Fig. 14 AFM images of the samples obtained with (a) sodium hydroxide; (b) PVP; (c) DTAB; (d) sodium chloride and (e) SDBS as the electrolytes. | ||
3.4 Yield of the production
The experiments with sodium hydroxide, PVP, DTAB, sodium chloride and SDBS as the electrolytes were carried out under the conditions mentioned in Section 3.1. The experiment with SDBS as the electrolyte under optimal conditions was also carried out. The concentrations of the graphene dispersions obtained from sodium hydroxide, PVP, DTAB sodium chloride and SDBS electrolytes were measured to be 1.313 g L−1, 1.360 g L−1, 1.811 g L−1, 3.102g L−1 and 3.341 g L−1, respectively. The concentration of the graphene dispersion obtained by the SDBS system under optimal conditions was 4.485 g L−1. The yields of the graphene products in sodium hydroxide, PVP, DTAB, sodium chloride and SDBS electrolyte systems were 32.9%, 34.0%, 45.2%, 77.5% and 83.5%, respectively. The yield of the graphene product in the SDBS system under optimal conditions was 89.7%.3.5 Recycling of the electrolyte
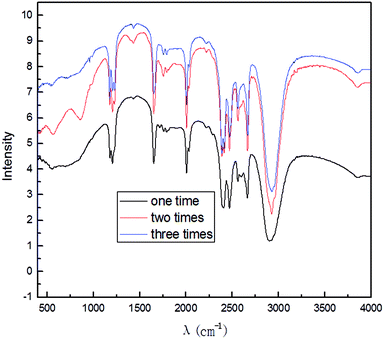
To study the effect of the reuse of electrolyte, we collected and recycled the electrolyte after the experiment. All the experiments were performed at optimal exfoliation conditions: SDBS electrolyte concentration, 10.0% (w/v); graphite dosage, 5.0 g; exfoliation time, 3 h; the applied current strength, 10 mA; air flow, 1.0 L h−1. The results of the electrolyte recycling for 3 times are shown in Fig. 16.It can be seen from Fig. 16 that the main absorption peaks are the same, which indicates that the electrolyte can be recycled and used. However, the absorption peaks of the products after recycling 2 times and 3 times were slightly weakened because of the loss of electrolyte during recycling. The recyclability of the electrolyte further proves that our production method can be a green and potential method for graphene production in industry.
4. Conclusion
In this work, we investigated a new method by the combination of hydroxyl radicals and electrical exfoliation to produce graphene from graphite. The roles of various electrolyte systems (sodium hydroxide, PVP, DTAB, sodium chloride and SDBS) for the exfoliation of graphite were investigated. The yields of the graphene products in sodium hydroxide, PVP, DTAB, sodium chloride and SDBS electrolyte systems were 32.9%, 34.0%, 45.2%, 77.5% and 83.5%, respectively, with SDBS showing the best exfoliation effect. The obtained optimal process parameters were as follows: graphite dosage, 5.0 g; SDBS solution concentration, 10.0% (w/v); applied current strength, 10 mA; air flow, 1.0 L h−1; and exfoliation time, 3 h. Under optimal conditions, the yield of the graphene product was 89.7%. Under optimal conditions, the layer number of the graphene product was about 2, while those of the products of sodium hydroxide, PVP, DTAB and sodium chloride systems were 30, 20, 4 and 3, respectively, at the same experimental conditions. The good quality of graphene could be attributed to good electrical conductivity and excellent hydrophilic properties of SDBS, which were favorable for the formation of hydroxyl radicals and enabled even dispersion of graphite. These two effects facilitated the exfoliation of graphite to form good-quality graphene. SDBS as the electrolyte did not corrode the electrode, and it could be recycled; also, it does not pollute the environment and reduces the production cost, which is favorable for mass production.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Materials Advanced Research Institute for their test supports in the process of characterization.References
- X. Li and W. F. Zhao, et al., Preparation and Characterization of Graphene, Mater. Lett., 2008, 22(8), 48 Search PubMed
.
- F. Liu and P. B. Ming, Ambition calculation of ideal strength and phonon instability of graphene in tension, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76(6), 064120 CrossRef
.
- R. R. Nair and P. Blake, et al., Fine structure constant defines visual transparency of grapheme, Science, 2008, 320, 1308 CrossRef CAS PubMed
.
- S. Sindhu and K. Shishir, et al., Million-fold decrease in polymer moisture permeability by a graphene monolayer, ACS Nano, 2016, 1–26 Search PubMed
.
- B. Seger and P. V. Kamat, Electro catalytically active graphene-platinum nanocomposites role of 2-D carbon support in pem fuel cells, J. Phys. Chem., 2009, 113(19), 7990 CAS
.
- K. S. Novoselov and A. K. Geim, et a1., Electric field effect in atomically thin carbon films, Science, 2004, 306, 666–669 CrossRef CAS PubMed
.
- M. D. Stoller and S. J. Park, et a1., Graphene-based ultra capacitors, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed
.
- J. C. Meyer and A. K. Geim, et al., The structure of suspended graphene sheets, Nature, 2007, 446(7131), 60–63 CrossRef CAS PubMed
.
- J. C. Meyer and A. K. Geim, et al., On the roughness of single-and bi-layer graphene membranes, Solid State Commun., 2007, 143(1–2), 101–109 CrossRef CAS
.
- A. Fasolino and J. H. Los, et al., Intrinsic ripples in grapheme, Nat. Mater., 2007, 6(11), 858–861 CrossRef CAS PubMed
.
- C. Knieke and K. Berger, et a1., Scalable production of graphene sheets by mechanical delimitation, Carbon, 2010, 48(11), 3196–3204 CrossRef CAS
.
- D. Sha and X. D. Qi, et al., A facile way to large-scale production of few-layered graphene via planetary ball mill, Chin. J. Polym. Sci., 2016, 34(10), 1270–1280 CrossRef
.
- G. R. Kumar and K. Jayasankar, et al., Shear-force-dominated dual-drive planetary ball milling for scalable production of graphene and its electro catalytic application with Pd nanostructures, RSC Adv., 2016, 6(24), 20067–20073 RSC
.
- H. H. Zhu and Y. L. Cao, et al., One-step preparation of graphene nanosheets via ball milling of graphite and the application in lithium-ion batteries, J. Mater. Sci., 2016, 51(8), 3675–3683 CrossRef CAS
.
- K. S. Kim and Y. Zhao, et al., Large-scale pattern growth of graphene films for stretchable transparent electrodes, Nature, 2009, 457(7230), 706–710 CrossRef CAS PubMed
.
- A. Reina and X. T. Jia, et al., Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition, Nano Lett., 2009, 9(1), 30–35 CrossRef CAS PubMed
.
- X. S. Li and W. W. Cai, et al., Large-area synthesis of high-quality and uniform graphene films on copper foils, Science, 2009, 324(5932), 1312–1314 CrossRef CAS PubMed
.
- X. S. Li and W. W. Cai, et al., Evolution of graphene growth on Ni and Cu by carbon isotope labeling, Nano Lett., 2009, 9(12), 4268–4272 CrossRef CAS PubMed
.
- J. Zheng and C. A. Di, et al., High quality graphene with large flakes exfoliated by oley amine, Chem. Commun., 2010, 46(31), 5728–5730 RSC
.
- T. V. Khaia and D. S. Kwaka, et al., Direct production of highly conductive graphene with low oxygen content by a microwave-assisted solvothermal method, Chem. Eng. J., 2013, 232(9), 346–355 CrossRef
.
- F. S. Al-Hazmi and G. H. Al-Harbi, et al., One pot synthesis of graphene based on microwave assisted solvothermal technique, Synth. Met., 2015, 200, 54–57 CrossRef CAS
.
- Y. Hemandez and V. Nicolosi, et al., High-yield production of graphene by liquid-phase exfoliation of graphite, Nat. Nanotechnol., 2008, 3(9), 563–568 CrossRef PubMed
.
- X. L. Li and G. Y. Zhang, et al., Highly conducting graphene sheets and langmuir-blodgett films, Nat. Nanotechnol., 2008, 3(9), 538–542 CrossRef CAS PubMed
.
- C. A. Amarnath and C. E. Hong, et a1., Efficient synthesis of graphene
sheets using pyrrole as a reducing agent, Carbon, 2011, 49(11), 3497–3502 CrossRef CAS
.
- Y. Y. Shao and J. Wang, et a1., Facile and controllable electrochemical reduction of graphene oxide and its applications, J. Mater. Chem., 2010, 20(4), 743–748 RSC
.
- H. L. Guo and X. F. Wang, et a1., A Green Approach to the Synthesis of Graphene Nanosheets, ACS Nano, 2009, 3(9), 2653–2659 CrossRef CAS PubMed
.
- W. C. Soon and W. L. Chin, et al., Green preparation of reduced graphene oxide using a natural reducing agent, Ceram. Int., 2015, 41, 9505–9513 CrossRef
.
- G. A. Tai and T. Zeng, et al., Temperature and pH effect on reduction of graphene oxides in aqueous solution, Mater. Res. Express, 2014, 1(3), 37–40 Search PubMed
.
- O. Mondal and S. Mitra, et al., Reduced graphene oxide synthesis by high energy ball milling, Mater. Chem. Phys., 2015, 161, 123–129 CrossRef CAS
.
- S. K. Sahoo and A. Mallik, Simple, fast and cost-effective electrochemical synthesis of few layer graphene nanosheets, Nano, 2015, 10(2), 3807–3809 CrossRef
.
- M. Min and M. M. Wang, et al., Simultaneous electrochemical synthesis of few-layer graphene flaks on both electrodes in portico ionic liquids, Chem. Commun., 2013, 49(46), 5301–5303 RSC
.
- M. H. Chakrabarti and N. S. A. Manan, et al., One-pot electrochemical gram-scale synthesis of graphene using deep eutectic solvents and acetonitrile, Chem. Eng. J., 2015, 274, 213–223 CrossRef CAS
.
- N. Azbar and T. Yonar, et al., Comparison of various advanced Oxidation processes and chemist treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent, Chemosphere, 2004, 55(1), 35–43 CrossRef CAS PubMed
.
- X. J. Ma and Y. Q. Cong, et al., Electrochemical induced hydroxyl radical degradation of phenolic pollutants in water, J. Chem. Ind., 2008, 59(1), 60–63 CAS
.
- H. F. Sun and J. Q. Du, Application of Hydroxyl Radical Active Oxygen in High Concentration Organic Wastewater Treatment, Mod. Chem. Ind., 2010, 30(1), 102–105 Search PubMed
.
- W. Li, Chemical Modification of Carbon Nanotubes with Hydroxyl Radical, MPhil thesis, East China Normal University, ShangHai, 2005
.
- G. Feng and P. Cheng, et al., Accelerated crystallization of zeolites via hydroxyl free radicals, Science, 2016, 351(6278), 1188–1191 CrossRef CAS PubMed
.
- L. Zhang and X. Wang, The method and apparatus for the preparation of graphene from graphite, CN201811179420.5, 2018.10.10 Search PubMed
.
- E. C. Ou, Preparation of graphene dispersion and its free organization, MPhil thesis, Hunan University, Hunan, 2012
.
- M. Lotya and P. J. King, et al., High-Concentration, Surfactant-Stabilized Graphene Dispersions, ACS Nano, 2010, 4(6), 3155–3162 CrossRef CAS PubMed
.
- Y. N. Cai and C. Yu, et al., Study on Dispensability of Graphene Aqueous Solution, J. Mater. Sci., 2014, 11 Search PubMed
.
- Z. F. Ma and Z. D. Yang, et al., Research progress of graphene-based super capacitors, Science and Energy Storage Technology, 2014, 1(3), 49–51 Search PubMed
.
- W. S. Yang and L. Zhang, et al., Progress in research on preparation and application of graphene composites, J. Mater. Eng., 2015, 43(3), 91–97 CAS
.
- U. Khan and A. O'Neil, et al., High-Concentration Solvent Exfoliation of Graphene, Small, 2010, 6(7), 864–871 CrossRef CAS PubMed
.
