Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation of mesoporous ZnAl2O4 nanoflakes by ion exchange from a Na-dawsonite parent material in the presence of an ionic liquid†
TongIl Kim ab,
HakSung Yuna,
GwangBok Hana,
Jiabiao Lianbc,
Jianmin Ma
ab,
HakSung Yuna,
GwangBok Hana,
Jiabiao Lianbc,
Jianmin Ma bd,
Xiaochuan Duan
bd,
Xiaochuan Duan be,
Lianjie Zhu*f and
Wenjun Zheng
be,
Lianjie Zhu*f and
Wenjun Zheng *b
*b
aInstitute of Chemistry and Biology, University of Science, Unjong District, Pyongyang, D. P. R. Korea
bDepartment of Materials Chemistry, Key Laboratory of Advanced Energy Materials Chemistry, TKL of Metal and Molecule-Baced Material Chemistry, College of Chemistry, Nankai University Tianjin, 300071, P. R. China. E-mail: zhwj@nankai.edu.cn
cInstitute for Energy Research, Jiangsu University, Zhenjiang 212013, P. R. China
dSchool of Physics and Electronics, Hunan University, Changsha 410082, P. R. China
ePen-Tung Sah Institute of Micro-Nano Science and Technology of Xiamen University, Xiamen, 361005, P. R. China
fSchool of Chemistry & Chemical Engineering, Tianjin University of Technology, Tianjin 300384, P. R. China. E-mail: zhulj@tjut.edu.cn
First published on 16th April 2019
Abstract
Herein, mesoporous ZnAl2O4 spinel nanoflakes were prepared by an ion-exchange method from a Na-dawsonite parent material in the presence of an ionic liquid, 1-butyl-2,3-dimethylimidazolium chloride ([bdmim][Cl]), followed by calcination at 700 °C for 2 h. The as-obtained products were characterized by several techniques such as X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and energy dispersive X-ray spectroscopy (EDX). The ZnAl2O4 nanoflakes with the thickness of ∼20 nm were composed of numerous nanoparticles, which resulted in a high specific surface area of 245 m2 g−1. The formation mechanism of the ZnAl2O4 nanoflakes was comprehensively investigated, and the results showed that a 2D growth process of the Zn6Al2(OH)16(CO3)·4H2O crystallites with the assistance of [bdmim][Cl] was the key for the induction of ZnAl2O4 nanoflakes. Moreover, mesopores were formed between adjacent nanoparticles due to the release of CO2 and H2O molecules from Zn6Al2(OH)16(CO3)·4H2O during the calcination process.
1. Introduction
Zinc aluminate (ZnAl2O4) spinel is a well-known wide band gap semiconductor (Eg = 3.8 eV)1 ceramic material with opto-mechanical property, which has been extensively studied as a catalyst and catalyst support,2–4 transparent conductor,5 dielectric material,6 optical material7 and sensor8 due to its high thermal stability, low surface acidity and high mechanical resistance.9 Several methods, for example, the solid state-reaction or ceramic method,10 wet chemical routes,11,12 the sol–gel method,13,14 the hydrothermal method,15 the solvothermal method,16 the plasma method,17 combustion in an aqueous solution18 and molten salt synthesis19 etc., have been applied for the preparation of ZnAl2O4 spinel. However, to date, it is still a challenge to synthesize 2D or 3D ZnAl2O4 spinel nanostructures via a solution route. Although hierarchical ZnO–Al2O3 microspheres have been reported,20,21 to date, the pure phase of 2D ZnAl2O4 nanoflakes has not been prepared via a facile ion-exchange method.Dawsonite (denoted by Na-Dw) is a mineralogical nomenclature that specifically refers to the naturally formed sodium hydroxyalumino-carbonate, NaAl(CO3)(OH)2.22 The Na-Dw compounds have been applied as ingredients in antacids,23 stabilizers in polymers,24,25 dry extinguishers in fuel leak fires,26 additives in synthetic fertilizers27 and precursors for pure alumina.28–30
Recently, the advantages of ionic liquids (ILs) have been gradually discovered in the synthetic processes of inorganic nanomaterials.31–37 Particularly, ILs have received significant attention as templates in the synthesis of numerous functional materials. Our research group has successfully synthesized various functional nanostructures using ionic liquids as soft templates, reactants or precursors.38–42
Herein, we present mesoporous ZnAl2O4 nanoflakes prepared by the ion-exchange method from a Na-Dw parent material in the presence of an ionic liquid, [bdmim][Cl], followed by calcination at 700 °C for 2 h. The product exhibits a thin flake-like morphology, composed of numerous nanoparticles, and has a high specific surface area, 245 m2 g−1. To the best of our knowledge, this is the first time that ZnAl2O4 spinel nanoflakes have been prepared by the ion-exchange method from a Na-Dw parent material.
2. Experimental
All the reagents were of analytical grade and used without further purification. The ionic liquid 1-butyl-2,3-dimethylimidazolium chloride ([bdmim][Cl]) was purchased from Lanzhou Greenchem ILS (LICP, CAS, China).2.1 Synthesis of the Na-Dw parent material
The Na-Dw parent material was prepared by the coprecipitation method reported in the literature.43 Typically, 2 mmol of AlCl3·6H2O was dissolved in 15 mL of deionized water under constant stirring, and then, 10 mmol of NaHCO3 was slowly added to the solution at room temperature. The obtained gel was hydrothermally treated at 120 °C for 12 h in a 20 mL Teflon-lined stainless steel autoclave. After the reaction was completed, the autoclave was naturally cooled down to room temperature. Then, the slurry was centrifuged and washed several times with distilled water and ethanol. The white solid residues were dried at 80 °C for 2 h, and thus, the Na-Dw parent material was obtained.2.2 Synthesis of the ZnAl2O4 nanoflakes
In a typical preparation of ZnAl2O4, 0.6 mmol (0.1 g) of Na-Dw and 1 mmol of the ionic liquid [bdmim][Cl] were added to 10 mL of 0.03 M Zn(NO3)2 aqueous solution, and then, the mixture was slowly stirred and maintained at 50 °C for 10 h. The as-obtained white precipitate was centrifuged, washed several times with distilled water and ethanol, dried at 80 °C for 2 h, and finally calcined at 700 °C for 2 h to obtain the ZnAl2O4 nanoflakes.2.3 Characterizations
The products were characterized by XRD, FTIR spectroscopy, SEM, TEM and EDX. XRD measurements were performed using the Rigaku D/max 2500 diffractometer with Cu Kα radiation (λ = 0.154056 nm) at V = 40 kV and I = 150 mA, and the scanning speed was 8° min−1. TGA measurements were performed using the DuPont Instruments 951 Thermogravimetric analyzer from room temperature to 725 °C in flowing nitrogen gas at the heating rate of 5 °C min−1. The FTIR spectroscopy of the sample was conducted at room temperature with a KBr pellet using the VECTOR-22 (Bruker) spectrometer in the range from 400 to 4000 cm−1. The morphologies of the samples were studied by field emission scanning electron microscopy (FE-SEM, JEOL JSM-6700F). The TEM and HR-TEM images and EDX spectra were obtained using the Tecnai G2 20S-Twin transmission electron microscope operating at the accelerating voltage of 120 kV. The specific surface areas (SBET) of the samples were calculated by following the multipoint Brunauer–Emmett–Teller (BET) procedure using the Quantachrome Nova 2000e sorption analyzer. The pore diameter and the pore size distribution were determined by the Barrett–Joyner–Halenda (BJH) method.3. Results and discussions
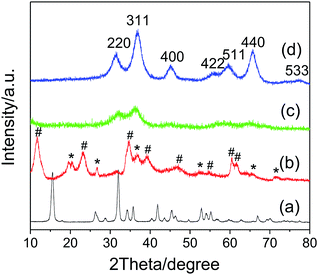
Fig. 1a shows the XRD pattern of the Na-Dw parent material prepared at 120 °C in 12 h. All detectable peaks in this pattern can be assigned by their peak positions to orthorhombic NaAl(CO3)(OH)2 (JCPDS: 45-1359). No evidence could be found for the existence of other impurities in the product after washing. The XRD pattern of the precursor prepared by ion exchange using the Zn(NO3)2 solution is shown in Fig. 1b, which clearly shows two types of characteristic diffraction peaks that can be indexed to monoclinic Al2O3·3H2O (JCPDS: 01-0259) and hexagonal Zn6Al2(OH)16(CO3)·4H2O (JCPDS: 38-0486). The XRD patterns of the samples obtained after calcination of the precursor at 500 and 700 °C for 2 h (Fig. 1c and d), respectively, present the characteristic diffraction peaks of the cubic phase ZnAl2O4 spinel (JCPDS: 05-0669); they indicate that the mixed crystalline phases of the precursor have been converted to the pure ZnAl2O4 spinel crystalline phase upon heat treatment, and higher calcination temperature is beneficial for the enhancement of crystallinity. As is well-known, the average size of the nanocrystal can be calculated via the Scherrer formula:44Dhkl = Kλ/(βhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θhkl) θhkl) |
| a2 = dhkl2(h2 + k2 + l2) |
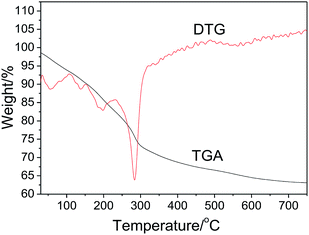
The thermal stability of the precursor prepared by ion exchange at 50 °C in 10 h was investigated by TGA and DTG. As shown in Fig. 2, the precursor exhibits the total weight loss of about 36.6%. Based on previous studies,46–49 we believe that the thermal decomposition process includes five steps as follows: (1) a 7.2% weight loss from 30 to 110 °C due to the removal of physically adsorbed water and part of crystal water from Al2O3·3H2O (eqn (2)), (2) a 2.7% weight loss from 110 to 150 °C due to the phase transition from Al2O3·3H2O to AlOOH (eqn (2)), (3) a 7.4% weight loss from 150 to 230 °C, assigned to the removal of structural interlayer water of the Zn6Al2(OH)16(CO3)·4H2O crystals (eqn (3)), (4) a 16.1% weight loss from 230 to 500 °C, attributed to the removal of residual crystal water, CO2 molecules and part of hydroxyl groups from the crystals of AlOOH and Zn6Al2(OH)16(CO3) (eqn (5) and (6)), and (5) a 3.2% weight loss above 500 °C due to the removal of residual hydroxyl groups. Based on the TGA results, we proposed the formation process of the ZnAl2O4 nanoflakes as follows:
| 12NaAl(OH)2CO3 + 6Zn2+ + H2O → 5Al2O3·3H2O + Zn6Al2(OH)16(CO3)·4H2O + 12Na+ + 11CO2 | (1) |
| Al2O3·3H2O → 2AlOOH + 2H2O [∼150 °C: Wloss = 11.1%] | (2) |
| Zn6Al2(OH)16(CO3)·4H2O → Zn6Al2(OH)16(CO3) + 4H2O [150–230 °C: Wloss = 4.5%] | (3) |
| Zn6Al2(OH)16(CO3) → 6ZnO + Al2O3 + 8H2O + CO2 [230–500 °C: Wloss = 11.6%] | (4) |
| 2AlOOH → Al2O3 + H2O [230–500 °C: Wloss = 5.6%] | (5) |
| ZnO + Al2O3 → ZnAl2O4 | (6) |
The theoretical total weight loss during the thermal-decomposition process is 32.8%, which is consistent with the TGA result.
The FTIR spectrum of the product obtained after calcination of the precursor at 700 °C for 2 h is shown in Fig. S1(a) in the ESI.† It displays a strong band around 3460 cm−1, which is attributed to the vibration of the OH group bonded to the surface. The band at 1610 cm−1 is associated with the vibration of Al–OH, characteristic of ZnAl2O4, and the weak peak at 1398 cm−1 is due to the HOH vibration of water. The wide band from 797 to 497 cm−1 is related to the inorganic network, including the Zn–O bending vibrations, Al–O stretching vibrations and Al–O–Zn stretching vibrations.45,50,51 As shown in Fig. S1(b) in the ESI,† the local composition EDX spectrum reveals that the stoichiometric atom concentration ratio is Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ≈ 15.6
O ≈ 15.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.3
29.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55.1% ≈ 1
55.1% ≈ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, confirming that the as-obtained product is ZnAl2O4. Moreover, a Cu signal located at 8.1 eV was revealed, which originated from a copper grid used in the HR-TEM measurement.
4, confirming that the as-obtained product is ZnAl2O4. Moreover, a Cu signal located at 8.1 eV was revealed, which originated from a copper grid used in the HR-TEM measurement.
The morphologies of the products were characterized by FE-SEM. The FE-SEM image of Na-Dw is shown in Fig. S2 in the ESI,† which exhibits a nanorod shape. However, well-developed nanoflakes were obtained after Zn2+ ion exchange reactions in the presence of the ionic liquid (Fig. 3a). To clarify the effect of the ionic liquid [bdmim][Cl] on the morphology of the product, a control experiment was carried out in the absence of the ionic liquid, and other reaction conditions were kept constant. The FE-SEM image of the as-obtained product is shown in Fig. S3 in the ESI,† which displays an irregular shape with few nanoflakes. These results imply that in the present reaction system, the ionic liquid has an important effect on the morphology of the product; moreover, ion exchange occurs between Zn2+ ions and Na-Dw molecules dissolved in solution, and then, new structures can be formed via recrystallization of the product molecules. There is no significant role of the ionic liquid in the ion exchange process, whereas in the crystal growth process, the ionic liquid plays a crucial role. Moreover, the abovementioned results reveal that the ILs can have an important effect on the morphologies of the inorganic materials at the very low temperature of about 50 °C; in an IL-templated system, the nanostructures of inorganic materials are generated by a hydrogen bonding-co-π–π stacking mechanism, as discussed in previous studies;40–42 the morphology of the sample after calcination at 700 °C for 2 h is well-preserved, and the sample still possesses a nanoflake shape (Fig. 3b).
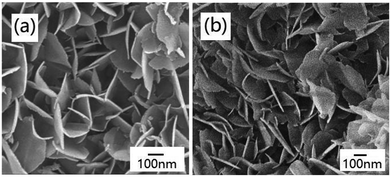 | ||
| Fig. 3 FE-SEM images of the products obtained (a) before and (b) after calcination at 700 °C for 2 h. | ||
Fig. 4 shows the TEM images of the product obtained by calcination at 700 °C for 2 h, which display a nanoflake-like morphology, and each nanoflake is composed of numerous nanoparticles with the diameters of about 20 nm. There are many mesopores between adjacent nanoparticles (Fig. 4b). The typical lattice spacing was determined to be 0.29 nm, corresponding to the (220) lattice plane of ZnAl2O4 (inset in Fig. 4b).
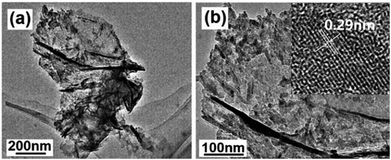 | ||
| Fig. 4 (a) Low- and (b) high-magnification TEM images of the product obtained by calcination at 700 °C for 2 h. Inset in (b) shows the HR-TEM image. | ||
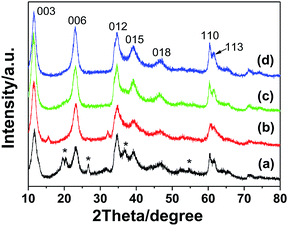
The effect of the mole ratios of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na-Dw on the crystal phase of the precursors was investigated on the basis of control experiments. As shown in Fig. 5, two crystal phases of Al2O3·3H2O and Zn6Al2(OH)16(CO3)·4H2O co-existed in the precursor when the mole ratio was 1
Na-Dw on the crystal phase of the precursors was investigated on the basis of control experiments. As shown in Fig. 5, two crystal phases of Al2O3·3H2O and Zn6Al2(OH)16(CO3)·4H2O co-existed in the precursor when the mole ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. As the mole ratio of Zn2+
2. As the mole ratio of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na-Dw was increased, the concentration of the Zn6Al2(OH)16(CO3)·4H2O phase gradually increased; when the mole ratio of Zn2+
Na-Dw was increased, the concentration of the Zn6Al2(OH)16(CO3)·4H2O phase gradually increased; when the mole ratio of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na-Dw reached 3
Na-Dw reached 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pure phase of the Zn6Al2(OH)16(CO3)·4H2O crystal was obtained. To further clarify the effect of the Zn2+
1, pure phase of the Zn6Al2(OH)16(CO3)·4H2O crystal was obtained. To further clarify the effect of the Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na-Dw mole ratio on the product structure, XRD analysis of the products obtained by calcination of the precursors at 700 °C for 2 h was carried out, as shown in Fig. S4 in the ESI.† When the mole ratio was 1
Na-Dw mole ratio on the product structure, XRD analysis of the products obtained by calcination of the precursors at 700 °C for 2 h was carried out, as shown in Fig. S4 in the ESI.† When the mole ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, pure ZnAl2O4 crystals could be obtained. In other cases, however, ZnO and ZnAl2O4 co-existed in the products. Moreover, as the mole ratio increased, the ZnO phase became the main crystal phase of the product. These results reveal that the mole ratio of Zn2+
2, pure ZnAl2O4 crystals could be obtained. In other cases, however, ZnO and ZnAl2O4 co-existed in the products. Moreover, as the mole ratio increased, the ZnO phase became the main crystal phase of the product. These results reveal that the mole ratio of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na-Dw significantly influences the product composition, and the optimal mole ratio is 1
Na-Dw significantly influences the product composition, and the optimal mole ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to obtain the pure phase of ZnAl2O4 nanoflakes. Fig. S5 in the ESI† shows the morphologies of the precursors obtained using Zn2+
2 to obtain the pure phase of ZnAl2O4 nanoflakes. Fig. S5 in the ESI† shows the morphologies of the precursors obtained using Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na-Dw at different mole ratios. It can be observed that there are no significant changes in the morphology of the precursors with a change in the mole ratios; this indicates that the nanoflakes are formed from Zn6Al2(OH)16(CO3)·4H2O rather than from Al2O3·3H2O or ZnO.
Na-Dw at different mole ratios. It can be observed that there are no significant changes in the morphology of the precursors with a change in the mole ratios; this indicates that the nanoflakes are formed from Zn6Al2(OH)16(CO3)·4H2O rather than from Al2O3·3H2O or ZnO.
