Open Access Article
Open Access ArticleDefected ZnWO4-decorated WO3 nanorod arrays for efficient photoelectrochemical water splitting†
Ya Cuiab,
Lun Pan *ab,
Ying Chenab,
Nisha Afzala,
Sana Ullaha,
Danyang Liuc,
Li Wangab,
Xiangwen Zhangab and
Ji-Jun Zou
*ab,
Ying Chenab,
Nisha Afzala,
Sana Ullaha,
Danyang Liuc,
Li Wangab,
Xiangwen Zhangab and
Ji-Jun Zou ab
ab
aKey Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: panlun76@tju.edu.cn
bCollaborative Innovative Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China
cPeople's Public Security University of China, Beijing 100038, China
First published on 13th February 2019
Abstract
The utilization of solar energy in photoelectrochemical water splitting is a popular approach to store solar energy and minimize the dependence on fossil fuels. Herein, defected ZnWO4-decorated WO3 nanorod arrays with type II heterojunction structures were synthesized via a two-step solvothermal method. By controlling the amount of Zn precursor, WO3 nanorods were decorated in situ with tunable amounts of ZnWO4 nanoparticles. Characterization confirmed the presence of abundant W5+ species in the defected ZnWO4-decorated WO3 samples, leading to enhanced light absorption and charge-separation efficiency. Therefore, the decorated WO3 nanorod arrays show much higher photoelectrochemical (PEC) activity than pure WO3 nanorod arrays. Specifically, the sample with optimal ZnWO4 decoration and surface defects exhibits a current density of 1.87 mA cm−2 in water splitting at 1.23 V vs. RHE under 1 sun irradiation (almost 2.36 times higher than that of pure WO3), a high incident photon-to-current efficiency of nearly 40% at 350 nm, and a relatively high photostability. However, the decoration of WO3 with too much ZnWO4 blocks the light absorption of WO3, inhibiting the PEC performance, even when many defects are present. This work provides a promising approach to rationally construct defected heterojunctions as highly active PEC anodes for practical applications.
1. Introduction
As a promising method to convert solar energy into storable chemical energy, photoelectrochemical (PEC) water splitting into H2 and O2 has attracted wide scientific and technological interest.1–4 Since the first discovery of PEC water splitting on TiO2 by Fujishima et al., many studies focused on fabricating efficient photoanodes have been conducted.5–8 Among the various semiconductor materials, n-type tungstic oxide (WO3) has emerged as a promising photoanode material for PEC water splitting because of its suitable bandgap (ca. 2.7 eV), which can utilize a portion of visible light, and its valence band (VB) edge (ca. 3 V vs. RHE), which is positive enough to provide a sufficient driving force for oxygen production.9,10However, one of the greatest challenges hindering the photoactivity of WO3 is its high rate of electron–hole recombination.11 To solve this problem, the construction of heterostructures with matching band structures on WO3 has been shown to be an efficient strategy. Type II heterojunctions, in which the both VB and conduction band (CB) of one semiconductor are either lower or higher than those of the other, are the most common composites. The formation of the proper band offset on the interface can drive photo-induced holes to the semiconductor with higher VB potential, while the electrons are driven to the semiconductor with lower CB potential. Thus, the recombination of charges is alleviated to some degree, and the spatial separation of charge carriers is enhanced.12–16 In recent years, several semiconductors have been applied to fabricate type II heterojunctions with WO3, such as BiVO4,17 BiOCl,18 and tungstate materials (e.g., NiWO4,19 CoWO4,14 and ZnWO4 (ref. 20)). Among them, tungstate materials have drawn considerable attention and been widely studied due to their superior compatibility with WO3 crystals. Using a modified scanning electrochemical microscope method, Leonard et al.20 found that the addition of Zn to WO3 resulted in the best photo-performance among 25 types of metals. Furthermore, several studies on the construction of zinc tungstate and WO3 have also been carried out.13,21,22 However, the PEC activity of WO3 requires further improvement. The introduction of W5+ species/oxygen vacancies is a promising approach because the dopant and shallow donors could prevent the recombination of photo-induced electron–hole pairs23,24 and create defect levels below the CB, inducing new channels for light absorption and thus improving the PEC performance.25,26
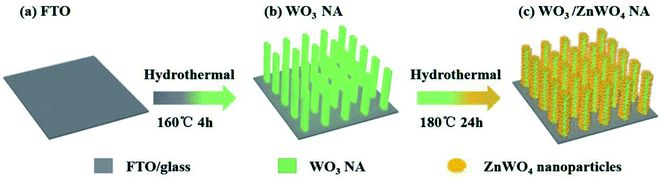
Herein, we develop a hydrothermal method with two steps, the formation of WO3 nanorod arrays (NAs) and the reaction of Zn precursor with WO3 NAs to form ZnWO4 nanoparticles (NPs), to finally synthesize ZnWO4-decorated WO3 NAs (WO3/ZnWO4, see Scheme 1). Importantly, the insertion of Zn atoms into the WO3 NA crystals leads to distortion and forms abundant W5+ species. By controlling the amount of Zn precursor, tunable amounts of ZnWO4 NPs were crystallized in situ on the surfaces of WO3 nanorods (named WZ-x, where x reflects the content of ZnWO4 NPs). Characterization confirmed the presence of abundant defects on the surface of ZnWO4. Therefore, with its continuous interface, the WO3/defected ZnWO4 NA shows a much higher PEC activity than that of pure WO3 NA. In particular, sample WZ-2 exhibits a current density as high as 1.87 mA cm−2 in PEC water splitting at 1.23 V vs. RHE under 1 sun irradiation (almost 2.36 times higher than that of pure WO3), a high incident photon-to-current efficiency (IPCE) of nearly 40% at 350 nm, and a relatively high photostability.
2. Experimental
2.1. Materials
Ammonium metatungstate, (NH4)6(H2W12O40)·xH2O, was obtained from Innochem Chemicals. Hydrochloric acid (36–38%), H2O2 (30%), acetone and absolute ethanol were obtained from Tianjin Yuanli Chemical Institute. Zinc acetate (Zn(CH3COO)2) was obtained from J&K Scientific. All reagents were analytical grade and used without further purification. Milli-Q ultrapure water (>18 mΩ cm) was used in all experiments.2.2. Sample preparation
2.3. Characterization
Crystal structures were characterized by X-ray diffraction (XRD) using a Rigaku D/MAX-2500 diffractometer equipped with Cu Kα radiation at 40 kV and 140 mA at a scanning rate of 5° min−1. Raman spectra were obtained using a Raman spectrometer (DXR Microscope) with a green semiconductor laser (532 nm) as the excitation source. Scanning electron microscopy (SEM) images were recorded using a field-emission scanning electron microscope (Hitachi, S-4800). High-resolution transmission electron microscopy (TEM) observations were made with a Tecnai G2 F-20 transmission electron microscope. X-ray spectroscopy (EDX) elemental maps were obtained using an EDX system attached to the TEM instrument. X-ray photoelectron spectroscopy (XPS) was carried out using a PHI-1600 X-ray photoelectron spectroscope equipped with Al Kα radiation. The binding energy was calibrated by the C1s peak (284.8 eV). Ultraviolet-visible (UV-Vis) diffuse reflectance spectroscopy (DRS) was conducted with a Shimadzu UV-2600 spectrometer equipped with a 60 mm-diameter integrating sphere using BaSO4 as the reflectance. Steady-state photoluminescence (PL) spectra were recorded using a Hitachi F-4600 instrument with excitation at 325 nm.2.4. Measurement of photoanode PEC performance
The PEC properties of the as-prepared WZ-x samples were evaluated in a typical three-electrode quartz cell with a Pt wire counter electrode and a saturated Ag/AgCl reference electrode in 0.5 M Na2SO4 electrolyte. The illumination source was a 300 W Xe arc lamp (100 mW cm−2, PLS-SXE300, Beijing Trusttech. Co. Ltd). Linear sweep voltammetry (LSV) was conducted by sweeping the potential to the positive direction at a scan rate of 10 mV s−1. Based on the Nernst equation, E (vs. RHE) = E (vs. Ag/AgCl) + 0.059 × pH + 0.197,27 the potential vs. Ag/AgCl reference electrode was converted to the potential vs. RHE.The PEC stability (I–t curve) was evaluated at a potential of 0.4 V vs. Ag/AgCl under AM 1.5 G simulated sunlight irradiation. Electrochemical impedance spectroscopy (EIS) measurements were recorded with a sinusoidal ac perturbation of 10 mV applied over the frequency range of 1–105 Hz.
The IPCE was calculated according to eqn (1):28
| IPCE (λ) = (1240 × I)/(λ × Jlight) × 100%, | (1) |
Supposing 100% faradaic efficiency, the applied bias photon-to-current efficiency (ABPE) was calculated by eqn (2):2
| ABPE = I × (1.23 − Vbias)/Jlight × 100%, | (2) |
3. Results and discussion
3.1. Crystal structure and morphology
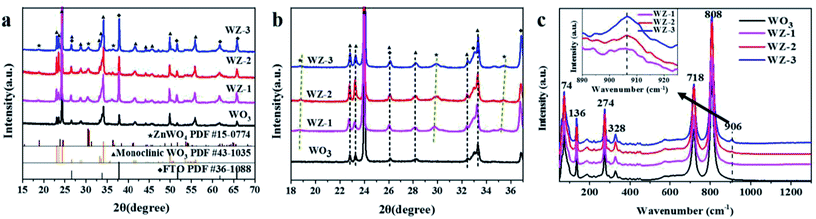
As shown in the XRD patterns (Fig. 1a), the pure WO3 NA film shows sharp and well-defined diffraction peaks corresponding to a typical monoclinic WO3 phase (JCPDS no. 43-1035).29 Compared to the pure WO3 NAs, the WZ-x samples show new diffraction peaks at 18.87°, 30.53° and 36.28°, respectively corresponding to the (100), (111) and (021) crystal planes of pure monoclinic sanmartinite ZnWO4, which has a space group of P2/c with C4 2h symmetry and two molecules per unit cell (Z = 2; JCPDS no. 15-0774).30 As shown in Fig. 1b, the diffraction peaks of ZnWO4 were slightly shifted to higher 2θ values as the content of Zn increased, resulting in smaller interplanar distances in ZnWO4. This may be attributed to the existence of defects formed during the synthetic process with the insertion/substitution of Zn atoms on the surface of WO3. The results in Table S1 (ESI†) indicate slight variations in the lattice parameters and cell volumes of ZnWO4 (compared with JCPDS card no. 15-0774). This suggests the distortion of the octahedral [ZnO6] and [WO6] clusters, generating crystal defects in the ZnWO4 lattice.31The crystal structures of the as-prepared photoanodes were also verified by Raman spectroscopy (Fig. 1c). Pure WO3 exhibits six main Raman peaks located at ca. 74, 136, 274, 328, 718 and 808 cm−1, which are attributed to the active Raman scattering modes of monoclinic WO3.23,32 The strong Raman peaks at 718 and 808 cm−1 are assigned to the W–O stretching modes, while the two other peaks at 274 and 328 cm−1 are related to bending vibrations (W–O–W).28 An extra peak at 906 cm−1 is observed for WZ-x samples and can be ascribed to symmetric stretching (←O←W→O→), which is contributed to by the newly generated ZnWO4 crystals.33 The intensity of the peak at 906 cm−1 increases gradually with increasing Zn amount (from WZ-1 to WZ-3). Furthermore, the synthesized crystals exhibit a relatively broad vibrational mode, indicating short-range structural disorder.34
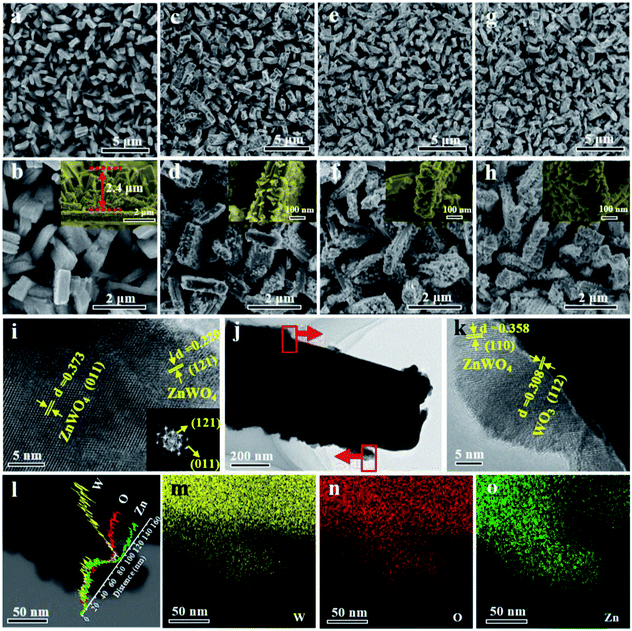
The surface morphologies of the WZ-x samples were characterized by SEM and TEM, as shown in Fig. 2. WO3 exhibits a uniform rod-like morphology. The rods are cuboid shaped with lengths of 500 nm to 1.5 μm, widths of 200–400 nm, and heights of ca. 2.4 μm (Fig. 2a and b). After secondary solvothermal crystallization and thermal calcination (WZ-x samples), the surface layers of the WO3 arrays are converted to ZnWO4 NPs, forming a well-contacted heterojunction structure (Fig. 2c–h). Compared to the pristine WO3 NAs, the WZ-x samples show much rougher surfaces after the formation of ZnWO4 NP layers. Compared to the other WZ-x samples, the lowest concentration of Zn precursor (3.125 mM; WZ-1) resulted in relatively fewer ZnWO4 NPs with crystal sizes of ca. 20–40 nm. When the Zn precursor concentration was increased to 6.25 mM (WZ-2), the amount of ZnWO4 NPs increased along with the crystal size (ca. 50 nm). Further increasing the concentration of Zn precursor to 12.5 mM (WZ-3) caused the ZnWO4 NPs to become much larger and denser, almost covering the surface of WO3 completely. This dense coverage might inhibit optical absorption and mass transfer for the PEC reaction.
The moderate coverage of ZnWO4 on WO3 in sample WZ-2 should promote optical absorption and facilitate the separation of generated electron–hole pairs for the constructed heterojunction. Moreover, this hierarchically structured film can provide sufficient surface active sites to effectively enable reactions at the interface between the photoanode and electrolyte, thus improving the PEC performance.35
The TEM images (Fig. 2i–k) confirm the formation of WO3/ZnWO4 NAs. The spacing of 0.308 nm is in good agreement with the interplanar spacing of the WO3 (112) plane,36 while the spacings of 0.358, 0.373 and 0.220 nm can be indexed to the (110), (011) and (121) planes of monoclinic ZnWO4.37 The corresponding fast Fourier transform (FFT) pattern (inset in Fig. 2i) clearly reveals that the ZnWO4 NPs are highly crystalline. It is worth noting that an compact interface in the WO3/ZnWO4 heterojunction was formed (Fig. 2k). This can be attributed to the in situ reaction of the Zn precursor with WO3 NAs, which is beneficial for the migration of photogenerated carriers between the two composites. Meanwhile, the elemental line scanning profiles (Fig. 2l) confirm that W and O are dispersed in the nanorods and branched ZnWO4 regions, while Zn exists only in the surrounding ZnWO4 regions. This further verifies that WO3 was decorated with well-defined defected ZnWO4 in sample WZ-2. Similarly, the EDX maps (Fig. 2m–o) also confirm the primary distribution of W (and O) and Zn in the rod core and on the surface, respectively.11,38
3.2. Chemical state
XPS was conducted to detect the surface chemical states of the as-prepared photoanodes. The survey spectra (Fig. S1a, ESI†) show the presence of W, O and Zn in the pure WO3 and WZ-x samples without any impurities. In the high-resolution O 1s XPS spectra (Fig. S1b, ESI†), the peak with a binding energy of 530.5 eV is attributed to O–W and O–Zn bonds.39,40 The Zn 2p XPS spectra (Fig. 3a) of the WZ-x samples show similar characteristic Zn 2p3/2 and Zn 2p1/2 peaks centered at 1021.9 and 1045.0 eV, respectively. These energies are slightly higher than those reported in the literature (1021.6 and 1044.7 eV, respectively),40 indicating that fewer electrons locate in the Zn atoms of WZ-x compared to in pristine ZnWO4. Because Zn has a lower electronegativity (1.65) than W (2.36), the shift in binding energy confirms the close interaction between WO3 and ZnWO4. As shown in Fig. 3b, the WZ-x samples exhibit obvious low-energy shifts in W 4f binding energy compared to pure WO3. For samples WZ-2 and WZ-3, a 0.3 eV negative shift in the W 4f binding energy is observed. This indicates that the formation of WZ-x heterojunctions increases the outer electron cloud density of the W atom, which results from W–O–Zn bonding and/or the presence of surface defects.41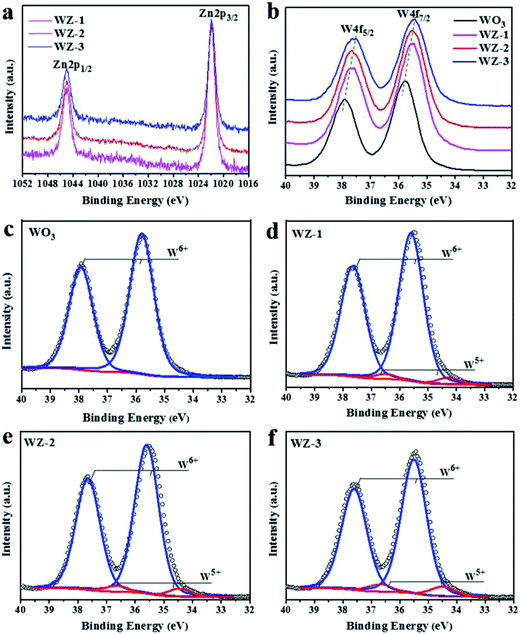 | ||
| Fig. 3 XPS spectra of WO3, WZ-1, WZ-2 and WZ-3: Zn2p (a) and W4f (b), and the fitted W4f XPS spectra of WO3 (c), WZ-1 (d), WZ-2 (e) and WZ-3 (f). | ||
Pristine WO3 shows only two symmetrical sharp peaks of W 4f (Fig. 3c), which is typical of W6+, while the W 4f peak of WZ-x can be divided into four peaks (Fig. 3d–f). The strong peaks at 35.4 and 37.6 eV correspond to W6+, while the weak peaks at 34.5 and 36.7 eV correspond to W5+.26 By fitting the XPS peak of W 4f, the contents of W5+ in samples WZ-1, WZ-2 and WZ-3 are estimated to be 3.9%, 4.9% and 7.2% (atomic percentage), respectively, and the content of W5+ is positively correlated with the amount of ZnWO4.
3.3. Optical properties
The UV-Vis DRS spectra of the WO3 and WZ-x photoanodes are shown in Fig. 4a. For pristine WO3, the absorption edge appears at ca. 460 nm, consistent with its intrinsic band-gap absorption.42 After coating with ZnWO4 NPs, the absorption intensity is obviously enhanced in the ultraviolet region compared to pure WO3. Among the WZ-x samples, WZ-2 achieves the best absorption due to the moderate decoration of defected ZnWO4 on WO3 in this sample. The enhanced optical absorption should be related to the enhanced light scattering by the rough surface. Moreover, the W5+ species in ZnWO4 create defect levels below the CB, giving rise to absorption in the far-visible and/or infrared regions and producing tailing at the onset of absorption.21 The high-resolution VB XPS spectra of WO3 and WZ-x were recorded to determine the VB edge. As shown in Fig. 4b, compared to pure WO3 (2.8 eV),43 the VB positions of WZ-x are clearly shifted toward a lower energy (0.1–0.2 eV), indicating that the ZnWO4 NPs have a lower VB edge (and lower CB on the basis of bandgap data16) than WO3, which benefits the formation of type II heterojunctions.26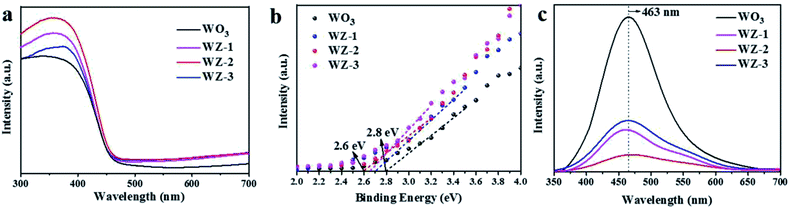 | ||
| Fig. 4 UV-Vis DRS spectra (a), high-resolution VB XPS spectra (b), and photoluminescence (PL) spectra (c) of WO3 and WZ-x. | ||
The recombination of photogenerated charge carriers was investigated through PL emission measurements. Generally, a higher PL intensity indicates a higher recombination rate of photoexcited electrons and holes, while a lower PL intensity indicates a lower recombination rate.14 Fig. 4c shows that pure WO3 and WZ-x exhibit a major PL emission peak centered at around 463 nm due to the intrinsic feature of WO3 and/or ZnWO4. This peak is ascribed to the radiative decay of self-trapped excitons in the crystals.35,44 However, the PL intensities of WZ-x are clearly lower than that of pristine WO3, indicating a much greater charge-separation efficiency of photoexcited electron–hole pairs. For surface ZnWO4, the PL peak at ca. 460 nm (Fig. S2, ESI†) is mainly caused by the charge transition between the O 2p orbitals and the empty d orbitals of the central W6+ ions in the WO62− complex, while the yellow-red emission is extrinsic and may be related to W5+.44 The defects induce the new energy states in the bandgap, which may benefit the separation efficiency of e−–h+ pairs.31 Fitting the PL peak indicated that the amount of W5+ species in WZ-x gradually increased with increasing Zn precursor concentration, consistent with the XPS results. However, for sample WZ-3, the total coverage of ZnWO4 on the WO3 surface will block light absorption and significantly inhibit the activity of WO3, even when a large number of defects are present. Thus, WZ-2 with abundant defects and a heterojunction structure exhibits the highest separation efficiency of photogenerated charge carriers.45
3.4. PEC performances of photoanodes
PEC catalysis is emerging as a promising method for solar water splitting (hydrogen and oxygen generation).9 In PEC water splitting, the oxidation and reduction processes are separated into two half-cell reactions [eqn (3) and (4)], with eqn (5) showing the overall reaction:| 4H+(aq) + 4e− → 2H2(g), Eo = 0 V vs. RHE | (3) |
| 2H2O(l) → O2(g) + 4H+(aq) + 4e−, Eo = 1.23 V vs. RHE | (4) |
| 2H2O(l) → O2(g) + 2H2(g), Eo = 1.23 V vs. RHE | (5) |
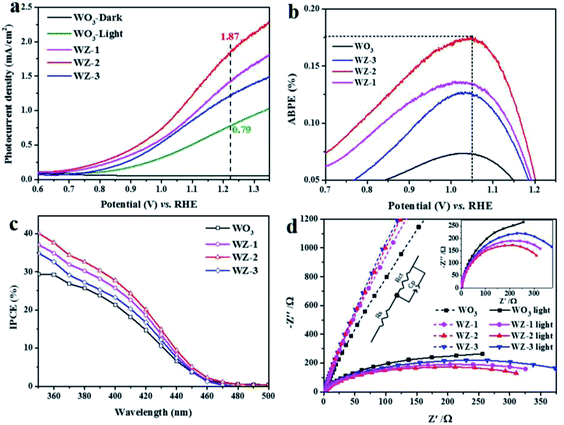
To identify the PEC performances of the pristine WO3 and WZ-x photoanodes, PEC measurements were performed under simulated sun light illumination (100 mW cm−2) in 0.5 M Na2SO4 electrolyte. As shown in Fig. 5a, no current was detected for pure WO3 in the dark (WO3-dark). Under light illumination, the photocurrent density of pure WO3 reaches 0.79 mA cm−2 at an applied bias 1.23 V vs. RHE. The ZnWO4-coated samples WZ-1 and WZ-3 show enhanced photocurrent densities of 1.44 and 1.24 mA cm−2, respectively, while WZ-2 exhibits the highest photocurrent density of ca. 1.87 mA cm−2 at 1.23 V vs. RHE, nearly 2.36 times higher than that of pure WO3. The PEC performances of recently reported WO3-based photoanodes are summarized in Table S2 (ESI†). Notably, WZ-2 shows a higher PEC current than the previously reported photoanodes. Moreover, the on-set potentials of the WZ-x photoanodes (0.70 V vs. RHE for WZ-1, 0.68 V for WZ-2, and 0.75 V for WZ-3) are also lower than that of pure WO3 (0.77 V vs. RHE). Based on the LSV results in Fig. 5a, WZ-2 achieved the maximum ABPE in this study (0.18% at 1.05 V vs. RHE; Fig. 5b), compared to only 0.07% for pure WO3.
The IPCE for PEC water splitting was carried out at 1.2 V vs. RHE, and the results are shown in Fig. 5c. The wavelengths of the initial light response are below 470 nm for the pure WO3 and WZ-x photoanodes and IPCE increases gradually as the irradiation wavelength decreases. Based on the IPCE values, the PEC decreases in the following order: WZ-2 > WZ-1 > WZ-3 > pure WO3. The IPCE value of WZ-2 reaches 40% at 350 nm. Sample WZ-2 also possesses relatively good photostability, with only a slight loss after long-term continuous illumination (Fig. S3, ESI†). After continuous PEC testing (over 3 h), WZ-2 shows nearly the same XRD peaks (Fig. S4a, ESI†) and surface morphology (Fig. S4b, ESI†) as those of a fresh sample, again confirming the relatively good photostability of WZ-2.
For the WO3 photoanode, the occurrence of hole-capture reactions (e.g., 2H2O + 2h+ → H2O2 + 2H+) on the surface is responsible for the corrosion of WO3 via the formation of peroxotungstates, which hinders charge transfer at the WO3/electrolyte interface. In WZ-x samples, the decoration of WO3 with defected ZnWO4 forms a type II heterogeneous structure. Since ZnWO4 possesses higher VB positions than WO3, the photo-induced holes will transfer rapidly to the ZnWO4 NPs, preventing the accumulation of holes on WO3 and preventing the photocorrosion of WO3. In addition, ZnWO4 has better chemical stability and photocorrosion resistance than WO3.20,31 Therefore, the decoration of WO3 with ZnWO4 benefits the stability of the photoanode.
To obtain more insight into the charge-transfer kinetics of the photoanodes, EIS measurements were carried out. The arc radii of the Nyquist plots can be used to evaluate the charge-transfer resistance at the semiconductor/electrolyte interface, with a smaller arc radius implying a smaller charge-transfer resistance.46 Based on the EIS plots (Fig. 5d), the light irradiation significantly decreases the arc radius of the photoanode, and the effect is more obvious for WZ-x than for pure WO3. This suggests that WZ-x exhibits more efficient light absorption and conversion than pure WO3. The charge-transfer resistances of the photoanodes were calculated by fitting the EIS spectra (see the inset in Fig. 5d), where Rs and Rct represent the series resistance and interfacial charge-transfer resistance across the electrode/electrolyte interface, respectively.47 The fitted values of each component are listed in Table S3 (ESI†). The Rs values for all photoanodes are similar, indicating that the series resistance effect is negligible. In the dark, Rct decreases as the amount of ZnWO4 NPs decreases (Rct = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552 Ω for WZ-3, 34
552 Ω for WZ-3, 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 Ω for WZ-2, 33
638 Ω for WZ-2, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 858 Ω for WZ-1, and 31
858 Ω for WZ-1, and 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 Ω for pure WO3). However, under light irradiation, the Rct values of all of the photoanodes are obviously decreased, and the order is also altered (Rct = 592 Ω for WO3, 488.5 Ω for WZ-3, 422.4 Ω for WZ-1, and 391.3 Ω for WZ-2, consistent with the PEC performances of the photoanodes). Notably, the construction of WZ-x heterojunctions benefits charge transfer under light irradiation, unless the coating of defected ZnWO4 is too thick (WZ-3).
347 Ω for pure WO3). However, under light irradiation, the Rct values of all of the photoanodes are obviously decreased, and the order is also altered (Rct = 592 Ω for WO3, 488.5 Ω for WZ-3, 422.4 Ω for WZ-1, and 391.3 Ω for WZ-2, consistent with the PEC performances of the photoanodes). Notably, the construction of WZ-x heterojunctions benefits charge transfer under light irradiation, unless the coating of defected ZnWO4 is too thick (WZ-3).
Scheme 2 shows the proposed process of charge separation and transfer for WO3/defected ZnWO4 NAs. As reported, the CB and VB energies of WO3 are 0.64 and 3.3 V, respectively, while the corresponding values for ZnWO4 are −0.1 and 3.05 V, respectively.13,20 As the shallow donors, the surface W5+ species in ZnWO4 will introduce a new band level below the CB (ca. 0.30 V, calculated based on the peak emission wavelength in the PL spectrum). Therefore, the matched band structures benefit the formation of interface heterojunctions between WO3 and defected ZnWO4 in the WZ-x samples. Under light illumination, the electrons are excited from the VBs of WO3 and ZnWO4 to their own CBs, leaving positive holes in the VBs. The photogenerated electrons in the CB of the ZnWO4 NPs would first migrate to the band of W5+ (or related oxygen vacancies) and then to the CB of WO3. The accumulated electrons will rapidly transfer from WO3 to the FTO substrate via back contact and finally reach the Pt counter electrode for hydrogen generation. Meanwhile, the photogenerated holes in the VB of WO3 have a higher probability of transferring to the surface of ZnWO4 because of the internal electric field of the heterojunction. Thus, the holes have a higher probability of reacting with water molecules to generate oxygen gas.
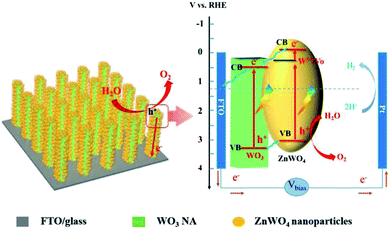 | ||
| Scheme 2 Schematic diagram of the WO3@defected ZnWO4 photoanode and the proposed charge-transfer processes. | ||
The good PEC performance of sample WZ-2 in this study should be closely related to the heterojunction and surface W5+ species. The heterojunction will force the spatial separation of electrons and holes in WO3 and ZnWO4, respectively, while the surface W5+ species will inhibit charge recombination.
4. Conclusions
Using a two-step solvothermal method, we successfully fabricated WO3/defected ZnWO4 as a photoanode for efficient PEC water splitting. By controlling the amount of Zn precursor, tunable amounts of ZnWO4 nanoparticles were decorated on WO3 nanorods. The type II heterojunction with abundant defects enhances the light absorption and charge-separation efficiency. Thus, the WZ-x samples show much higher PEC activities than those of pure WO3 NA. Among the samples, WZ-2 exhibits the highest photocurrent, with a current density of 1.87 mA cm−2 in PEC water splitting at 1.23 V vs. RHE (almost 2.36 times higher than that of pure WO3), a high IPCE of ca. 40% at 350 nm, and a relatively high photostability. This work paves the way for fabricating highly active PEC anodes with defected heterojunction structures for practical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors appreciate the support from the National Natural Science Foundation of China (21506156, 21676193, 51661145026) and the Tianjin Municipal Natural Science Foundation (16JCQNJC05200, 15JCZDJC37300).Notes and references
- S. Ye, C. Ding, R. Chen, F. Fan, P. Fu, H. Yin, X. Wang, Z. Wang, P. Du and C. Li, J. Am. Chem. Soc., 2018, 140, 3250–3256 CrossRef CAS PubMed.
- Y. Bi, B. Zhang, L. Wang, Y. Zhang and Y. Ding, Angew. Chem., Int. Ed., 2018, 57, 2248–2252 CrossRef PubMed.
- L. Pan, S. Wang, J. Xie, L. Wang, X. Zhang and J.-J. Zou, Nano Energy, 2016, 28, 296–303 CrossRef CAS.
- X.-T. Xu, L. Pan, X. Zhang, L. Wang and J.-J. Zou, Adv. Sci., 2019, 6, 1801505 CrossRef PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- L. Pan, T. Muhammad, L. Ma, Z.-F. Huang, S. Wang, L. Wang, J.-J. Zou and X. Zhang, Appl. Catal., B, 2016, 189, 181–191 CrossRef CAS.
- S. Wang, X. Zhang, S. Li, Y. Fang, L. Pan and J.-J. Zou, J. Hazard. Mater., 2017, 331, 235–245 CrossRef CAS PubMed.
- D. K. Lee and K.-S. Choi, Nat. Energy, 2018, 3, 53–60 CrossRef CAS.
- J. Huang, Y. Zhang and Y. Ding, ACS Catal., 2017, 7, 1841–1845 CrossRef CAS.
- N. Zhang, X. Li, H. Ye, S. Chen, H. Ju, D. Liu, Y. Lin, W. Ye, C. Wang, Q. Xu, J. Zhu, L. Song, J. Jiang and Y. Xiong, J. Am. Chem. Soc., 2016, 138, 8928–8935 CrossRef CAS PubMed.
- P. M. Rao, L. Cai, C. Liu, I. S. Cho, C. H. Lee, J. M. Weisse, P. Yang and X. Zheng, Nano Lett., 2014, 14, 1099–1105 CrossRef CAS PubMed.
- Y. Hou, F. Zuo, A. P. Dagg, J. K. Liu and P. Y. Feng, Adv. Mater., 2014, 26, 5043–5049 CrossRef CAS PubMed.
- K. Yuan, Q. Cao, X. Li, H.-Y. Chen, Y. Deng, Y.-Y. Wang, W. Luo, H.-L. Lu and D. W. Zhang, Nano Energy, 2017, 41, 543–551 CrossRef CAS.
- H. Zhang, W. Tian, Y. Li, H. Sun, M. O. Tade and S. Wang, J. Mater. Chem. A, 2018, 6, 6265–6272 RSC.
- S. Cao, X. Yan, Z. Kang, Q. Liang, X. Liao and Y. Zhang, Nano Energy, 2016, 24, 25–31 CrossRef CAS.
- X. Cheng, S. Cao, Y. Huan, Z. Bai, M. Li, H. Wu, R. Zhang, W. Peng, Z. Ji and X. Yan, Energy Technol., 2018 DOI:10.1002/ente.201800899.
- J. H. Baek, B. J. Kim, G. S. Han, S. W. Hwang, D. R. Kim, I. S. Cho and H. S. Jung, ACS Appl. Mater. Interfaces, 2017, 9, 1479–1487 CrossRef CAS PubMed.
- W. Yang, Y. Wen, D. Zeng, Q. Wang, R. Chen, W. Wang and B. Shan, J. Mater. Chem. A, 2014, 2, 20770–20775 RSC.
- S. F. Anis, B. S. Lalia, G. Palmisano and R. Hashaikeh, J. Mater. Sci., 2017, 53, 2208–2220 CrossRef.
- K. C. Leonard, K. M. Nam, H. C. Lee, S. H. Kang, H. S. Park and A. J. Bard, J. Phys. Chem. C, 2013, 117, 15901–15910 CrossRef CAS.
- Y. Keereeta, S. Thongtem and T. Thongtem, Powder Technol., 2015, 284, 85–94 CrossRef CAS.
- Y. Keereeta, T. Thongtem and S. Thongtem, J. Alloys Compd., 2011, 509, 6689–6695 CrossRef CAS.
- G. M. Wang, Y. C. Ling, H. Y. Wang, X. Y. Yang, C. C. Wang, J. Z. Zhang and Y. Li, Energy Environ. Sci., 2012, 5, 6180–6187 RSC.
- P. Chen, M. Baldwin and P. R. Bandaru, J. Mater. Chem. A, 2017, 5, 14898–14905 RSC.
- A. Naldoni, M. Allieta, S. Santangelo, M. Marelli, F. Fabbri, S. Cappelli, C. L. Bianchi, R. Psaro and V. Dal Santo, J. Am. Chem. Soc., 2012, 134, 7600–7603 CrossRef CAS PubMed.
- X. Jia, M. Tahir, L. Pan, Z.-F. Huang, X. Zhang, L. Wang and J.-J. Zou, Appl. Catal., B, 2016, 198, 154–161 CrossRef CAS.
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356–8359 CrossRef CAS PubMed.
- H. Zhang, W. Zhou, Y. Yang and C. Cheng, Small, 2017, 13, 1603840 CrossRef PubMed.
- J. Zhang, X. Chang, C. Li, A. Li, S. Liu, T. Wang and J. Gong, J. Mater. Chem. A, 2017, 6, 3267–3756 Search PubMed.
- Y. Huang, Y. Gao, Q. Zhang, J.-j. Cao, R.-j. Huang, W. Ho and S. C. Lee, Appl. Catal., A, 2016, 515, 170–178 CrossRef CAS.
- P. F. S. Pereira, A. F. Gouveia, M. Assis, R. C. de Oliveira, I. M. Pinatti, M. Penha, R. F. Gonçalves, L. Gracia, J. Andrés and E. Longo, Phys. Chem. Chem. Phys., 2018, 20, 1923–1937 RSC.
- N. Datta, N. Ramgir, M. Kaur, M. Roy, R. Bhatt, S. Kailasaganapathi, A. K. Debnath, D. K. Aswal and S. K. Gupta, Mater. Chem. Phys., 2012, 134, 851–857 CrossRef CAS.
- D. Errandonea, F. J. Manjón, N. Garro, P. Rodríguez-Hernández, S. Radescu, A. Mujica, A. Muñoz and C. Y. Tu, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 054116 CrossRef.
- E. Longo, D. P. Volanti, V. M. Longo, L. Gracia, I. C. Nogueira, M. A. P. Almeida, A. N. Pinheiro, M. M. Ferrer, L. S. Cavalcante and J. Andrés, J. Phys. Chem. C, 2014, 118, 1229–1239 CrossRef CAS.
- X. Fan, B. Gao, T. Wang, X. Huang, H. Gong, H. Xue, H. Guo, L. Song, W. Xia and J. He, Appl. Catal., A, 2016, 528, 52–58 CrossRef CAS.
- L. Santos, P. Wojcik, J. V. Pinto, E. Elangovan, J. Viegas, L. Pereira, R. Martins and E. Fortunato, Adv. Electron. Mater., 2015, 1, 1400002 CrossRef.
- S. Huang, Y. Feng, L. Han, W. Fan, X. Zhao, Z. Lou, Z. Qi, B. Yu and N. Zhu, RSC Adv., 2014, 4, 61679–61686 RSC.
- X. Shi, I. Y. Choi, K. Zhang, J. Kwon, D. Y. Kim, J. K. Lee, S. H. Oh, J. K. Kim and J. H. Park, Nat. Commun., 2014, 5, 4775 CrossRef CAS PubMed.
- B. Jin, E. Jung, M. Ma, S. Kim, K. Zhang, J. I. Kim, Y. Son and J. H. Park, J. Mater. Chem. A, 2018, 6, 2585–2592 RSC.
- J. Lu, M. Liu, S. Zhou, X. Zhou and Y. Yang, Dyes Pigm., 2017, 136, 1–7 CrossRef CAS.
- S. S. Kalanur, I.-H. Yoo, K. Eom and H. Seo, J. Catal., 2018, 357, 127–137 CrossRef.
- Y. Liu, C. Xie, H. Li, H. Chen, T. Zou and D. Zeng, J. Hazard. Mater., 2011, 196, 52–58 CrossRef CAS PubMed.
- M. Ma, K. Zhang, P. Li, M. S. Jung, M. J. Jeong and J. H. Park, Angew. Chem., Int. Ed., 2016, 55, 11819–11823 CrossRef CAS PubMed.
- P. Siriwong, T. Thongtem, A. Phuruangrat and S. Thongtem, CrystEngComm, 2011, 13, 1564–1569 RSC.
- W. He, R. Wang, L. Zhang, J. Zhu, X. Xiang and F. Li, J. Mater. Chem. A, 2015, 3, 17977–17982 RSC.
- S. Wang, L. Pan, J.-J. Song, W. Mi, J.-J. Zou, L. Wang and X. Zhang, J. Am. Chem. Soc., 2015, 137, 2975–2983 CrossRef CAS PubMed.
- A. Thapa, J. Zai, H. Elbohy, P. Poudel, N. Adhikari, X. Qian and Q. Qiao, Nano Res., 2014, 7, 1154–1163 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Lattice parameters, unit cell volumes, fitted values of Rs and Rct, XPS survey spectra of WO3 and WZ-x, fitting of PL data for WZ-x, and PEC stabilities of WO3 and WZ-2. See DOI: 10.1039/c8ra10060h |
| This journal is © The Royal Society of Chemistry 2019 |