- M. Min and M. M. Wang, et al., Simultaneous electrochemical synthesis of few-layer graphene flaks on both electrodes in protic ionic liquids, Chem. Commun., 2013, 49(46), 5301–5303 RSC
.
- M. N. Schuchmann and C. V. Sonntag, Hydroxyl Radical-Induced Oxidation of 2-Methyl-2-propanol In Oxygenated Aqueous Solution. A Product and Pulse Radiolysis Study, J. Phys. Chem., 1979, 83(7), 780–784 CrossRef CAS
.
- J. M. Chen and W. W. Pan, et al., Research Progress on the Mechanism and Detection of Hydroxyl Radicals in Electrochemical System, J. Zhejiang Univ. Technol., 2008, 36(4), 416–422 CAS
.
- W. S. Ma, et al., Preparation and Characterization of Graphene, J. Chem. Eng. Chin. Univ., 2010, 24(4), 719–722 CAS
.
- H. He, J. Klinowski and A. Lcrf, et a1., A new structural model for graphite oxide, Chem. Phys. Lett., 1998, 2870(2), 53–56 CrossRef
.
- X. X. Ma, Enzymatic Hydrolysis of Natural Cellulose, MPhil thesis, Beijing Chemical University, Beijing, 2010
.
- L. L. Wang and G. T. Han, et al., Comparative study of composition, structure and properties of Apocynum venetum fibers under different pretreatments carbohyder, Polymer, 2007, 69, 391–397 CAS
.
- Y. H. Yang and H. J. Sun, et al., Preparation and Characterization of Graphene by Redox, Chin. J. Inorg. Chem., 2010, 26(11), 2083–2090 CAS
.
- K. Bissessur and S. F. Scully, Intercalation of solid polymer electrolytes into graphite oxide, Solid State Ionics, 2007, 178(11–12), 877–882 CrossRef
.
- J. Lee and N. Koo, et al., Reactive oxygen species, aging, and ant oxidative nutraceuticals, Compr. Rev. Food Sci. Food Saf., 2004, 3, 21–33 CrossRef CAS
.
- C. Li, et al., Hydroxyl radical generating device and its using method, China, CN 104496003 A, 2015 Search PubMed
.
- M. Zhang, Hydroxyl radicals react with inorganic ox acids, MPhil thesis, Dalian Maritime University, Dalian, 2015
.
- M. Jiang, Study on the Influence of Electrochemical Detection of Hydroxyl Free Radicals and Generations, PhD thesis, Xi'an University of Architecture and Technology, Xi'an, 2015
.
- K. Bissessur and S. F. Scully, Intercalation of solid polymer electrolytes into graphite oxide, Solid State Ionics, 2007, 178(11–12), 877–882 CrossRef
.
- S. Hummers and R. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80(6), 1339 CrossRef
.
- J. Wang and Z. D. Han, The combustion behavior of polyacrylate ester graphite oxide composites, Polym. Adv. Technol., 2006, 17(4), 335–340 CrossRef CAS
.
- P. J. Yen and C. C. Ting, et al., Facile production of graphene nanosheets comprising nitrogen-doping through in situ cathodic plasma formation during electrochemical exfoliation, J. Mater. Chem. C, 2017, 5, 2597–2602 RSC
.
- C. C. Yang and M. H. Tsai, et al., Carbon nanotube/nitrogen-doped reduced Graphene oxide nanocomposites and their application in supercapacitors, J. Nanosci. Nanotechnol., 2017, 17, 5366–5373 CrossRef CAS
.
- L. J. Li and C. W. Chu, et al., Plasma-assisted electrochemical exfoliation of graphite for rapid production of graphene sheets, RSC Adv., 2014, 4, 6946–6949 RSC
.
- C. A. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61(20), 14095–14107 CrossRef
.
- C. Gomez-Navarro and R. T. Weitz, et al., Electronic transport properties of individual chemically reduced graphene oxide sheets, Nano Lett., 2007, 7(11), 3499–3503 CrossRef CAS PubMed
.
- A. C. Femri and J. C. Meyer, et al., A Study on Graphene-Metal Contact, Phys. Rev. Lett., 2006, 97(18), 7401 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2019 |