As is well-known, well-developed alumina nanostructures, such as AlOOH or Al2O3, can only be obtained at higher reaction temperatures by hydrothermal synthesis52 or solvothermal synthesis.53 Thus, in the present reaction system, it is impossible for the alumina crystals to develop well because of the low reaction temperature of 50 °C.
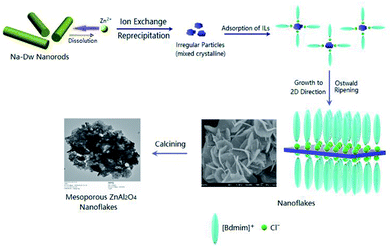
Zn6Al2(OH)16(CO3)·4H2O, known as a layered double hydroxide (LDHs) or hydrotalcite-like compound, exists either as a natural mineral or a synthesized material. It has a sandwich structure composed of a cation (Zn2+ and Al3+) layer (octahedron) and an anion (CO32−) interlayer, both of which are quite tunable (Scheme S1 in the ESI†).54,55 Considering its structural characteristics, in the present reaction system, the crystal growth of Zn6Al2(OH)16(CO3)·4H2O is a dominant factor in the formation of well-developed 2D flake-like nanostructures via the recrystallization process of the mixed crystals obtained after ion-exchange reactions. According to the abovementioned discussions, we believe that the ionic liquid molecules adsorbed on the surface of the Zn6Al2(OH)16(CO3)·4H2O crystallites play an important role as templates or structural indicators; however, they also adsorb on the surface of the Al2O3·3H2O crystallites. Since the pH value of the present reaction system is about 7 and the PZCs of Zn6Al2(OH)16(CO3)·4H2O and Al2O3·3H2O crystals are about 11.5 and 9.7,56,57 respectively, the surfaces of the abovementioned two kinds of crystallites are positively charged. Therefore, the ionic liquid molecules adsorb on the surfaces of the crystals through an anionic dominant model.39 The schematic of adsorption is shown in Scheme 1.
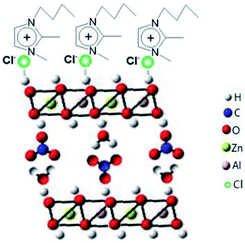 | ||
| Scheme 1 Schematic for the adsorption of [bdmim]Cl molecules on the surface of the Zn6Al2(OH)16(CO3)·4H2O crystallite. | ||
To investigate the formation process of the flake-like ZnAl2O4 nanostructures, we carried out analogous experiments for different reaction durations, as shown in Fig. 6. Fig. 6a shows that irregular particles are first formed after reaction for 1 h at 50 °C. The morphology of these particles is entirely different from that of the Na-Dw parent material; this indicates that the ion-exchange process is accompanied by the dissolution of precursor molecules rather than simple in situ ion exchange. When the reaction time was extended to 2 h, some nanoflake-like structures appeared (Fig. 6b). Large-scale underdeveloped nanoflakes were formed after a 4 h reaction (Fig. 6c), and well-developed nanoflake-like structures could be obtained after an 8 h reaction (Fig. 6d). Moreover, there was no significant change in the morphology after a 10 h reaction.
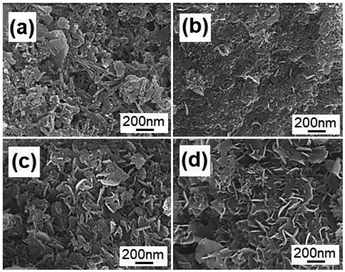 | ||
| Fig. 6 FE-SEM images of the products obtained by ion exchange from Na-Dw at 50 °C for (a) 1 h, (b) 2 h, (c) 4 h and (d) 8 h. | ||
Based on the abovementioned experimental results, a possible formation process of the flake-like ZnAl2O4 was proposed. In the first stage, irregular particles were formed via dissolution of the Na-Dw parent material, which underwent ion exchange with Zn2+ ions and reprecipitated in sequence, as shown in Scheme 2. In the subsequent stage, the dissolution–recrystallization process dominated, and the [bdmim][Cl] molecules adsorbed on the surface of the irregular particles as a soft template to control the direction of the crystal growth, as illustrated in Scheme 1. Herein, the Cl− ions from [bdmim]Cl preferentially adsorbed on the building blocks of hydrotalcite-like Zn6Al2(OH)16(CO3)·4H2O due to the formation of hydrogen bonds between Cl− ions and Zn6Al2(OH)16(CO3)·4H2O molecules; then, the [bdmim]+ ions also adsorbed on the abovementioned building blocks due to electrostatic interactions. As previously reported, the [bdmim]+ ions have a great tendency to self-assemble into ordered structures that are stabilized by additional π–π interactions along the aligned hydrogen bonds.40 In the last stage, an Ostwald ripening process dominates, and consequently, well-developed 2D flake-like nanostructures are obtained. Based on the abovementioned discussions, we proposed the formation mechanism of the mesoporous ZnAl2O4 spinel nanoflakes, as illustrated in Scheme 3.
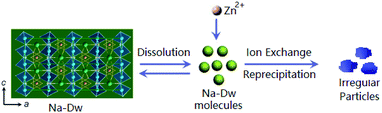 | ||
| Scheme 2 Schematic for the ion-exchange process and the reprecipitation process occurring in the present reaction system by the addition of zinc salt. | ||
To investigate the specific surface area and porous nature of the ZnAl2O4 spinel nanoflakes, Brunauer–Emmett–Teller (BET) gas-sorption measurements were carried out. The nitrogen adsorption/desorption isotherm obtained for the product shows significant hysteresis at the relative pressure P/P0 of above 0.71 (Fig. 7). Moreover, the BET specific surface area of the product was calculated, which was about 245 m2 g−1, higher than the previous research results: 183.5 m2 g−1,18 182.8 m2 g−1 (ref. 45) and 147 m2 g−1.58 The Barrett–Joyner–Halenda (BJH) calculations for the pore-size distribution, derived from the desorption data, reveal a narrow pore distribution with one apex centered at 14.5 nm (inset of Fig. 7), indicating that the as-obtained ZnAl2O4 spinel product has mesopores. These mesopores presumably arise from the spaces between adjacent nanoparticles formed during the calcination process due to the release of CO2 and H2O molecules from Zn6Al2(OH)16(CO3)·4H2O.
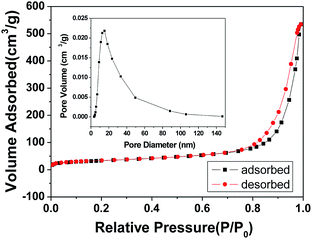 | ||
| Fig. 7 N2 adsorption–desorption isotherm and the pore-size distribution curve (inset) for the ZnAl2O4 spinel nanoflakes obtained by calcination at 700 °C for 2 h. | ||
4. Conclusions
In summary, the well-developed mesoporous ZnAl2O4 spinel nanoflakes were successfully prepared by the ion-exchange method using an aqueous solution of Zn(NO3)2 and Na-Dw parent materials in the presence of an ionic liquid, [bdmim][Cl], at 50 °C, followed by calcination at 700 °C for 2 h. The formation mechanism of the ZnAl2O4 nanoflakes was explored on the basis of control experiments and structure analyses. The results demonstrate that [bdmim][Cl] plays a crucial role in the formation of the flake-like morphology at the abovementioned low temperature. The optimal mole ratio of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na-Dw is 1
Na-Dw is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to obtain the ZnAl2O4 spinel nanoflakes. The BET-specific surface area of the mesoporous ZnAl2O4 nanoflakes, constructed by numerous nanoparticles, is as high as 245 m2 g−1. Since Na-Dw is a cheap natural mineral, the synthesis of ZnAl2O4 spinel nanostructures at low temperatures using Na-Dw as a parent material can be applied as an economical and significant industrial method. The mesoporous ZnAl2O4 spinel nanoflakes are expected to be used in some applications such as in catalysts and catalyst supports.
2 to obtain the ZnAl2O4 spinel nanoflakes. The BET-specific surface area of the mesoporous ZnAl2O4 nanoflakes, constructed by numerous nanoparticles, is as high as 245 m2 g−1. Since Na-Dw is a cheap natural mineral, the synthesis of ZnAl2O4 spinel nanostructures at low temperatures using Na-Dw as a parent material can be applied as an economical and significant industrial method. The mesoporous ZnAl2O4 spinel nanoflakes are expected to be used in some applications such as in catalysts and catalyst supports.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 20971070 and 21073095), the 111project (B12015) and the National Foundation of Science and Technology (2017012012) of DPRK.References
- S. K. Sampath and J. F. Cordaro, J. Am. Ceram. Soc., 1998, 81, 649 CrossRef CAS.
- R. Roesky, J. Weiguny, H. Bestgen and U. Dingerdissen, Appl. Catal., A, 1999, 176, 213 CrossRef CAS.
- C. G. Anchieta, D. Sallet, E. L. Foletto, S. S. Da Silva, O. Chiavone-Filho and C. A. do Nascimento, Ceram. Int., 2014, 40, 4173 CrossRef CAS.
- S. Battidton, C. Rigo, E. Cruz Severo, M. A. Mazutti, R. C. Kuhn, A. Gundel and E. L. Foletto, Mater. Res., 2014, 17, 735 Search PubMed.
- M. Garcia-Hipolito, J. Guzman-Mendoza, E. Martinez, O. Alvarez-Fregoso and C. Falcony, Phys. Status Solidi A, 2004, 201, 1510 CrossRef CAS.
- N. J. Van der Laag, M. D. Snel, P. C. M. M. Magusin and G. De With, J. Eur. Ceram. Soc., 2004, 24, 2417 CrossRef CAS.
- S. Mathur, M. Veith, M. Haas, A. Shen, N. Lecerf, V. Huch, S. Hufner, R. Haberkorn, H. P. Beck and M. Jilavi, J. Am. Ceram. Soc., 2001, 84, 1921 CrossRef CAS.
- H. Matsui, C. Xu and H. Tateyama, Appl. Phys. Lett., 2001, 78, 1068 CrossRef CAS.
- D. L. Ge, Y. J. Fan, C. L. Qi and Z. X. Sun, J. Mater. Chem. A, 2013, 1, 1651 RSC.
- S. Pin, M. Suardelli, F. D'Acapito, G. Spinolo, M. Zema, S. C. Tarantino, L. Barba and P. Ghigna, J. Phys. Chem. C, 2013, 117, 6113 CrossRef CAS.
- A. D. Ballarini, S. A. Bocanegra, A. C. Castro, S. R. Miguel and O. A. Scelza, Catal. Lett., 2009, 129, 293 CrossRef CAS.
- E. L. Foletto, S. Battiston, J. M. Simões, M. M. Bassaco, L. S. Pereira, E. M. Flores and E. I. Müller, Microporous Mesoporous Mater., 2012, 163, 29 CrossRef CAS.
- F. Davar and M. Salavati-Niasari, J. Alloys Compd., 2011, 509, 2487 CrossRef CAS.
- A. V. Belyaev, I. I. Evdokimov, V. V. Drobotenko and A. A. Sorokin, J. Eur. Ceram. Soc., 2017, 37, 2747 CrossRef CAS.
- S. G. Menon, K. S. Choudhari, S. A. Shivashankar, S. Chidangila and S. D. Kulkarni, New J. Chem., 2017, 41, 5420 RSC.
- D. Zhang, Y. H. Qiu, Y. R. Xie, X. C. Zhou, Q. R. Wang, Q. Shi, S. H. Li and W. J. Wang, Mater. Des., 2017, 115, 37 CrossRef CAS.
- S. Sommer, E. D. Bøjesen, A. B. Blichfeld and B. B. Iversen, J. Solid State Chem., 2017, 256, 45 CrossRef CAS.
- H. P. Macedo, R. L. Medeiros, A. L. Medeiros, A. A. S. Oliveira, G. P. Figueredo, M. A. Melo and D. M. Melo, Mater. Res., 2017, 20, 29 CrossRef.
- Z. Li, S. Zhang and W. Lee, J. Eur. Ceram. Soc., 2007, 27, 3407 CrossRef CAS.
- M. Obaidullaha, T. Furusawa, I. A. Siddiquey, M. Sato and N. Suzuki, Adv. Powder Technol., 2017, 28, 2678 CrossRef.
- C. S. Lei, M. Pi, W. Zhou, Y. Q. Guo, F. Zhang and J. Q. Qin, Chem. Phys. Lett., 2017, 687, 143 CrossRef CAS.
- L. B. Railsback, Carbonates Evaporites, 1999, 14, 1 CrossRef CAS.
- C. J. Serna, J. L. White and S. L. Hem, J. Pharm. Sci., 1978, 67, 324 CrossRef CAS PubMed.
- P. V. Bonsignore, Plast. Eng., 1976, 32, 41 CAS.
- E. A. Woycheshin, R. J. Rigge and I. Sobolev, US Pat. 3 878 166, 1975.
- R. L. Altman, L. A. Mayer and A. C. Ling, US Pat. 4 406 797, 1983.
- G. Gillman and A. Noble, Environ. Qual. Manag., 2005, 15, 59 CrossRef.
- G. Ci. Li, Y. Q. Liu, L. L. Guan, X. F. Hu and C. G. Liu, Mater. Res. Bull., 2012, 47, 1073 CrossRef CAS.
- M. Shoaib, M. Mazhar, A. Mushtaq, A. Jamil, T. T. Muhammad, A. Izhar and M. H. Syed, Mater. Chem. Phys., 2017, 191, 62 CrossRef.
- X. X. Zou, P. Wang, Z. Wang, G. Fan, S. Chen, S. Liao, H. Si and M. Lu, Ceram. Int., 2018, 44, 10847 CrossRef CAS.
- I. B. Malham, P. Letellier and M. Turmine, J. Phys. Chem. B, 2006, 110, 14212 CrossRef CAS PubMed.
- X. C. Duan, J. M. Ma, Y. Shen and W. J. Zheng, Inorg. Chem., 2012, 51, 914 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Soc. Rev., 2013, 42, 1096 RSC.
- L. Xu, J. Xia, L. Wang, J. Qian, H. Li, K. Wang, K. Sun and M. He, Chem.–Eur. J., 2014, 20, 2244 CrossRef CAS PubMed.
- R. Hayes, G. G. Warr and R. Atkin, Chem. Rev., 2015, 115, 6357 CrossRef CAS PubMed.
- X. C. Duan, H. Huang, S. H. Xiao, J. W. Deng, G. Zhou, Q. H. Lia and T. H. Wang, J. Mater. Chem. A, 2016, 4, 8402 RSC.
- H. Roohi, H. Iloukhani and F. Rouhani, J. Mol. Liq., 2017, 240, 138 CrossRef CAS.
- X. C. Duan, T. I. Kim, D. Li, J. Ma and W. J. Zheng, Chem.–Eur. J., 2013, 19, 5924 CrossRef CAS PubMed.
- J. Zhang, H. J. Feng, J. Q. Yang, Q. Qin, H. M. Fan, C. Y. Wei and W. J. Zheng, ACS Appl. Mater. Interfaces, 2015, 7, 21735 CrossRef CAS PubMed.
- Q. Qin, T. I. Kim, X. C. Duan, J. B. Lian and W. J. Zheng, Cryst. Growth Des., 2016, 16, 6139 CrossRef CAS.
- W. H. Luo, G. F. Zhang, Y. X. Cui, Y. Sun, Q. Qin, J. Zhang and W. J. Zheng, J. Mater. Chem. A, 2017, 5, 11278 RSC.
- Q. Qin, J. Hao and W. J. Zheng, ACS Appl. Mater. Interfaces, 2018, 10, 17827 CrossRef CAS PubMed.
- X. C. Duan, T. I. Kim, L. L. Han, J. M. Ma, X. W. Du and W. J. Zheng, Sci. Rep., 2013, 3, 3218 CrossRef PubMed.
- B. D. Cullity and S. R. Stock, Elements of X-Ray Diffraction, Pearson, London, 3rd edn, 2014, p. 664 Search PubMed.
- X. L. Du, L. Q. Li, W. X. Zhang, W. C. Chen and Y. T. Cui, Mater. Res. Bull., 2015, 61, 64 CrossRef CAS.
- M. Mokhtar, A. Inayat, J. Ofili and W. Schwieger, Appl. Clay Sci., 2010, 50, 176 CrossRef CAS.
- A. Smalenskaitea, D. E. L. Vieirab, A. N. Salakb, M. G. S. Ferreirab, A. Katelnikovasc and A. Kareiva, Appl. Clay Sci., 2017, 143, 175 CrossRef.
- C. R. Chen, P. X. Ding, S. Xu, J. Z. Du, H. Y. Zeng and Y. X. Sun, Appl. Clay Sci., 2018, 161, 404 CrossRef CAS.
- Y. Y. Zhang, J. Chang, J. Y. Zhao and Y. F. Fang, J. Am. Ceram. Soc., 2018, 101, 4262 CrossRef CAS.
- M. Barroso, M. Gomez, G. J. Andrade, L. Arrua and M. Abello, J. Phys. Chem. Solids, 2006, 67, 1583 CrossRef CAS.
- X. Z. Guo, P. A. Yin, W. Lei and H. Yang, New J. Chem., 2017, 41, 11998 RSC.
- J. K. Lee, E. J. Jang, H. Y. Jeong and J. H. Kwak, Appl. Catal., A, 2018, 556, 121 CrossRef CAS.
- J. B. Lian, J. M. Ma, X. C. Duan, T. I. Kim, H. B. Li and W. J. Zheng, Chem. Commun., 2010, 46, 2650 RSC.
- M. Shamim and K. Dana, J. Mol. Struct., 2016, 1125, 27 CrossRef CAS.
- M. Zhou, L. Yan, H. Ling, Y. P. Diao, X. L. Pang, Y. Wang and K. Gao, Appl. Surf. Sci., 2017, 404, 246 CrossRef CAS.
- W. Cai, J. Yu and M. Jaroniec, J. Mater. Chem., 2010, 20, 4587 RSC.
- R. Rojas Delgado, C. P. De Pauli, C. Barriga Carrasco and M. J. Avena, Appl. Clay Sci., 2008, 40, 27 CrossRef CAS.
- T. R. Mandlimath and K. I. Sathiyanarayanan, RSC Adv., 2016, 6, 3117 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra10524c |
| This journal is © The Royal Society of Chemistry 2019 |