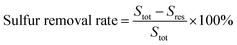Open Access Article
Open Access Article[HDMF]Cl-based DES as highly efficient extractants and catalysts for oxidative desulfurization of model oil
Rong-xiang Zhao,
Xiu-ping Li *,
Chun-feng Mao
*,
Chun-feng Mao ,
Liangpei Hou and
Xiaohan Gao
,
Liangpei Hou and
Xiaohan Gao
College of Chemistry, Chemical Engineering and Environmental Engineering, Liaoning Shihua University, Fushun 113001, China. E-mail: zylhzrx@126.com; lilili_171717@126.com
First published on 8th May 2019
Abstract
N,N-Dimethylformamide hydrochloric acid/XMCln ([HDMF]Cl/XMCln, M = Zn or Fe, n = 2 or 3) was synthesized by stirring the mixture of [HDMF]Cl and metal chloride. [HDMF]Cl-based DES was characterized by FT-IR spectroscopy, ESI-MS and 1H-NMR spectroscopy. The oxidative desulfurization activity was investigated using [HDMF]Cl/0.2FeCl3 and [HDMF]Cl/ZnCl2 as the extractant and catalyst, and hydrogen peroxide (H2O2) as the oxidant. The desulfurization rate can reach up to 98.08% and 99.2% for DBT using [HDMF]Cl/0.2FeCl3 and [HDMF]Cl/ZnCl2, respectively. After recycling for 7 times, the removal rate of DBT still can reach more than 97%.
1 Introduction
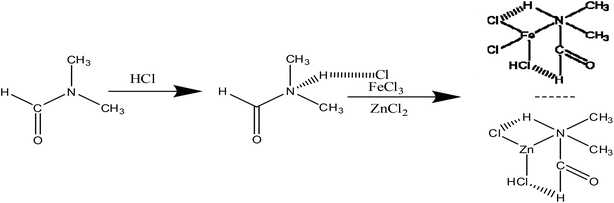
Ultra-desulfurization of gasoline and diesel oil is important because the sulfur compounds have brought many negative influences on the weather and health. Many countries have formulated strict environmental regulations to limit the sulfur content in the fuels (sulfur content < 10 ppm).1,2In order to remove organic sulfur from petroleum products, the hydrodesulfurization (HDS) technology3 has been widely applied in the refining enterprises. However, HDS needs strict operating conditions,4 such as high temperature and high pressure, which result in the increase in the operational cost. Meanwhile, it is difficult to remove dibenzothiophene (DBT) and its derivatives due to space hindrance.5 In order to overcome the shortcomings of HDS, some non-HDS technologies such as adsorption desulfurization (ADS),6,7 oxidative desulfurization (ODS),8–10 biological desulfurization (BDS)11 and extractive desulfurization12 have been widely investigated. Among them, the oxidative desulfurization has become a hot topic because of its potential advantages such as mild reaction conditions and high desulfurization rate for dibenzothiophene (DBT) and its derivatives. In the oxidative desulfurization process, sulfur is oxidized into sulfone by oxidants such as molecular oxygen,13 H2O2,14 NO2 (ref. 15) and substitute solid.16 Among these, H2O2 is a widely used oxidant because of its economic and environmental benefits.
DES as an ionic liquid is composed of a cation and an anion, and has some desirable properties17–20 such as excellent solubility, non-volatility, and non-flammability. Moreover, DES containing hydrogen bonding networks is liquid at room temperature. DES as an environment-friendly solvent is applied in synthesis,21 catalysis,22 separation23 and electrochemistry.24 In last several years, DES has been applied to the desulfurization process due to higher desulfurization rate.25–28 For instance, Li et al.29 synthesized 1.6Et3NHCl·FeCl3 extract, which facilitated the desulfurization rate up to 87%. Chen et al.30 reported that [Hnmp]Cl/ZnCl2 was applied in oxidative desulfurization and its desulfurization rate reached up to 99.9% for the model diesel oil. Li et al.31 reported that C5H9NO·0.3FeCl3 was used as an extractant as well as a catalyst in ODS and the removal rate of DBT was 97% in 3 h. Chen et al.32 synthesized [C4mim]Cl/3ZnCl2 and 99.9% sulphur-removal rate could be obtained. Dong et al.33 found that the removal rate of DBT can reach up to 100% using [C63MPy]Cl/FeCl3 as extractants and catalysts in ODS. In the process of desulfurization of oil, N,N-dimethylformamide (DMF) is often selected as an effective extractant.34,35 However, it is difficult to industrialize because the DMF dosage is too large. In this work, [HDMF]Cl-based DES is synthesized by a simple method. A small amount of DES used as catalysis and extractant in the oxidative desulfurization system can significantly improve desulfurization activity. The effects of the molar ratio of [HDMF]Cl to MCln, temperature, oxygen to sulfur (O/S) molar ratio, amount of DES, and types of sulfur on the desulfurization activity were studied. The mechanism of desulfurization was investigated.
2 Experiment
2.1 Materials
2.2 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and stirred for 3 hours at 50 °C. Water in the solution was removed by rotary evaporation after stirring. Finally, the [HDMF]Cl solution was obtained.
1 and stirred for 3 hours at 50 °C. Water in the solution was removed by rotary evaporation after stirring. Finally, the [HDMF]Cl solution was obtained.2.3 Desulfurization experiment
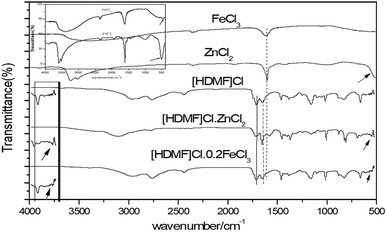
Model oil (500 μg g−1) was prepared by dissolving 1.437 g DBT in 500 mL n-octane. To implement the ODS process, the model oil, DES and 30 wt% H2O2 were added into a three-necked flask. The mixture was stirred at 50 rpm in a water bath at a particular temperature. The oil taken out from the upper layer in every 20 min was analyzed by gas chromatography on an Agilent 7890A GC with an FID detector using a 30 m packed HP5 column. The removal rate was calculated by the formula:where Stot (500 μg g−1) is the total content of the sulfur compound in the model oil, Sres is the residual content of the sulfur compound after the ODS process.
3 Results and discussion
3.1 ESI-MS spectra of [HDMF]Cl·0.2FeCl3
In order to determine the structures of [HDMF]Cl·0.2FeCl3 and [HDMF]Cl/ZnCl2, the ESI-MS spectra of [HDMF]Cl·0.2FeCl3 and [HDMF]Cl/ZnCl2 were obtained. The results are shown in Fig. 2. From the ESI-MS spectrum of [HDMF]Cl·0.2FeCl3, the peaks observed at m/z = 163 and 198 correspond to FeCl3 and FeCl4−, respectively. The peak at m/z = 201 can be attributed to [HDMF]FeCl2+. The peaks at m/z = 163 and 198 correspond to those in a previous report.29,30 The peak at m/z = 201 demonstrates that there is a reaction between [HDMF]Cl and FeCl3. The lone pair electrons of N in [HDMF] and an unoccupied orbital of Fe in FeCl3 form a covalent bond. 4 mol of [HDMF] supplies four electrons and 1 mol Fe supplies four half-unoccupied orbitals resulting in the molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for [HDMF]Cl and FeCl3. Fig. 2(b) also shows that peaks of ZnCl3−, Zn2Cl5− and Zn3Cl7− appeared at m/z 171, 307 and 448. However, these peaks cannot verify the reaction between [HDMF]Cl and ZnCl2. The ESI-MS spectrum of the cation is shown in Fig. 2(c). The peaks at m/z 174, 244, 347 and 478 are the peaks of [HDMF]Cl/Zn2+, [HDMF]Cl/ZnCl2, [HDMF]2Cl2/ZnCl2 and 2([HDMF]Cl/ZnCl2) losing a –CH3, respectively. These results confirm that [HDMF]Cl/ZnCl2 has been synthesized. [HDMF]Cl·0.2FeCl3 only has anion spectrum show that all species of [HDMF]Cl·0.2FeCl3 are anion.
1 for [HDMF]Cl and FeCl3. Fig. 2(b) also shows that peaks of ZnCl3−, Zn2Cl5− and Zn3Cl7− appeared at m/z 171, 307 and 448. However, these peaks cannot verify the reaction between [HDMF]Cl and ZnCl2. The ESI-MS spectrum of the cation is shown in Fig. 2(c). The peaks at m/z 174, 244, 347 and 478 are the peaks of [HDMF]Cl/Zn2+, [HDMF]Cl/ZnCl2, [HDMF]2Cl2/ZnCl2 and 2([HDMF]Cl/ZnCl2) losing a –CH3, respectively. These results confirm that [HDMF]Cl/ZnCl2 has been synthesized. [HDMF]Cl·0.2FeCl3 only has anion spectrum show that all species of [HDMF]Cl·0.2FeCl3 are anion.
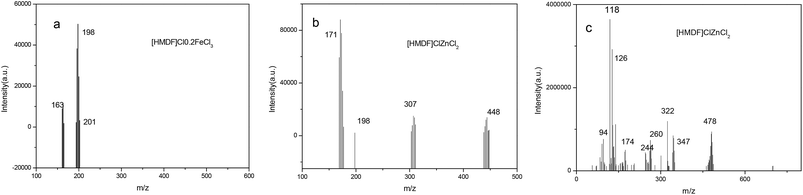
3.2 FTIR characterization
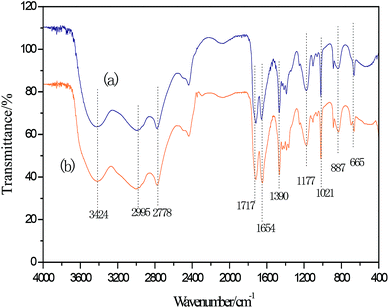
Infrared spectrum peaks of ZnCl2, FeCl3, [HDMF]Cl, [HDMF]Cl·0.2FeCl3 and [HDMF]Cl/ZnCl2 have been displayed in Fig. 3. The peaks correlated to the bending of N–H at 665 cm−1, bending of C–H at 887 and 1390 cm−1, the stretching vibrations of C–N at 1021 and 1177 cm−1, and that of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1654 and 1717 cm−1, the stretching vibration of C–H at 2778 and 2995 cm−1, and that of N–H at 3421 cm−1 in [HDMF]Cl are shown in Fig. 3. It can be observed that some infrared spectrum-peaks of [HDMF]Cl·0.2FeCl3 and [HDMF]Cl are identical. Peaks of FeCl3 and ZnCl2 appear at about 550 cm−1. Meanwhile, the stretching vibration of C
O at 1654 and 1717 cm−1, the stretching vibration of C–H at 2778 and 2995 cm−1, and that of N–H at 3421 cm−1 in [HDMF]Cl are shown in Fig. 3. It can be observed that some infrared spectrum-peaks of [HDMF]Cl·0.2FeCl3 and [HDMF]Cl are identical. Peaks of FeCl3 and ZnCl2 appear at about 550 cm−1. Meanwhile, the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1654 cm−1 strengthens and a new peak corresponding to N–H–C–N at 1560 cm−1 appears in the infrared spectrum of [HDMF]Cl/ZnCl2. These results show that [HDMF]Cl still retain the original structure in [HDMF]Cl·0.2FeCl3 and [HDMF]Cl/ZnCl2.
O at 1654 cm−1 strengthens and a new peak corresponding to N–H–C–N at 1560 cm−1 appears in the infrared spectrum of [HDMF]Cl/ZnCl2. These results show that [HDMF]Cl still retain the original structure in [HDMF]Cl·0.2FeCl3 and [HDMF]Cl/ZnCl2.
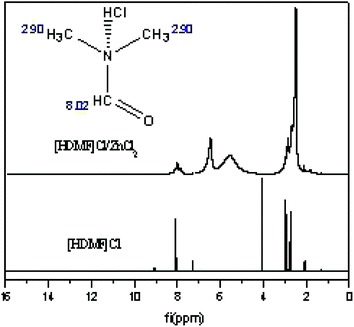
3.3 Hydrogen spectrum of [HDMF]Cl/ZnCl2
The hydrogen spectrum of [HDMF]Cl·0.2FeCl3 cannot be detected due to the magnetic properties of iron. The hydrogen spectrum of [HDMF]Cl/ZnCl2 was recorded and the results are displayed in Fig. 4. This is because the zinc chloride and [HDMF]Cl formed a hydrogen bond. The formation of hydrogen bond leads to the disappearance and the shift of hydrogen bonds in [HDMF]Cl. The δ = 2.90 and 8.02 are attributed to the hydrogen bonds of –CH3 and –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. The δ = 4.05 is the hydrogen bond formed between N and HCl. As seen from Fig. 4, these peaks of hydrogen bonds broaden and are shifted. The new peak is attributed to the hydrogen bond between Cl in ZnCl2 and H in [HDMF]Cl. These results are also seen in another ref. 36.
O, respectively. The δ = 4.05 is the hydrogen bond formed between N and HCl. As seen from Fig. 4, these peaks of hydrogen bonds broaden and are shifted. The new peak is attributed to the hydrogen bond between Cl in ZnCl2 and H in [HDMF]Cl. These results are also seen in another ref. 36.
3.4 Different desulfurization system
In order to investigate the influence of H2O2, [HDMF], [HDMF]Cl, [HDMF]Cl/ZnCl2 and [HDMF]Cl·0.2FeCl3 on desulfurization activity, different desulfurization systems were selected as shown in Table 1. The removal rate of DBT in model oil increased with an increase in H2O2 and DES. [HDMF] is a good extractant for sulfide in oil. The desulfurization rate could reach up to 75% when the volume ratio of [HDMF] and model oil was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The high dose of [HDMF] is harmful for the environment. Hence, [HDMF]Cl/MCln was synthesized and applied to desulfurization, and the volume ratio of [HDMF] and model oil was decreased to 0.2. As shown in Table 1, desulfurization systems of [HDMF]Cl·ZnCl2 + H2O2 and [HDMF]Cl·0.2FeCl3 + H2O2 showed excellent desulfurization activities. Lewis acids can accelerate H2O2 degradation to hydroxyl radicals35 in the system of oxidative desulfurization and result in high desulfurization activity. However, very high molar ratio of [HDMF]Cl to MCln decreased the desulfurization rate. The strong acidity accelerate decomposition of H2O2 (ref. 37) result in decrease of oxidant ability in the desulfurization system. Therefore, [HDMF]Cl·0.2FeCl3 and [HDMF]Cl·1.0ZnCl2 are the most suitable ones for the desulfurization system. The desulfurization rates of [HDMF]Cl·0.2FeCl3 and [HDMF]Cl·1.0ZnCl2 are 20.36% and 15.3%, respectively, without the addition of H2O2. However, the rates are only 9.2% and 9.6% in the system of [HDMF]Cl and [HDMF]Cl + H2O2, respectively. These experimental results demonstrate that H2O2 and Lewis acids are indispensable for the desulfurization system.
1. The high dose of [HDMF] is harmful for the environment. Hence, [HDMF]Cl/MCln was synthesized and applied to desulfurization, and the volume ratio of [HDMF] and model oil was decreased to 0.2. As shown in Table 1, desulfurization systems of [HDMF]Cl·ZnCl2 + H2O2 and [HDMF]Cl·0.2FeCl3 + H2O2 showed excellent desulfurization activities. Lewis acids can accelerate H2O2 degradation to hydroxyl radicals35 in the system of oxidative desulfurization and result in high desulfurization activity. However, very high molar ratio of [HDMF]Cl to MCln decreased the desulfurization rate. The strong acidity accelerate decomposition of H2O2 (ref. 37) result in decrease of oxidant ability in the desulfurization system. Therefore, [HDMF]Cl·0.2FeCl3 and [HDMF]Cl·1.0ZnCl2 are the most suitable ones for the desulfurization system. The desulfurization rates of [HDMF]Cl·0.2FeCl3 and [HDMF]Cl·1.0ZnCl2 are 20.36% and 15.3%, respectively, without the addition of H2O2. However, the rates are only 9.2% and 9.6% in the system of [HDMF]Cl and [HDMF]Cl + H2O2, respectively. These experimental results demonstrate that H2O2 and Lewis acids are indispensable for the desulfurization system.
| ILs | Sulfur removal/% | Sulfur removal/% | |
|---|---|---|---|
| [HDMF]Cl·0.1FeCl3 + H2O2 | 83.46 | [HDMF]Cl·0.5ZnCl2 + H2O2 | 63.8 |
| [HDMF]Cl·0.2FeCl3 + H2O2 | 98.08 | [HDMF]Cl·1.0ZnCl2 + H2O2 | 98.6 |
| [HDMF]Cl·0.3FeCl3 + H2O2 | 95.96 | [HDMF]Cl·1.5ZnCl2 + H2O2 | 98 |
| [HDMF]Cl·0.4FeCl3 + H2O2 | 62.12 | [HDMF]Cl·2ZnCl2 + H2O2 | 87 |
| [HDMF]Cl·0.5FeCl3 + H2O2 | 29.18 | ||
| [HDMF]Cl·0.2FeCl3 | 20.36 | [HDMF]Cl·1.0ZnCl2 | 15.3 |
| [HDMF]Cl | 9.2 | ||
| [HDMF]Cl + H2O2 | 9.6 | ||
v[HDMF]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vmodel oil = 1 vmodel oil = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, extractive desulfurization rate = 75% 1, extractive desulfurization rate = 75% |
|||
We investigated the influence of temperature, O/S, volume ratio of DES and model oil on desulfurization activity. The optimal desulfurization conditions are listed in Table 2. High temperature, O/S molar ratio and volume ratio of DES and the model oil can accelerate the reaction rate and improve the desulfurization activity. However, very high temperature leads to the decomposition of H2O2 into H2O and O2.38 A very high O/S molar ratio results in the high production of H2O.39 Too high volume ratio of DES and model oil results in more sulfide being extracted into the DES phase, resulting in the decrease of O/S molar ratio. This reduces the desulfurization activity of the system. Thus, the optimal conditions of desulfurization are listed in Table 2.
| Condition | [HDMF]Cl·0.2FeCl3 | Removal rate of DBT% | [HDMF]Cl/ZnCl2 | Removal rate of DBT% |
|---|---|---|---|---|
| T (°C) | 40 | 98.08 | 60 | 99.21 |
| O/S | 6 | 98.12 | 6 | 99.12 |
| Volume ratio of DES/model oil | 0.2 | 98.0 | 0.15–0.2 | 99.08 |
| t (min) | 30 | 98.10 | 60 | 99.16 |
3.5 Influence of different sulfur compounds on the desulfurization system
To investigate the influence of [HDMF]Cl·0.2FeCl3–H2O2 and [HDMF]Cl/ZnCl2–H2O2 on the different sulfur compounds, four sulfur compounds, namely, as DBT, 4,6-DMDBT, BT and TH were selected and the reactions were carried out under optimal conditions. From the ref. 40, it was known that the order of electron cloud density is 4,6-DMDBT (5.760) > DBT (5.758) > BT (5.739) > TH (5.696). As shown in Table 3, the order of oxidative desulfurization is DBT > 4,6-DMDBT > BT > TH in the systems of [HDMF]Cl·0.2FeCl3–H2O2 and [HDMF]Cl/ZnCl2–H2O2. It can be concluded that the bigger electron cloud density can easily remove sulfur. The electron cloud densities of DBT and 4,6-DMDBT were 5.758 and 5.760, respectively. The desulfurization rate of DBT was higher than that of 4,6-DMDBT. This could be attributed to the steric hindrance of two methyls of 4,6-DMDBT, which inturn hinders the desulfurization reaction.| [HDMF]Cl·0.2FeCl3 t (min) | Removal rate% | [HDMF]Cl/ZnCl2 t (time) | Removal rate% | |
|---|---|---|---|---|
| DBT | 30 | 98.08 | 60 | 99.2 |
| 4,6 DMDBT | 30 | 95.4 | 120 | 95.6 |
| BT | 60 | 42.99 | 140 | 54.6 |
| HT | 30 | 13.91 | 140 | 26.5 |
3.6 Recovery-regeneration of ILs
The upper oil phase was removed using a separating funnel after the oxidative desulfurization reaction. The water in the DES was removed by rotary evaporation. The DES was extracted three times using carbon tetrachloride (CCl4) of equal volume. The recycling experiments were carried out by adding fresh H2O2, model oil, and the recovered DES at the optimal conditions. As shown in Table 4, the desulfurization activity of [HDMF]Cl·0.2FeCl3 decreased from 98.08% to 96.01%, and the desulfurization rate of [HDMF]Cl/ZnCl2 decreased from 99.2% to 97.6% after seven cycles. This can be attributed to a little loss of DES and the residual oxidation products of DBT in the DES during the recovery process.| Recycle time/n | [HDMF]Cl·0.2FeCl3 | [HDMF]Cl/ZnCl2 |
|---|---|---|
| 1 | 98.08 | 99.2 |
| 2 | 97.95 | 99.1 |
| 3 | 97.69 | 98.9 |
| 4 | 97.31 | 98.8 |
| 5 | 97.04 | 98.5 |
| 6 | 96.74 | 98.3 |
| 7 | 96.01 | 97.6 |
3.7 FT-IR characterization of oxidation products
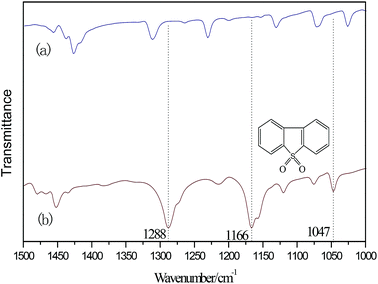
According to the above experiment, DES was extracted by CCl4. Then, CCl4 was removed by rotary evaporation. Thereafter, a white powder was obtained. The infrared analysis of the white powder is shown in Fig. 5. The three infrared absorption peaks at 1166, 1047 and 1288 cm−1 correspond to the three characterization peaks of dibenzothiophene sulfone (DBTO2).35,41,42 It can be concluded that the oxidation product was DBTO2 in the oxidative desulfurization system. DES was analyzed by FT-IR spectroscopy in order to study its stability. From Fig. 6, the FT-IR spectrum of used DES shows peaks at 665, 887, 1390, 1021, 1177, 1654, 1717, 2778, 2995 and 3424 cm−1. The new peak at 3424 cm−1 is the stretching vibration of OH. This shows that the used DES absorbs a little bit of water in air. Water also can decrease the activity of DES, resulting in the decrease of desulfurization rate.3.8 Mechanism of catalyzed oxidative desulfurization
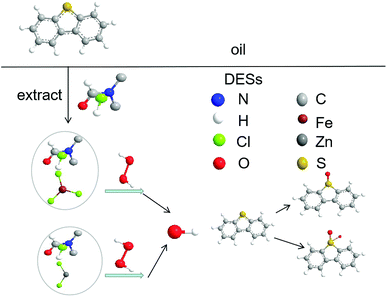
DBT in model oil was chosen as a representative of sulfur compounds in the oxidative desulfurization system. DBT in model oil was partially extracted by the DMF of DES. FeCl3 and ZnCl2 can catalyze H2O2 to produce hydroxyl radicals (˙OH). Hydroxyl radicals as a strong oxidant can oxidize DBT into dibenzothiophene sulfone (DBTO2). DBT continued to be extracted and oxidized in the subsequent cycles until DBT was completely transformed to DBTO2. The mechanism43–45 of the catalytic oxidative desulfurization is shown in Fig. 7.4 Conclusion
In this work, [HDMF]Cl/XMCln was synthesized by a stirring method at low temperature. The sulfur compounds in the model oil were removed using DES as the extractant and catalyst, and H2O2 as the oxidant. The experimental results showed that at a low O/S molar ratio and low volume ratio of DES/model oil, the desulfurization rates of [HDMF]Cl·0.2FeCl3 and [HDMF]Cl·ZnCl2 can be achieved up to 98.08% and 99.2%, respectively. Two systems of desulfurization saved lots of N,N-dimethylformamide and improved the desulfurization activities. Moreover, [HDMF]Cl·0.2FeCl3 can attain high desulfurization rate within a short time at low temperature.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support of the Natural Science Foundation of China (Project no. 21003069) and Doctoral Fund of Liaoning Province (201501105).References
- W. N. W. Abdullah, W. A. Bakar and R. Ali, Catalytic oxidative desulfurization of diesel fuel utilizing a polymolybdate alumina supported catalyst: characterization, catalytic activity and mechanistic study, React. Kinet., Mech. Catal., 2015, 114(2), 547–560 CrossRef CAS.
- J. Zhang, W. Zhu and H. Li, et al., Deep oxidative desulfurization of fuels by Fenton-like reagent in ionic liquids, Green Chem., 2009, 11(11), 1801–1807 RSC.
- S. Brunet, D. Mey and G. Pérot, et al., On the hydrodesulfurization of FCC gasoline: a review, Appl. Catal., A, 2005, 278(2), 143–172 CrossRef CAS.
- H. Lü, J. Gao and Z. Jiang, et al., Ultra-deep desulfurization of diesel by selective oxidation with [C18H37N(CH3)3]4[H2NaPW10O36] catalyst assembled in emulsion droplets, J. Catal., 2006, 239(2), 369–375 CrossRef.
- C. Shen, Y. J. Wang and J. H. Xu, et al., Synthesis of TS-1 on porous glass beads for catalytic oxidative desulfurization, Chem. Eng. J., 2015, 259, 552–561 CrossRef CAS.
- J. Gao, X. Chen and N. Ren, et al., Acylation desulfurization of oil via reactive adsorption, AIChE J., 2013, 59(8), 2966–2976 CrossRef CAS.
- G. Miao, F. Ye and L. Wu, et al., Selective adsorption of thiophenic compounds from fuel over TiO2/SiO2 under UV-irradiation, J. Hazard. Mater., 2015, 300, 426–432 CrossRef CAS PubMed.
- C. Wang, Z. Chen and X. Yao, et al., Decavanadates anchored into micropores of graphene-like boron nitride: efficient heterogeneous catalysts for aerobic oxidative desulfurization, Fuel, 2018, 230, 104–112 CrossRef CAS.
- W. Zhu, P. Wu and L. Yang, et al., Pyridinium-based temperature-responsive magnetic ionic liquid for oxidative desulfurization of fuels, Chem. Eng. J., 2013, 229, 250–256 CrossRef CAS.
- W. Zhu, C. Wang and H. Li, et al., One-pot extraction combined with metal-free photochemical aerobic oxidative desulfurization in deep eutectic solvent, Green Chem., 2015, 17(4), 2464–2472 RSC.
- M. Soleimani, A. Bassi and A. Margaritis, Biodesulfurization of refractory organic sulfur compounds in fossil fuels, Biotechnol. Adv., 2007, 25(6), 570–596 CrossRef CAS PubMed.
- H. Gao, S. Zeng and X. Liu, et al., Extractive desulfurization of fuel using N-butylpyridinium-based ionic liquids, RSC Adv., 2015, 5(38), 30234–30238 RSC.
- H. Lü, J. Gao and Z. Jiang, et al., Oxidative desulfurization of dibenzothiophene with molecular oxygen using emulsion catalysis, Chem. Commun., 2007,(2), 150–152 RSC.
- S. S. Cheng and T. F. Yen, Use of ionic liquids as phase-transfer catalysis for deep oxygenative desulfurization, Energy Fuels, 2008, 22(2), 1400–1401 CrossRef CAS.
- P. S. Tam, J. R. Kittrell and J. W. Eldridge, Desulfurization of fuel oil by oxidation and extraction. 1. Enhancement of extraction oil yield, Ind. Eng. Chem. Res., 1990, 29(3), 321–324 CrossRef CAS.
- P. De Filippis and M. Scarsella, Functionalized hexagonal mesoporous silica as an oxidizing agent for the oxidative desulfurization of organosulfur compounds, Ind. Eng. Chem. Res., 2008, 47(3), 973–975 CrossRef CAS.
- A. Arce, M. Francisco and A. Soto, Evaluation of the polysubstituted pyridinium ionic liquid [hmmpy][Ntf2] as a suitable solvent for desulfurization: phase equilibria, J. Chem. Thermodyn., 2010, 42(6), 712–718 CrossRef CAS.
- J. Ranke, S. Stolte and R. Störmann, et al., Design of sustainable chemical products the example of ionic liquids, Chem. Rev., 2007, 107(6), 2183–2206 CrossRef CAS PubMed.
- Y. Nie, C. Li and A. Sun, et al., Extractive desulfurization of gasoline using imidazolium-based phosphoric ionic liquids, Energy Fuels, 2006, 20(5), 2083–2087 CrossRef CAS.
- M. A. P. Martins, C. P. Frizzo and D. N. Moreira, et al., Ionic liquids in heterocyclic synthesis, Chem. Rev., 2008, 108(6), 2015–2050 CrossRef CAS PubMed.
- L. Zhang, Y. Cui and C. Zhang, et al., Biodiesel production by esterification of oleic acid over brønsted acidic ionic liquid supported onto Fe-incorporated SBA-15, Ind. Eng. Chem. Res., 2012, 51(51), 16590–16596 CrossRef CAS.
- H. Lü, C. Deng and W. Ren, et al., Oxidative desulfurization of model diesel using [(C4H9)4N]6Mo7O24 as a catalyst in ionic liquids, Fuel Process. Technol., 2014, 119, 87–91 CrossRef.
- Q. Wang, L. Lei and J. Zhu, et al., Deep desulfurization of fuels by extraction with 4-dimethyl- aminopyridinium-based ionic liquids, Energy Fuels, 2013, 27(8), 4617–4623 CrossRef CAS.
- N. Liu, F. Luo and H. Wu, et al., One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite, Adv. Funct. Mater., 2008, 18(10), 1518–1525 CrossRef CAS.
- A. Bösmann, L. Datsevich and A. Jess, et al., Deep desulfurization of diesel fuel by extraction with ionic liquids, Chem. Commun., 2001,(23), 2494–2495 RSC.
- S. Zhang, Q. Zhang and Z. C. Zhang, Extractive desulfurization and denitrogenation of fuels using ionic liquids, Ind. Eng. Chem. Res., 2004, 43(2), 614–622 CrossRef CAS.
- C. Huang, B. Chen and J. Zhang, et al., Desulfurization of gasoline by extraction with new ionic liquids, Energy Fuels, 2004, 18(6), 1862–1864 CrossRef CAS.
- L. Alonso, A. Arce and M. Francisco, et al., Solvent extraction of thiophene from n-alkanes (C7, C12, and C16) using the ionic liquid [C8mim][BF4], J. Chem. Thermodyn., 2008, 40(6), 966–972 CrossRef CAS.
- F. Li, Y. Liu and Z. Sun, et al., Deep Extractive Desulfurization of Gasoline with xEt3NHCl·FeCl3 Ionic Liquids, Energy Fuels, 2010, 24(8), 4285–4289 CrossRef CAS.
- X. Chen, H. Guo and A. A. Abdeltawab, et al., Brønsted–Lewis Acidic Ionic Liquids and Application in Oxidative Desulfurization of Diesel Fuel, Energy Fuels, 2015, 29(5), 2998–3003 CrossRef CAS.
- F. Li, B. Wu and R. Liu, et al., An inexpensive N-methyl-2-pyrrolidone-based ionic liquid as efficient extractant and catalyst for desulfurization of Dibenzothiophene, Chem. Eng. J., 2015, 274, 192–199 CrossRef CAS.
- X. Chen, D. Song and C. Asumana, et al., Deep oxidative desulfurization of diesel fuels by Lewis acidic ionic liquids based on 1-n-butyl-3-methylimidazolium metal Chloride, J. Mol. Catal. A: Chem., 2012, 359, 8–13 CrossRef CAS.
- Y. Dong, Y. Nie and Q. Zhou, Highly efficient oxidative desulfurization of fuels by Lewis acidic ionic liquids based on iron chloride, Chem. Eng. Technol., 2013, 36(3), 435–442 CrossRef CAS.
- L. F. Ramirez-Verduzco, E. Torres-Garcia and R. Gomez-Quintana, et al., Desulfurization of diesel by oxidation/extraction scheme: influence of the extraction solvent, Catal. Today, 2004, 98(1), 289–294 CrossRef CAS.
- S. Otsuki, T. Nonaka and N. Takashima, et al., Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction, Energy Fuels, 2000, 14(6), 1232–1239 CrossRef CAS.
- Y. Nie, X. Gong and H. S. Gao, et al., Simultaneous desulfurization and denitrogen of liquid fuels using two functionalized group ionic liquids, Sci. China: Chem., 2014, 57(12), 1766–1773 CrossRef CAS.
- Y. Jiang, W. Zhu and H. Li, et al., Oxidative Desulfurization of Fuels Catalyzed by Fenton-Like Ionic Liquids at Room Temperature, ChemSusChem, 2011, 4(3), 399–403 CrossRef CAS PubMed.
- W. Zhu, H. Li and X. Jiang, et al., Commercially available molybdic compound-catalyzed ultra-deep desulfurization of fuels in ionic liquids, Green Chem., 2008, 10(6), 641–646 RSC.
- W. Jiang, W. Zhu and H. Li, et al., Deep oxidative desulfurization of fuels catalyzed by magnetic Fenton-like hybrid catalysts in ionic liquids, RSC Adv., 2013, 3(7), 2355–2361 RSC.
- C. Komintarachat and W. Trakarnpruk, Oxidative desulfurization using Polyoxometalates, Ind. Eng. Chem. Res., 2006, 45(6), 1853–1856 CrossRef CAS.
- C. Mao, R. Zhao and X. Li, et al., Trifluoromethanesulfonic acid-based DESs as extractants and catalysts for removal of DBT from model oil, RSC Adv., 2017, 7(21), 12805–12811 RSC.
- M. Zhang, W. Zhu and S. Xun, et al., Deep oxidative desulfurization of dibenzothiophene with POM-based hybrid materials in ionic liquids, Chem. Eng. J., 2013, 220, 328–336 CrossRef CAS.
- J. Zhang, W. Zhu and H. Li, et al., Deep oxidative desulfurization of fuels by Fenton-like reagent in ionic liquids, Green Chem., 2009, 11(11), 1801–1807 RSC.
- Y. Nie, X. Gong and H. S. Gao, et al., Simultaneous desulfurization and denitrogen of liquid fuels using two functionalized group ionic liquids, Sci. China: Chem., 2014, 57(12), 1766–1773 CrossRef CAS.
- H. Azimzadeh, A. Akbari and M. R. Omidkhah, Catalytic oxidative desulfurization performance of immobilized NMP. FeCl3 ionic liquid on γ-Al2O3 support, Chem. Eng. J., 2017, 320, 189–200 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |