Open Access Article
Open Access ArticleBoron nitride nanochannels encapsulating a water/heavy water layer for energy applications
Farzaneh Shayeganfar *ab,
Javad Beheshtianc and
Rouzbeh Shahsavari*ad
*ab,
Javad Beheshtianc and
Rouzbeh Shahsavari*ad
aDepartment of Civil and Environmental Engineering, Rice University, Houston, TX 77005, USA. E-mail: rouzbeh@rice.edu; fs24@rice.edu
bDepartment of Energy Engineering and Physics, Amirkabir University of Technology, 14588 Tehran, Iran
cDepartment of Chemistry, Shahid Rajaee Teacher Training University, 16875-163 Tehran, Iran
dDepartment of Material Science and NanoEngineering, Rice University, Houston, TX 77005, USA
First published on 18th February 2019
Abstract
Water interaction and transport through nanochannels of two-dimensional (2D) nanomaterials hold great promises in several applications including separation, energy harvesting and drug delivery. However, the fundamental underpinning of the electronic phenomena at the interface of such systems is poorly understood. Inspired by recent experiments, herein, we focus on water/heavy water in boron nitride (BN) nanochannels – as a model system – and report a series of ab initio based density functional theory (DFT) calculations on correlating the stability of adsorption and interfacial properties, decoding various synergies in the complex interfacial interactions of water encapsulated in BN nanocapillaries. We provide a comparison of phonon vibrational modes of water and heavy water (D2O) captured in bilayer BN (BLBN) to compare their mobility and group speed as a key factor for separation mechanisms. This finding, combined with the fundamental insights into the nature of the interfacial properties, provides key hypotheses for the design of nanochannels.
Hexagonal boron nitride (h-BN) is a synthetic material, similar to graphene in structure and layered form,1,2 but with alternating B and N atoms. Among others properties, h-BN is considered for its hydrophobicity, remarkable chemical and thermodynamic stability (air stable up to 1000 °C),3 and exceptional mechanical4 and optical properties.5 Thin BN sheets have been fabricated by high-energy electron beam irradiation,6 ultrasonication,7 Lewis acid–base,8 hydrolysis of lithium based materials,9 liquid alloys of alkali metals at room temperature10 and chemical vapor deposition2 methods.
Nanochannels by design is an appealing idea due to their potential applications in filtration and separation as well as molecular sieving.11,12 Recently, Agrawal et al.13 revealed the water phase transition encapsulated within carbon nanotubes of different radius. Specifically, fabricating artificial capillaries for molecular transport led to emergence of nanofluidics.14 Shultz et al.15 studied the local water structure by investigation of local and long-range response of hydrogen-bond network of water with surface. Water is polar due to the high electronegativity of oxygen atom and two weakly electropositive hydrogen atoms besides the buckled structure.16 Graphene-based materials such as graphene oxide (GO) membranes can have nanometer pores and tunable sieving of ions,12 indicating low frictional water flow. Sun et al.17 studied on electro/magneto modulated ion transport through GO membranes. While several experimental works demonstrate promising applications of nanochannels, the fundamental subatomic and electronic phenomena at the heart of such nanochannels are poorly understood.
Herein, we present a comprehensive ab initio study to decode the complex interfacial interactions of water and heavy water encapsulated in bilayer BN (BLBN) as a model system. Our findings demonstrated that 2D BN can be used as nanochannels with tunable interlayer distance that is determined by in/out-plane hydrogen bonds of water layers. To discover the mechanism for separating water and heavy water, we further investigate phonon spectrum for water layers between BLBN and for heavy water, deuterium oxide D2O. This nanometer-scale BN capillaries is a flagship architecture in fabricating atomically channels for nanofluidic technology.18
BN nanosheet could be a good candidate for water cleaning and purification as experimentally has been reported by Lei, et al.19 Moreover, Hao et al. in their paper20 reported the properties of atomic layer deposition (ALD) boron nitride nanotube, which is relevant for water purification. Li et al.21 reported that porous boron nitride nanosheets show an excellent performance for water purification.
