Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: Facile preparation of bithiazole-based material for inkjet printed light-emitting electrochemical cell†
Jingpei Huo *,
Wanying Zou,
Yubang Zhang,
Weilan Chen,
Xiaohong Hu,
Qianjun Deng and
Dongchu Chen*
*,
Wanying Zou,
Yubang Zhang,
Weilan Chen,
Xiaohong Hu,
Qianjun Deng and
Dongchu Chen*
Electrochemical Corrosion Institute, College of Materials Science and Energy Engineering, Foshan University, Foshan, 528000, People’s Republic of China. E-mail: johnhome222@163.com; cdcever@163.com
First published on 20th February 2019
Abstract
Light-emitting electrochemical cell of bithiazole-based material was fabricated by solution processing rendered high external quantum efficiency over 12.8% and luminance of 1.8 104 cd m−2.
It is well known that inkjet printing works as precise and versatile patterning method for printed electronics.1,2 As for its advantages, it is already being exploited widespread for printing electronics.3,4 Those merits are easily processed from solutions and conveniently used for air-stable electrodes.5,6
Light-emitting electrochemical cells (LECs) have emerged as an active layer,7,8 arousing tremendous attention over the years.9–11 Nevertheless, LECs are simpler than organic light-emitting diodes (OLEDs).12,13 Due to its single layer architecture, low fabrication price and operating voltages, LECs are considered as a promising, next-generation, emissive thin-film technology.14–16
The most efficient device used to date for LECs are biscyclometalated iridium(III) (Ir) complexes, because they have highly efficient and stable devices spanning the whole visible range.17–19 However, avoiding the use of Ir is strongly desired because of its high cost and limited supply.20–22 Till now, thiazole has worked as active component in a LECs system with a long-lived charge-separation molecule, without additional ions in its active layer.23–26 As for its advantages, it can reduce recombination of charge carriers and facilitate carrier transfer.27–29 More and more importance has been attached to thiazole compounds, especially bithiazole-based ones.30–33 Therefore, these have aroused interest in the syntheses of bithiazoles.34–36
Based on our previous research,37–41 the use of bithiazole ligands already explored by our group for photocatalytic technology has the advantage of an easy transformation of neutral complexes into charged ones by substitution on the N-atom of the thiazole moiety.42,43 Furthermore, we demonstrate the fabrication of LEC devices by a combination of inkjet printing and spin coating, on A4 paper substrates for low cost, disposable and flexible conductive pattern.44,45 Their corresponding material characteristics were closely investigated, a detailed report of the material properties of the resulting coatings, and an envisaged proof of concept application are disclosed in this paper. These fully printed devices demonstrate the potential upscaling of the fabrication of optoelectronic devices.
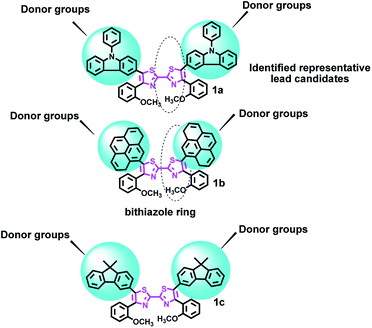
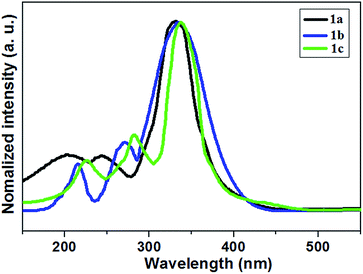
Herein we report the design for a range of bithiazole derivatives 1a–1c (Fig. 1, their synthesis is shown in Scheme S1†), and explore their properties. First of all, the UV-visible diffuse-reflectance (UV-vis DRS) spectra of those specimens at room temperature are displayed in Fig. 2 and the data are collected in Table S1.† This enhancement was ascribed to the increase of aromatic rings, which has an intense absorption in the visible-light region. And their bandgap energies (Eg) are between 2.97–3.01 eV (Table S1†), and are estimated from these absorption spectra according to the Kubelka–Munk method. Expanding the conjugated system and electron density with these donor groups, it can lead to a larger bathochromic shift of the absorption maximum.46,47
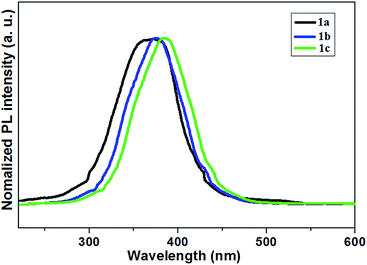
All of the three compounds are photoluminescent at room temperature, the relevant fluorescence peak maximum ranges from 360 to 398 nm (Fig. 3). They emit blue light when they are being excited. In agreement with Fig. 3, the presence of electron-releasing groups would shift the emission maximum. Among them, the most electron-donating carbazole unit would give the most red-shifted peak at 398 nm for 1c (Table S1†). The PL quantum yield (ϕPL) studies show that both 1a and 1b are as high as 62 and 78%, while another one is 85% (Table S1†). The data for their fluorescent quantum yields largely depends on interaction effect with molecules 1a–1c in the crystal packing. In this context, π–π intermolecular interactions can inhibit the fluorescence.48
It may indicate that enlarging the conjugation length and electron density with these donor groups plays an important role in increasing the ϕPL.49 Moreover, those with high ϕPL may be suitable for application as efficient light-emitting material in LECs.50
In addition, time-resolved measurements of the donor lifetime (τ) in 1a–1c were carried out, and their corresponding values are given in Table S1.† The bithiazole derivatives 1a–1c showed long-lived singlet fluorescence lifetimes (τF), ranging between 1.04 and 9.54 ns (Table S1†). This was on average more than double the fluorescence lifetime of the bithiazole starting material.51 These three samples possess relatively high decay time, as a result of their inhomogeneity.52 Compound 1c exhibited the longest fluorescence lifetime decay τF = 9.54 ns. The sensitivity of the emission to the polarity of the solvent is beneficial for an intramolecular charge transfer (ICT)-like emission.53 This difference probably accounts for the high concentration of donor units in the bithiazole backbone, low rates of intersystem crossing to reactive triplet states.48,54
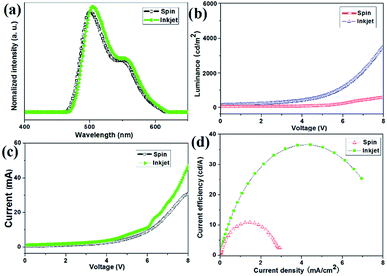
Consequently, the composite 1c was the best performing LEC and chosen for the below test. The EL spectra obtained for both devices were almost identical, with a main peak at 498 nm and other peak at 504 nm wavelength as illustrated in Fig. 4a. It may account for trapping, cavity, and self-absorption effects from within the LEC device 1c multilayer-structure. The spin-coated emission is quantified by the commission Internationale de L’Eclairage (CIE) coordinates of (0.28, 0.42), and a colour rendering index (CRI) of 65 (Table S2†). While inkjet-printed light emission with CIE coordinates of (0.34, 0.43) and a CRI value of 83 was achieved for device 1c (Table S2†). It exhibits good colour rendering indices (CRI > 70), this device exhibited a warm-white appearance, and the colour rendering is considered sufficient for indoor lighting applications.
The time-dependent brightness and current density under constant biases of 2.9–3.3 V for device 1c are shown in Fig. 4b and c. Interestingly, the device with the inkjet-printed emitting layer (EML) produced a light output of around 3600 cd m−2, whereas 4600 cd m−2 was achieved with the spin-coated EML. In other words, the device with the inkjet printed EML obtained about 78.3% of brightness compared with the reference device. The differences between the two devices regarding current efficiency (J) and power efficiency (PE) were rather closed, as listed in the Table S2.† A luminous efficiency much larger than 10 cd A−1 was achieved for the inkjet-printed EML, which tended to be 73.9%, and is similar to the spin-coated emitting layer.
The performance gap between the devices (Fig. 4d) can be attributed to differences in the height and surface roughness of the emitting layer.55 Lateral sizing histograms (Fig. S11†) show that the spin-coated EML possessed a thickness of 35 nm and a very smooth surface. The inferior performance of the inkjet-printed EML is caused by a less homogeneous surface morphology and an overall fatter layer.56 The depth of the inkjet-printed EML ranged from 25 nm (pixel centre) to 35 nm (pixel edges). As a consequence, it is an inkjet deposition process and the related evaporation dynamics reduces the light output and efficiency, but also postpones the stability of the device in the system.10,57
We then shifted our attention to the turn-on kinetics, efficiency and long-term stability of the LEC device for 1c; the typical evolution of current density and light emission is presented in Fig. 5a and b, showing a slow turn-on followed by a progressive decay over time.
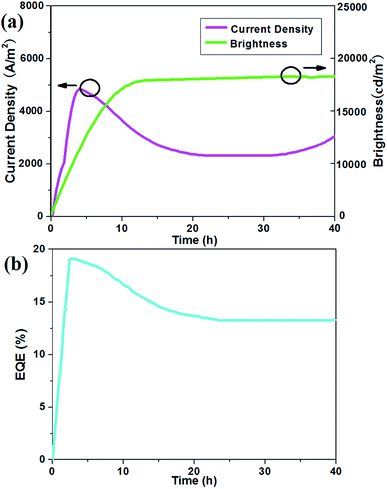 | ||
| Fig. 5 (a) Current density (closed symbols) and brightness (open symbols) versus time at 3 V for a device with the representative sample 1c; (b) EQE versus time at an applied voltage of 3 V. | ||
The build-up of the light output is synchronous with that of the current density. This time-delayed response is one of the striking features of the operation of an electrochemical cell and reflects the mechanism of device operation.22 The champion device in this set exhibited a peak power efficiency (PE) of 17.4 lm W−1 at a luminance of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2. Meanwhile, the current density began to decrease, possibly due to the electrochemical oxidation or electro corrosion that occurred on component 1c through accumulated electroinduced holes, and then it kept up a durative datum.58 Apparently it exhibited outstanding long-term operation stability beyond 40 h (Fig. 5b).
000 cd m−2. Meanwhile, the current density began to decrease, possibly due to the electrochemical oxidation or electro corrosion that occurred on component 1c through accumulated electroinduced holes, and then it kept up a durative datum.58 Apparently it exhibited outstanding long-term operation stability beyond 40 h (Fig. 5b).
The time-dependent external quantum efficiency (EQE) of LEC for the representative substrate 1c are shown in Fig. 5b. It also exhibited similar temporal tendency in device efficiency. When a bias was applied on the LECs, the EQE quickly increased since balanced carrier injection was achieved by the formation of the doped layers.59 After attaining the crest value, the device current was still rising while the EQE reduced little by little. It implied that both growing the doped layers and weakening of the EQE was resulting from exciton quenching near the bithiazlole core in persistently extended doped layers.60 Doping-induced self-absorption was rather adaptable to the temporal roll-off in device efficiency. This configuration shows the best performance (EQE = 12.8%) compared to those devices prepared with the single compounds, which is given in Table S3.† The reason may be that the steady-state recombination zone in 1c device was close to the central active layer.61 Besides it contains fluorenes on its side chains, constructing plane structure, benefiting from good electron injection abilities, leading to enhancing the EQE itself.62
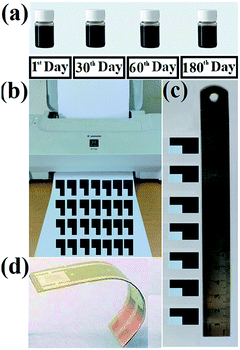
The fabrication of inkjet-printed bithiazole interdigitated electrode (IDE) is illustrated in Fig. 6. All the prepared varieties of bithiazole-based ink have been found to be highly stable with nil or miniscule precipitation in over 180 days being stored on the shelf (Fig. 6a). After HCl treatment of the paper substrate, the prepared inks have filled within ink cartridges of a low-cost desktop printer Canon iP1188 (Fig. 6b). Fig. 6c shows the photograph of inkjet-printed conductive patterns.
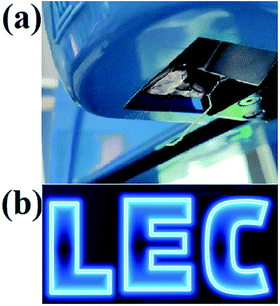
In addition, the most attractive prospect of the bilayer device structure at this stage is the possibility for patterned emission for the creation of a static display. Fig. 7 presents a photograph of a small portion of a larger static display, with a resolution of 170 PPI, which repeatedly exhibits a message in the form of the word “LEC”. As shown in Fig. 7, the pixel array produced are fairly luminous. The EL spectra were collected from each pixel on the substrate, the emission peak wavelength was about 510 nm and the full width at half maximum was about 170 nm. The low variation in emission intensity in the different pixels implies a small variation in thickness of the solution-coated layers. More specifically, if we assume a minimum diameter of 20 μm for an inkjetted electrolyte droplet, and a smallest inter-droplet distance of 10 μm, we attain a pitch of 30 μm, which corresponds to a high display resolution of 850 PPI. Finally, we draw attention to the herein presented static-display LEC that comprises solely air-stabile materials, and that we routinely fabricate the bilayer stack under ambient atmosphere.
Conclusions
In this work, we demonstrate the fabrication of conductive patterns by inkjet printing the bithiazole ink. The developed supercapacitor device displays distinctly improved performance with onset voltages for the current as well as for the luminance slightly above 3 V and remarkable enhanced light output. Further, by depositing electrolyte droplets in a well-defined binary lattice with an inkjet printer, we are able to realize uniform light emission. It is achieving a brightness of >18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 and stable > 1000 cycles, displaying static messages with a pixel density of 170 PPI and high contrast. With such superior characteristics, the emitter can be a promising candidate in realizing energy-saving, high-quality full-colour displays and solid-state lighting by utilizing inkjet-printed technology.
000 cd m−2 and stable > 1000 cycles, displaying static messages with a pixel density of 170 PPI and high contrast. With such superior characteristics, the emitter can be a promising candidate in realizing energy-saving, high-quality full-colour displays and solid-state lighting by utilizing inkjet-printed technology.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the High-Level Talent Start-Up Research Project of Foshan University (Gg040947), Guangdong Natural Science Foundation of China (Grant No. 2018A030310350), the Key Project of Department of Education of Guangdong Province (2016GCZX008), the Key Research Platform Project of Department of Education of Guangdong Province (gg041002), the Project of Engineering Research Centre of Foshan (20172010018) for financial support. We thank Benjamin J. Deibert for proofreading this article.Notes and references
- T. J. Wallin, J. Pikul and R. F. Shepherd, Nat. Rev. Mater., 2018, 3, 84 CrossRef.
- Y. Kim, H. Yuk, R. K. Zhao, S. A. Chester and X. H. Zhao, Nature, 2018, 558, 274 CrossRef CAS PubMed.
- M. Tavakoli, M. H. Malakooti, H. Paisana, Y. Ohm, D. G. Marques, P. A. Lopes, A. P. Piedade, A. T. de Almeida and C. Majidi, Adv. Mater., 2018, 30, 1801852 CrossRef PubMed.
- Y. Wang, Y.-Z. Zhang, D. Dubbink and J. E. ten Elshof, Nano Energy, 2018, 49, 481 CrossRef CAS.
- G. H. Hu, J. Kang, L. W. T. Ng, X. X. Zhu, R. C. T. Howe, C. G. Jones, M. C. Hersam and T. Hasan, Chem. Soc. Rev., 2018, 47, 3265 RSC.
- B. H. Wang, W. Huang, L. F. Chi, M. Al-Hashimi, T. J. Marks and A. Facchetti, Chem. Rev., 2018, 118, 5690 CrossRef CAS PubMed.
- E. M. Zhao, Y. F. Zhang, J. Mehl, H. Park, M. A. Lalwani, J. E. Toettcher and J. L. Avalos, Nature, 2018, 555, 683 CrossRef CAS PubMed.
- S. H. Kim, C. S. Haines, N. Li, K. J. Kim, T. J. Mun, C. Choi, J. T. Di, Y. J. Oh, J. P. Oviedo, J. Bykova, S. L. Fang, N. Jiang, Z. F. Liu, R. Wang, P. Kumar, R. Qiao, S. Priya, K. Cho, M. Kim, M. S. Lucas, L. F. Drummy, B. Maruyama, D. Y. Lee, X. Lepró, E. L. Gao, D. Albarq, R. Ovalle-Robles, S. J. Kim and R. H. Baughman, Science, 2017, 357, 773 CrossRef CAS PubMed.
- S. Tang, A. Sandström, P. Lundberg, T. Lanz, C. Larsen, S. van Reenen, M. Kemerink and L. Edman, Nat. Commun., 2017, 8, 1180 CrossRef PubMed.
- H. Yang and M. A. M. Gijs, Chem. Soc. Rev., 2018, 47, 1391 RSC.
- H. Cho, J. S. Kim, C. Wolf, Y.-H. Kim, H. J. Yun, S.-H. Jeong, A. Sadhanala, V. Venugopalan, J. W. Choi, C.-L. Lee, R. H. Friend and T.-W. Lee, ACS Nano, 2018, 12, 2883 CrossRef CAS PubMed.
- J. Liu, J. Oliva, K. Tong, F. C. Zhao, D. Chen and Q. B. Pei, Sci. Rep., 2017, 7, 1524 CrossRef PubMed.
- J. Zimmermann, L. Porcarelli, T. Rödlmeier, A. Sanchez-Sanchez, D. Mecerreyes and G. Hernandez-Sosa, Adv. Funct. Mater., 2018, 28, 1705795 CrossRef.
- S. Tang, A. Sandström, P. Lundberg, T. Lanz, C. Larsen, S. van Reenen, M. Kemerink and L. Edman, Nat. Commun., 2017, 8, 1190 CrossRef PubMed.
- H. Cho, Y.-H. Kim, C. Wolf, H.-D. Lee and T.-W. Lee, Adv. Mater., 2018, 30, 1704587 CrossRef PubMed.
- C. D. Ertl, C. Momblona, A. Pertegás, J. M. Junquera-Hernández, M.-G. La-Placa, A. Prescimone, E. Ortí, C. E. Housecroft, E. C. Constable and H. J. Bolink, J. Am. Chem. Soc., 2017, 139, 3237 CrossRef CAS PubMed.
- C. E. Housecroft and E. C. Constable, Coord. Chem. Rev., 2017, 350, 155 CrossRef CAS.
- P. Murto, S. Tang, C. Larsen, X. F. Xu, A. Sandström, J. Pietarinen, B. Bagemihl, B. A. Abdulahi, W. Mammo, M. R. Andersson, E. Wang and L. Edman, ACS Appl. Energy Mater., 2018, 1, 1753 CrossRef CAS.
- J. Yeonah, C. D. Sunesh, R. K. Chitumalla, J. Jang and Y. Choe, Org. Electron., 2018, 54, 167 CrossRef CAS.
- J. E. Namanga, N. Gerlitzki, V. Smetana and A.-V. Mudring, ACS Appl. Mater. Interfaces, 2018, 10, 11026 CrossRef CAS PubMed.
- M. Martínez-Alonso, J. Cerdá, C. Momblona, A. Pertegás, J. M. Junquera-Hernández, A. Heras, A. M. Rodríguez, G. Espino, H. Bolink and E. Ortí, Inorg. Chem., 2017, 56, 10298 CrossRef PubMed.
- S. H. Kong, J. I. Lee, S. Kim and M. S. Kang, ACS Photonics, 2018, 5, 267 CrossRef CAS.
- M. Nazima, S. Ameen, M. S. Akhtar and H.-S. Shin, Sol. Energy, 2018, 171, 366 CrossRef.
- A. K. Pal, D. B. Cordes, A. M. Z. Slawin, C. Momblona, A. Pertegás, E. Ortí, H. J. Bolink and E. Zysman-Colman, RSC Adv., 2017, 7, 31833 RSC.
- Z. B. Yuan, C. Buckley, S. Thomas, G. Y. Zhang, I. Bargigia, G. Wang, B. Y. Fu, C. Silva, J.-L. Brédas and E. Reichmanis, Macromolecules, 2018, 51, 7320 CrossRef CAS.
- M. T. Nguyen, R. A. Jones and B. J. Holliday, Coord. Chem. Rev., 2018, 377, 237 CrossRef CAS.
- J. Gruszczyk, U. Kanjee, L.-J. Chan, S. Menant, B. Malleret, N. T. Y. Lim, C. Q. Schmidt, Y.-F. Mok, K.-M. Lin, R. D. Pearson, G. Rangel, B. J. Smith, M. J. Call, M. P. Weekes, M. D. W. Griffin, J. M. Murphy, J. Abraham, K. Sriprawat, M. J. Menezes, M. U. Ferreira, B. Russell, L. Renia, M. T. Duraisingh and W.-H. Tham, Science, 2018, 359, 48 CrossRef CAS PubMed.
- B. Krismer, C. Weidenmaier, A. Zipperer and A. Peschel, Nat. Rev. Microbiol., 2017, 15, 675 CrossRef CAS PubMed.
- B. Lüscher, M. Bütepage, L. Eckei, S. Krieg, P. Verheugd and B. H. Shilton, Chem. Rev., 2018, 118, 1092 CrossRef PubMed.
- D. Yang, Hindra, L.-B. Dong, I. Crnovcic and B. Shen, J. Antibiot., 2017, 71, 97 CrossRef PubMed.
- E. Azzali, D. Machado, A. Kaushik, F. Vacondio, S. Flisi, C. S. Cabassi, G. Lamichhane, M. Viveiros, G. Costantino and M. Pieroni, J. Med. Chem., 2017, 60, 7108 CrossRef CAS PubMed.
- A. K.-W. Chan, M. Ng, Y.-C. Wong, M.-Y. Chan, W.-T. Wong and V. W.-W. Yam, J. Am. Chem. Soc., 2017, 139, 10750 CrossRef CAS PubMed.
- S. Benz, J. López-Andarias, J. Mareda, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 2017, 40, 812 CrossRef PubMed.
- Y. Wang, T. Hasegawa, H. Matsumoto, T. Mori and T. Michinobu, Adv. Funct. Mater., 2018, 28, 1706517 CrossRef.
- H. Aoki, H. Saito, Y. Shimoyama, J. Kuwabara, T. Yasuda and T. Kanbara, ACS Macro Lett., 2018, 7, 90 CrossRef CAS.
- S. H. Wang, H. D. Sun, T. Erdmann, G. Wang, D. Fazzi, U. Lappan, Y. Puttisong, Z. H. Chen, M. Berggren, X. Crispin, A. Kiriy, B. Voit, T. J. Marks, S. Fabiano and A. Facchetti, Adv. Mater., 2018, 30, 1801898 CrossRef PubMed.
- J. P. Huo, Q. J. Deng, T. Fan, G. Z. He, X. H. Hu, X. X. Hong, H. Chen, S. H. Luo, Z. Y. Wang and D. C. Chen, Polym. Chem., 2017, 8, 7438 RSC.
- J. P. Huo, H. W. Hu, M. Zhang, X. H. Hu, M. Chen, D. C. Chen, J. W. Liu, G. F. Xiao, Y. Z. Wang and Z. L. Wen, RSC Adv., 2017, 7, 2281 RSC.
- J. P. Huo, H. W. Hu, G. Z. He, X. X. Hong, Z. H. Yang, S. H. Luo, X. F. Ye, Y. L. Li, Y. B. Zhang, M. Zhang, H. Chen, T. Fan, Y. B. Zhang, B. Y. Xiong, Z. Y. Wang, Z. B. Zhu and D. C. Chen, Appl. Surf. Sci., 2017, 423, 951 CrossRef CAS.
- J. P. Huo, Z. D. Hu, D. C. Chen, S. H. Luo, Z. Y. Wang, Y. H. Gao, M. Zhang and H. Chen, ACS Omega, 2017, 2, 5557 CrossRef CAS.
- J. P. Huo and H. P. Zeng, J. Mater. Chem. A, 2015, 3, 6258 RSC.
- L. M. T. FrijaArmando, J. L. Pombeiro and M. N. Kopylovich, Coord. Chem. Rev., 2016, 308, 32 CrossRef.
- X. Y. Chen, G. H. Zhou, S. Mao and J. H. Chen, Environ. Sci.: Nano, 2018, 5, 837 RSC.
- J. Freudenberg, D. Jänsch, F. Hinkel and U. H. F. Bunz, Chem. Rev., 2018, 118, 5598 CrossRef CAS PubMed.
- D. R. Wang, Y. K. Zhang, X. Lu, Z. J. Ma, C. Xie and Z. J. Zheng, Chem. Soc. Rev., 2018, 47, 4611 RSC.
- J. W. Yu, J. L. Ornelas, Y. M. Tang, M. A. Uddin, H. Guo, S. M. Yu, Y. L. Wang, H. Y. Woo, S. M. Zhang, G. C. Xing, X. G. Guo and W. Huang, ACS Appl. Mater. Interfaces, 2017, 9, 42167 CrossRef CAS PubMed.
- H.-L. Su, D. N. Sredojevic, H. Bronstein, T. J. Marks, B. C. Schroeder and M. Al-Hashimi, Macromol. Rapid Commun., 2017, 38, 1600610 CrossRef PubMed.
- M. A. Potopnyk, R. Lytvyn, Y. Danyliv, M. Ceborska, O. Bezvikonnyi, D. Volyniuk and J. V. Gražulevičius, J. Org. Chem., 2018, 83, 1095 CrossRef CAS PubMed.
- S. Mukherjee, D. E. Torres and E. Jakubikova, Chem. Sci., 2017, 4, 8115 RSC.
- T. Hosokai, H. Matsuzaki, H. Nakanotani, K. Tokumaru, T. Tsutsui, A. Furube, K. Nasu, H. Nomura, M. Yahiro and C. Adachi, Sci. Adv., 2017, 3, e1603282 CrossRef PubMed.
- H. Leopold and T. Strassner, Dalton Trans., 2017, 46, 7800 RSC.
- A. N. Woodward, J. M. Kolesar, S. R. Hall, N.-A. Saleh, D. S. Jones and M. G. Walter, J. Am. Chem. Soc., 2017, 139, 8467 CrossRef CAS PubMed.
- K. I. Lugovik, A. K. Eltyshev, P. O. Suntsova, P. A. Slepukhin, E. Benassi and N. P. Belskaya, Chem.–Asian J., 2018, 13, 311 CrossRef CAS PubMed.
- P. J. Waller, Y. S. AlFaraj, C. S. Diercks and N. N. Jarenwattananon, J. Am. Chem. Soc., 2018, 140, 9099 CrossRef CAS PubMed.
- S. Yamamoto, N. Nishina, J. Matsui, T. Miyashita and M. Mitsuishi, Langmuir, 2018, 34, 10491 CrossRef CAS PubMed.
- F. Mathies, H. Eggers, B. S. Richards, G. Hernandez-Sosa, U. Lemmer and U. W. Paetzold, ACS Appl. Energy Mater., 2018, 1, 1834 CrossRef CAS.
- J. Kim, W. Yang, Y. Oh, H. Lee, S. Lee, H. Shin, J. Kim and J. Moon, J. Mater. Chem. A, 2017, 5, 2180 RSC.
- L. B. Boinovich, K. A. Emelyanenko, A. G. Domantovsky, E. V. Chulkova, A. A. Shiryaev and A. M. Emelyanenko, Adv. Mater. Interfaces, 2018, 5, 1801099 CrossRef.
- J.-H. Jou, J.-L. Li, S. Sahoo, D. K. Dubey, R. A. K. Yadav, V. Joseph, K. R. Justin Thomas, C.-W. Wang, J. Jayakumar and C.-H. Cheng, J. Phys. Chem. C, 2018, 122, 24295 CrossRef CAS.
- T. M. Zhang, C. S. Shi, C. Y. Zhao, Z. B. Wu, J. S. Chen, Z. Y. Xie and D. G. Ma, ACS Appl. Mater. Interfaces, 2018, 10, 8148 CrossRef CAS PubMed.
- E. Fresta and R. D. Costa, J. Mater. Chem. C, 2017, 5, 5643 RSC.
- H. Shahroosvand, S. Abaspour, B. Pashaei, E. Radicchi, F. D. Angelis and F. Bonaccorso, Chem. Commun., 2017, 53, 6211 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00093c |
| This journal is © The Royal Society of Chemistry 2019 |