More recently Falin et al.22 synthesized single crystalline of mono/few layer BN nanosheets. They experimentally measured the Young's modulus of high quality 1–9 layer BN.21 Mechanical properties of BN nanosheets placed them among the strongest insulating materials, indicating the fracture strength of 70.5 ± 5.5 GPa.21
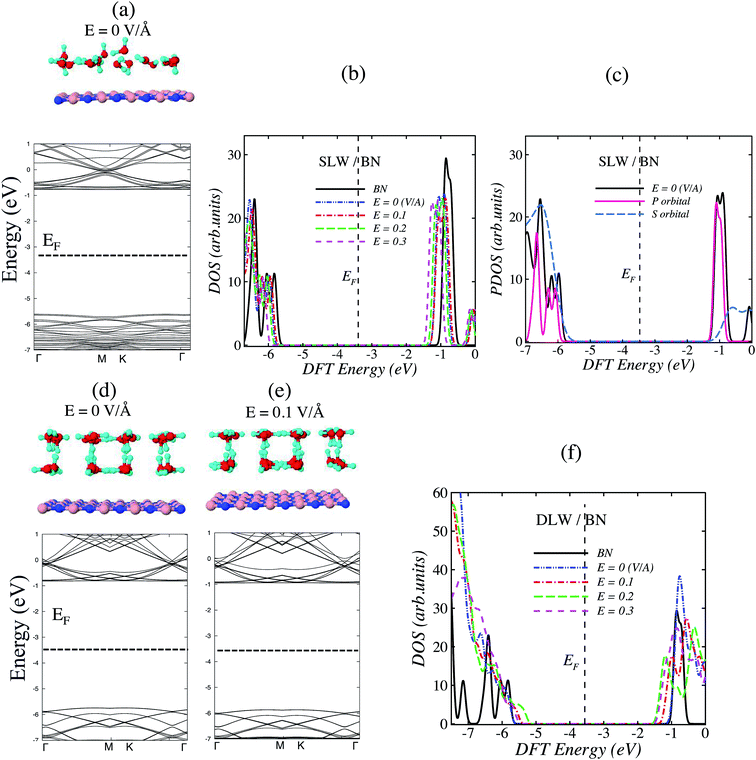
To obtain fundamental insights on the interfacial interactions, a series of DFT calculations were performed on single layer water (SLW) molecules and double layer water (DLW) molecules settled on BN layers and encapsulated in BLBN (see Methods). We first examined the molecular models of SLW settled on BN layers with and without external field. Fig. 1a and b show SLW/BN, including (H2O)16, representing 16 water molecules and the substrate containing 120 B, N atoms. In order to take into account the effect of structural boundary conditions and compare the interfacial properties of SLW and DLW on BN surface, we employed the energy minimized structures for the SLW and DLW on BN layer (Fig. 1d and e), containing 32H2O molecules. The electrical dipole moment (Table 1) enhances with increased external electric field Eext. For instance, the magnitude of dipole moment of SLW/BN system is 3.1 (debye) for Eext = 0 V Å−1, while it increases to 11.4 (debye) for Eext = 0.3 V Å−1. Furthermore, the net charge transfer will increase as a direct result of increased dipole moment and Eext.
| SLW/BN | DLW/BN | |||||||
|---|---|---|---|---|---|---|---|---|
| External electric field Eext (V Å−1) | 0.0 | 0.1 | 0.2 | 0.3 | 0.0 | 0.1 | 0.2 | 0.3 |
| Eint (eV) | −2.41 | −2.57 | −2.73 | −2.95 | −2.11 | −2.23 | −2.37 | −2.53 |
| dO–H (Å) | 0.97 | 1.0 | 1.02 | 1.04 | 0.99 | 1.0 | 1.01 | 1.02 |
| Deformation (BN) (Å) | 0.1 | 0.13 | 0.15 | 0.17 | 0.033 | 0.098 | 0.11 | 0.14 |
| Electrical dipole (pz) (debye) | 3.1 | 7 | 9.3 | 11.4 | 2 | 5 | 8 | 11 |
| Pressure (GPa) | 0.17 | 0.13 | 0.1 | 0.08 | 0.5 | 0.41 | 0.24 | 0.16 |
| Bandgap (eV) | 4.8 | 4.9 | 4.7 | 4.8 | 5 | 4.93 | 5 | 4.9 |
| Water net charge (|e|) | −0.12 | −0.15 | −0.18 | −0.2 | −0.1 | −0.13 | −0.18 | −0.2 |
Table 1 shows the DFT results in terms of the intermolecular energy (Eint), the structural deformation (dO–H), the electrical dipole moment (pz), the intermediate pressure between adsorbate and surface (P = (Σi,jFi,j)/A, where Fi,j is the force on ith atom of adsorbate due to jth atom of substrate and A is the substrate area), energy gap Eg and lastly net charge on water layer.
In view of Table 1, the following observations are in order: (i) SLW/BN is energetically more stable than DLW/BN, since the formation of several directional hydrogen bonds between the water molecules of DLW compensates the stability of the water layer on the BN substrate, (ii) the structural deformation for SLW/BN is greater than DLW/BN due to the strength of intermolecular interaction, (iii) the vdW pressure for the stable system i.e., SLW/BN is 0.17 GPa, which is less than DLW/BN 0.5 GPa. To obtain deep insights on electronic properties of SLW/BN and DLW/BN, Fig. 1 shows the electronic band structure and total density of states (DOS) of these systems.
Fig. 1b shows that DOS of SLW/BN has an energy shift while the presence of SLW does not significantly affect the electron density, confirming physisorption of SLW/BN.23 This finding suggests that the interaction of water with monolayer BN is weak, consistent with the hydrophobicity of BN. The interfacial SLW can infuse positive charges on the monolayer BN, i.e., some electrons from the valence band (VB) of BN are transferred to SLW because of the existence of OH groups. This charge induction cause the DOS of SLW/BN system to be shifted by 0.15 eV below the Fermi energy of the isolated BN, indicating p-doping of the contacting BN.
The electron density of states are experimentally measured by using scanning tunneling microscopy (STM). Therefore, to analyze orbital contribution of designed nanochannel, we plotted projected density of state (PDOS) in Fig. 1c for SLW/BN. This figure (PDOS) reveals that the interfacial interaction of water and BN layer is dominated by P-orbitals.
An analogous DOS information can be obtained for DLW/BN. Applying Eext in the system enhances the distance between H and O atoms dO–H. For instance, when Eext is 0.1 V Å−1, dO–H elongates to 1.0 Å, or 1.02 Å for Eext = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Table 1).
2 (Table 1).
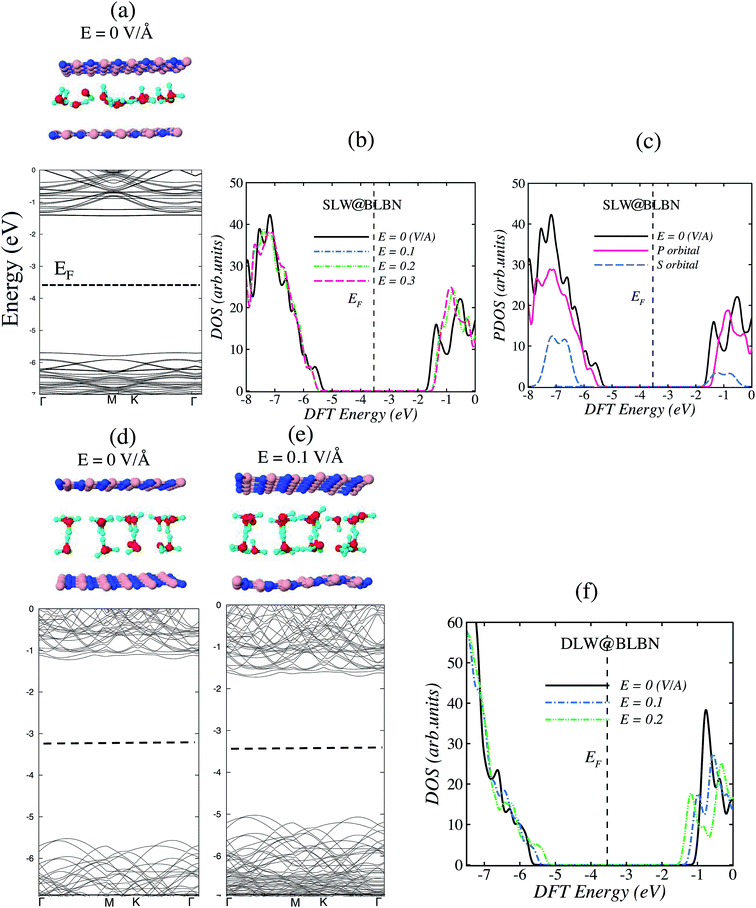
Now we turn our attention to SLW and DLW encapsulated in BLBN. We are interested in comparing the variation of interfacial properties induced by SLW and DLW encapsulated between BLBN. Two structures were studied in Fig. 2 with and without applying Eext. In order to clarify the influence of BLBN on the interfacial properties of water layers, we are presenting electronic band structure and DOS for SLW (DLW)@BLBN in Fig. 2. DFT results of the interfacial properties of these systems are presented in Table 2. The presence of Eext has not significantly affected the interactions of SLW with the bilayer surfaces. Nevertheless, the interfacial properties of water layer on monolayer and bilayer BN model indicates that these water layers constitute stable arrangement. This suggests that the use of BLBN would be advantageous in describing the mass transport interactions in nanochannel systems.
| SLW/BLBN | DLW/BLBN | ||||||
|---|---|---|---|---|---|---|---|
| External electric field Eext (V Å−1) | 0.0 | 0.1 | 0.2 | 0.3 | 0.0 | 0.1 | 0.2 |
| Eint (eV) | −1.96 | −2.04 | −2.18 | −2.78 | −0.6 | −1.43 | −1.62 |
| dO–H (Å) | 0.97 | 0.99 | 1.0 | 1.02 | 1.0 | 1.01 | 1.03 |
| Deformation (BN) (Å) | 0.13 | 0.15 | 0.15 | 0.14 | 0.8 | 0.97 | 0.96 |
| Electrical dipole (pz) (debye) | 4.5 | 8.3 | 12.3 | 28.3 | 0.07 | 4.6 | 9.6 |
| Pressure (GPa) | 0.24 | 0.18 | 0.12 | 0.09 | 0.42 | 0.28 | 0.09 |
| Bandgap (eV) | 4.3 | 4.23 | 4.3 | 4.3 | 4.5 | 3.4 | 3 |
| Water net charge (|e|) | −0.19 | −0.22 | −0.3 | −0.39 | −0.11 | −0.19 | −0.28 |
Comparing the intermolecular energy (Eint) and vdW pressure of SLW or DLW @BLBN in Table 2, we find that the vdW pressure is more important for DLW@BLBN (0.42 GPa) given that DLW is less stable (Eint = −0.6 eV). This finding indicates that vdW pressure in nanoscale system plays a key role to tune their electronic properties.
Fig. 2b describes the total DOS, while Fig. 2c shows the orbital contribution of SLW@BLBN. Again, one can observe that P-orbital is dominating over S-orbital for BLBN and confirms our obtained electronic properties. For instance, SLW@BLBN system shows 4.3 eV band gap smaller than SLW/BN (4.8 eV), arising from hybridization of S-orbital and P-orbital and creating π bond in BLBN weaker than SLW/BN. This weaker π bond yields to correlation of electrons in conduction and valance band, creating smaller band gap.
Fig. 2f shows a few Fermi energy shift for 2D water networks encapsulated in BLBN with Eext and small differences in their electronic properties. The band structure and DOS associated with the SLW@BLBN (Fig. 2a–c) support the adsorption of SLW, suggesting that the weakly dispersed states in the BN can be linked to the hydrogen networks between the water molecules.
Table 2 shows several contrasting features in the optimized structural parameters of DLW@BLBN. As an example, increasing electric field in DLW@BLBN leads to the band gap closing from 4.5 to 3.0 eV. This bandgap closing may significantly contribute to the magnitude of enhanced electric dipole moment due to the external electric field.
To identify the source of band gap closing in DLW@BLBN, we examine the profile of electronic band structure in Fig. 2d and e. Dispersed bands in conduction bands (CB) (Fig. 2e) indicate increasing group velocity and electron mobility, imparting the polar character in these structures and band gap reduction, as reported previously.24 In particular, a slight displacement of the VB and CB toward Fermi level region is observed with increasing the electric field (Fig. 2f).25
Finally, we study the double layer heavy water (deuterium oxide) (DLD) between BLBN (Fig. 3). Phonon dispersion plots of isolated DLW (as a reference) and DLW encapsulated in BLBN are depicted in Fig. 3a and b, respectively. Fig. 3b shows that variation of the vibrational phonon modes of DLW@ BLBN includes additional details on the intermolecular interaction.
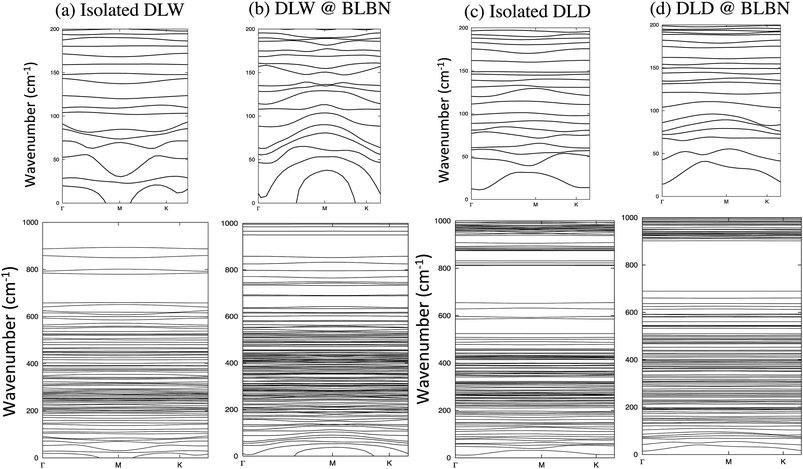 | ||
| Fig. 3 Phonon spectrum for (a) isolated double layer water (DLW), (b) DLW@BLBN, (c) isolated deuterium oxide (heavy water) (DLD) and (d) DLD@BLBN. | ||
First and foremost, no negative phonon vibrations exist for both cases. Second, we observe that splitting occurs for long wavelengths of DLW@BLBN, as compared to isolated DLW. The origin of this splitting is the interior electric filed created by charge transfer in such system, in line with previous reports for ice systems.22 Simply put, the charge transfer between DLW and BLBN induces interior electric field, arising from ionic displacements. Then, the long range Coulomb interactions between DLW water with BLBN cause splitting of the long wavelength. Third, there is some stop bands (bandgaps) in frequency spectra due to non-unity mass ratio of DLW.26 The dispersion of bands is strongly influenced by intermolecular interaction of DLW trapped inside BLBN, while the stop bands are shifted up. Note that the acoustic phonon modes (small frequency extent) are dispersed for DLW@BLBN due to the interactions of DLW with localized electrons of BN.
These plots confirm that disperse bands lead to higher carrier mobility in contrast to localized carriers presented by flat bands.27 A common trend in these phonon spectra is that the contribution of optical phonons (large frequency extent) to the intermolecular interaction is negligible due to the flat bands, considering the low phonon group velocity.
Fig. 3d presents our results for phonon dispersion of double layer deuterium oxide (heavy water) (DLD)@BLBN, and Fig. 3c extends our findings for isolated DLD. For isolated DLD, we observe the expected phonon spectra data as experimentally reported. Interestingly, comparison of Fig. 3c and d indicate that the phonon modes differ for isolated DLD and DLW. Different trends can be secured from these phonon plots.
The first significant result lies in comparison of isolated DLD (Fig. 3c) and phonon spectra of DLD between BLBN (Fig. 3d), which is not drastically affected. The perceived reason can be considered as greater closed shell character of deuterium oxide than water, causing weaker interaction between DLD@BLBN than DLW@BLBN. The number of flat bands increases for large frequencies, indicating more localized carrier.28 Another consequence of phonon spectra is the distribution of stop bands in Fig. 3d, which differs from that of isolated DLD where the stop bands are shifted up.
Our results for deuterium oxide and water interaction are in line with experimental reports on scalable methods for hydrogen isotopes enrichment, due to the high level energy barrier of deuterons compared to proton.29 For instance, it is reported that the mobility of hydrogen is greater than deuterium ions in parent gases30 or deuterons permeate through BN nanostructures more slowly than protons, achieving a separation factor of ∼10.28 Exclusive properties of water encapsulated between 2D materials such as BN could lead to wide applications of nanochannels (e.g., separation, drug delivery, energy harvesting) by making nanometer-sized capillaries. Overall, these fundamental insights could be useful to understand, correlate and predict experimental results.
In summary, we investigated the interfacial and electronic properties of single/double layer water and heavy water settled on monolayer and double layer BN using DFT calculations. The formation of DLW as a network reduces the extent of OH deformation, and their interfacial interactions on BN become less prevalent. Thus, SLW/BN is energetically more stable than DLW/BN. The formation of several directional hydrogen bonds between the water molecules in DLW compensates the stability of the water layer on the BN substrate. The phonon spectra of DLW@BLBN have strong dispersion related to their high mobility and group speed, supporting higher rate of water interaction through BLBN, in line with experiments.28 Considering deuterium oxide, the phonon bands are less dispersed than isolated water spectra, suggesting that slower DLD mobility than water, thus an effective separation tool for D2O and H2O through such 2D materials. Moreover, phonon spectra of DLW@BLBN reveals that high mobility of water inside BLBN is originated from the weak interaction of water with BLBN. Broadly, the findings and methods of this work can have implications on fundamental understanding and designing effective filtration tools using a host of other 2D mono- and multi-layer atomic sheets (e.g. molybdenum disulfide, niobium diselenide, layered double hydroxides, etc.) for separation, drug delivery, and energy harvesting.
Methods
To describe the structural, electronic and interfacial properties of single layer water (SLW) and double layer water (DLW) settled on monolayer BN and between BLBN, density functional theory (DFT) is used, via the projector augmented-wave (PAW) formalism31 by using VASP simulation package.32To account the dispersion interaction, generalized gradient (GG) approximation associated with vdW-DF2 for exchange–correlation (XC) energies was performed, which is based on the algorithm of Román-Pérez and Soler33 and refitted Perdew–Wang 86 (rPW86).34 Plane wave basis set and norm conserving Troullier–Martins pseudopotentials was employed to solve Kohn–Sham equations. We adopt a 12 × 12 × 1 Monkhorst–Pack sampling grid, regarding the k-points for all structures.35 Two criteria were set to optimize total energy and force, 10−5 eV and 0.01 eV Å−1 respectively.22
Fig. 2 shows the supercell of water within BN layers, containing 240 B, N atoms of BLBN and 32H2O molecules (whose 32 O atoms and 64 H atoms). The intermolecular energy of monolayer and double layer water on the surfaces, Eint, is defined by
| Eint = E(water layer/substrate) − E(substrate) + E(water layer) | (1) |
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The supercomputer machines utilized in this work were supported in part by NIH award NCRR S10RR02950 and an IBM Shared Rice University Research (SUR).References
- L. Ci, L. Song, C. H. Jin, D. Jariwala, D. X. Wu, Y. J. Li, A. Srivastava, Z. F. Wang, K. Storr, L. Balicas, F. Liu and P. M. Ajayan, Atomic layers of hybridized boron nitride and graphene domains, Nat. Mater., 2010, 9, 430 CrossRef CAS PubMed.
- L. Song, L. Ci, H. Lu, P. B. Sorokin, C. Jin, J. Ni, A. G. Kvashnin, D. G. Kvashnin, J. Lou, B. I. Yakobson and P. M. Ajayan, Large Scale Growth and Characterization of Atomic Hexagonal Boron Nitride Layers, Nano Lett., 2010, 10(8), 3209–3215 CrossRef CAS PubMed.
- M. J. Rand and J. F. Roberts, Preparation and Properties of Thin Film Boron Nitride, J. Electrochem. Soc., 1968, 115, 423 CrossRef CAS.
- K. Miyoshi, D. H. Buckley, J. J. Pouch, S. A. Alterovitz and H. E. Sliney, Mechanical Strength and Tribological Behavior of Ion-Beam Deposited Boron Nitride Films on Non-Metallic Substrates, Surf. Coat. Technol., 1987, 33, 221 CrossRef CAS.
- L. H. Li, J. Cervenka, K. Watanabe, T. Taniguchi and Y. Chen, Strong Oxidation Resistance of Atomically Thin Boron Nitride Nanosheets, ACS Nano, 2014, 8, 1457 CrossRef CAS PubMed.
- C. Jin, F. Lin, K. Suenaga and S. Iijima, Fabrication of a Freestanding Boron Nitride Single Layer and Its Defect Assignments, Phys. Rev. Lett., 2009, 102, 195505 CrossRef PubMed.
- C. Zhi, Y. Bando, C. Tang, H. Kuwahara and D. Golberg, Large Scale Fabrication of Boron Nitride Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Mechanical Properties, Adv. Mater., 2009, 21, 2889–2893 CrossRef CAS.
- L. Cao, S. Emami and K. Lafdi, Large-Scale Exfoliation of Hexagonal Boron Nitride Nanosheets in Liquid Phase, Mater. Express, 2014, 4, 165–171 CrossRef CAS.
- J. Zhu, H. Wang, J. Liu, L. Ouyang and M. Zhu, Exfoliation of MoS2 and h-BN Nanosheets by Hydrolysis of LiBH4, Nanotechnology, 2017, 28, 115604 CrossRef PubMed.
- H. Feng, Z. Hua and X. Liu, Facile and Efficient Exfoliation of Inorganic Layered Materials Using Liquid Alkali Metal Alloys, Chem. Commun., 2015, 51, 10961–10964 RSC.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes, Science, 2014, 343, 752 CrossRef CAS PubMed.
- J. Abraham, K. S. Vasu, C. D. Williams, K. Gopinadhan, Y. Su, C. Cherian, J. Dix, E. Prestat, S. J. Haigh, I. V. Grigorieva, P. Carbone, A. K. Geim and R. R. Nair, Tunable Sieving of Ions Using Graphene Oxide Membranes, Nat. Nanotechnol., 2017, 12, 546–550 CrossRef CAS PubMed.
- K. V. Agrawal, S. Shimizu, L. W. Drahushuk, D. Kilcoyne and M. S. Strano, Observation of Extreme Phase Transition Temperatures of Water Confined Inside Isolated Carbon Nanotube, Nat. Nanotechnol., 2017, 12, 267–273 CrossRef CAS PubMed.
- B. Radha, A. Esfandiar, F. C. Wang, A. P. Rooney, K. Gopinadhan, A. Keerthi, A. Mishchenko, A. Janardanan, P. Blake, L. Fumagalli, M. Lozada-Hidalgo, S. Garaj, S. J. Haigh, I. V. Grigorieva, H. A. Wu and A. K. Geim, Molecular Transport Through Capillaries Made with Atomic-Scale Precision, Nature, 2016, 538, 222–225 CrossRef CAS PubMed.
- M. J. Shultz, T. H. Vu, B. Meyer and P. Bisson, Water: A Responsive Small Molecule, Acc. Chem. Res., 2012, 45, 15–22 CrossRef CAS PubMed.
- V. Vaida, H. G. Kjaergaard, P. E. Hintze and D. J. Donaldson, Photolysis of Sulfuric Acid Vapor by Visible Solar Radiation, Science, 2003, 299, 1566–1568 CrossRef CAS PubMed.
- P. Sun, F. Zheng, K. Wang, M. Zhong, D. Wu and H. Zhu, Electro- and Magneto-Modulated Ion Transport Through Graphene Oxide Membranes, Sci. Rep., 2014, 4, 6798 CrossRef CAS PubMed.
- A. Morelos-Gomez, R. Cruz-Silva, H. Muramatsu, J. Ortiz-Medina, T. Araki, T. Fukuyo, S. Tejima, K. Takeuchi, T. Hayashi, M. Terrones and M. Endo, Effective NaCl and Dye Rejection of Hybrid Graphene Oxide/Graphene Layered Membranes, Nat. Nanotechnol., 2017, 12, 1083–1088 CrossRef CAS PubMed.
- W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Porous boron nitride nanosheets for effective water cleaning, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- W. Hao, C. Marichy and A. Brioude, Promising properties of ALD boron nitride nanotube mats for water purification, Environ. Sci.: Nano, 2017, 4, 2311–2320 RSC.
- J. Li, P. Jin, W. Dai, C. Wang, R. Li, T. Wu and C. Tang, Excellent performance for water purification achieved by activated porous boron nitride nanosheets, Mater. Chem. Phys., 2017, 196, 186–193 CrossRef CAS.
- A. Falin, Q. Cai, J. G. Elton, D. Scullion, D. Qian, R. Zhang, Z. Yang, S. Huang, K. Watanabe, T. Taniguchi, M. R. Barnett, Y. Chen, R. S. Ruoff and L. H. Li, Mechanical properties of atomically thin boron nitride and the role of interlayer interactions, Nat. Commun., 2017, 8, 15815 CrossRef CAS PubMed.
- F. Shayeganfar and R. Shahsavar, Electronic and pseudomagnetic properties of hybrid carbon/boron-nitride nanomaterials via ab initio calculations and elasticity theory, Carbon, 2016, 99, 523–532 CrossRef CAS.
- Z. Zhang, W. Guoa and B. I. Yakobson, Self-Modulated Band Gap in Boron Nitride Nanoribbons and Hydrogenated Sheets, Nanoscale, 2013, 5, 6381–6387 RSC.
- F. Shayeganfar and A. Rochefort, Tuning the Electronic Properties of a Boron-Doped Si(111) Surface by Self-Assembling of Trimesic Acid, J. Phys. Chem. C, 2015, 119(27), 1574215748 CrossRef.
- A. J. H. McGaughey, M. I. Husseinz, E. S. Landry, M. Kaviany and G. M. Hulbert, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 104304 CrossRef.
- F. Shayeganfar and R. Shahsavari, Electronic and Pseudomagnetic Properties of Hybrid Carbon/Boron-Nitride Nanomaterials via Ab Initio Calculations and Elasticity Theory, Carbon, 2016, 99, 523–532 CrossRef CAS.
- F. Shayeganfar, J. Beheshtian and R. Shahsavari, First-Principles Study of Water Nanotubes Captured Inside Carbon/Boron Nitride Nanotubes, Langmuir, 2018, 34(37), 11176–11187 CrossRef CAS PubMed.
- M. Lozada-Hidalgo, S. Hu, O. Marshall, A. Mishchenko, A. N. Grigorenko, R. A. W. Dryfe, B. Radha, I. V. Grigorieva and A. K. Geim, Sieving Hydrogen Isotopes Through Two-Dimensional Crystals, Science, 2016, 351, 6268 CrossRef PubMed.
- D. J. Rose, Mobility of Hydrogen and Deuterium Positive Ions in their Parent Gases, J. Appl. Phys., 1960, 31, 643 CrossRef CAS.
- P. E. Blchl, Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef.
- G. Kresse and J. Hafner, Ab Initio Molecular Dynamics for Liquid Metals, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558 CrossRef CAS.
- G. Román-Pérez and J. M. Soler, Efficient Implementation of a van der Waals Density Functional: Application to Double-Wall Carbon Nanotubes, Phys. Rev. Lett., 2009, 103, 096102 CrossRef PubMed.
- E. D. Murray, K. Lee and D. C. Langreth, Investigation of Exchange Energy Density Functional Accuracy for Interacting Molecules, J. Chem. Theory Comput., 2009, 5, 2754 CrossRef CAS PubMed.
- F. Shayeganfar, K. S. Vasu, R. R. Nair, F. M. Peeters and M. Neek-Amal, Monolayer Alkali and Transition Metal Monoxides: MgO, CaO, MnO and NiO, Phys. Rev. B, 2017, 99, 523–532 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |