Open Access Article
Open Access ArticleApplication of the combination of ball-milling and hot-melt extrusion in the development of an amorphous solid dispersion of a poorly water-soluble drug with high melting point
Wenling Fan *abc,
Wenjing Zhu
*abc,
Wenjing Zhu ab,
Xinyi Zhang
ab,
Xinyi Zhang ab,
Yan Xu
ab,
Yan Xu ab and
Liuqing Di
ab and
Liuqing Di ab
ab
aLaboratory of Pharmaceutical Engineering, College of Pharmacy, Nanjing University of Chinese Medicine, Nanjing 210023, China. E-mail: fanwl.happy@163.com
bInstitute of Jiangsu Engineering Research Center for Efficient Delivery System of Traditional Chinese Medicine, Nanjing 210023, China
cJiangsu Collaborative Innovation Center of Chinese Medicine Resources Industrialization, Nanjing 210023, China
First published on 17th July 2019
Abstract
The aim of the study was to develop an amorphous solid dispersion of a poorly water-soluble drug with high melting point by ball milling and hot melt extrusion as a co-processing method. Solid dispersion systems were prepared by ball milling-hot melt extrusion and then compared with those prepared with hot melt extrusion. The effects of three process parameters in the co-processing method, namely, barrel temperature, screw speed, and cooling rate, were systematically studied. The physical state of prepared solid dispersion was characterized by differential scanning calorimetry, X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, phase solubility, and dissolution study. The Resveratrol–Eudragit® EPO system exhibited good miscibility and significant dissolution enhancement. Resveratrol in the amorphous solid dispersion existed in an amorphous state and had molecular interactions with Eudragit® EPO. Stability studies showed no apparent difference in the physical state of the solid dispersion after 6 months. In conclusion, combining ball milling with hot melt extrusion is a promising method for preparing the amorphous solid dispersion of a poorly water-soluble drug with high melting point.
Introduction
Approximately 70% of active pharmaceutical ingredients (APIs) have poor aqueous solubility, and thus their development in the pharmaceutical industry is limited.1 Overcoming the obstacle of poor water solubility is necessary. Various methods for improving the dissolution property of poorly water-soluble drugs have been developed, including salt formation; particle size reduction; micro/nano emulsion; solubilization with a surfactant/hydrotrope; complexation; solid dispersion; and lipid formulation.2–6 Solid dispersion is one of the most commonly used pharmaceutical approaches owing to its simple preparation procedures.7A large number of studies have proven that solid dispersion is effective in improving the solubility of poorly water-soluble drugs, especially amorphous solid dispersion drugs.8,9 Solid dispersion drugs can be classified into crystalline or amorphous solid dispersion drugs depending on their forms. The crystalline drugs have high purity and physical or chemical stability.9 However, crystalline drugs need to break the lattice energy barrier in the solubility/dissolution process.10 Amorphous drugs exhibit disordered structures in contrast to crystalline drugs and thus possess higher free energy (thermodynamic driving force) and water solubility.11 Therefore, amorphous solid dispersion is highly desirable for the improvement of the dissolution properties of poorly water-soluble drugs.
Hot melt extrusion (HME) and spray drying technology are the methods for preparing amorphous solid dispersion systems that can be applied to industrial production.12 Hot melt extrusion technology offers a prominent advantage over spray drying technology: the absence of solvents during processing. During the hot melt extrusion process, thermal and mechanical energy from heated barrels and rotating screws converts a drug and polymer blend into an amorphous solid dispersion. One major drawback of hot melt extrusion is the thermal degradation of drugs or polymers.13 High process temperature is required to convert a drug with a high melting point (above 200 °C) into amorphous one but may lead to the polymer or drug degradation.1 Strategies for preventing degradation during the extrusion of an amorphous solid dispersion for a drug substance with high melting point are few. Lakshman et al. used the solvent method to convert a drug with high melting point to an amorphous form before preparing a solid dispersion by hot melt extrusion.13 Hughey et al. utilized KinetiSol Dispersing-a patent technology, which is about a high shear with a very short residence time, to prepare an amorphous solid dispersion for a drug with high melting point.14 Zhang et al. used a carrier and a plasticizer to reduce the melting point of a drug; they found that an amorphous solid dispersion can be prepared at low process temperature.15 However, a solvent is introduced in the first method, and achieving the amorphous form of some drugs is difficult. In the second method, the type of carrier or plasticizer is limited, and determining suitable ones that can reduce the melting point of the drug is uncertain. Hence, exploring the application of hot melt extrusion for the preparation of the amorphous solid dispersion of drugs with high melting points is necessary.
Resveratrol (RES) [3,5,4′-trihydroxystilbene] is a naturally polyphenolic compound found in many plants, including grapes, peanuts, Polygonum cuspidatum.16 Its structure is shown in Fig. 1. This natural compound has aroused public concern due to its broad physiological activities, especially antitumor biological activity. RES is a new green anticancer drug apart from paclitaxel, which has inhibitory effects on a variety of cancers, such as hepatic cancer, lymph cancer, and ovarian cancer.17,18 RES demonstrates several cellular mechanisms for inhibiting tumor initiation, promotion, and development in the process of carcinogenesis.19 Unfortunately, despite the significant anticancer effect of RES, its therapeutic efficiency has been highly limited due to its low aqueous solubility (0.05 mg ml−1 in water) and low oral bioavailability (1.5%).16,20 Numerous attempts to increase RES solubility have been made, including the use of liposomes, nanoparticles, cyclodextrin complexes, and solid dispersion.21–24 Resveratrol solid dispersion systems prepared by solvent methods, such as solvent evaporation and fluid bed coating techniques have been reported.
In the present study, a nonhygroscopic hydrophilic polymer Eudragit® EPO (EPO) (Fig. 2), which is a cationic copolymer with dimethyl-amino ethyl methacrylate as a functional group, was selected as the carrier. The polymer is pH dependent and has maximum solubility in gastric fluids with pH of 5.25
The prime objective was to prepare an amorphous solid dispersion through the co-processing of ball milling and hot melt extrusion. First, the reformulation studies of the drug and polymer were researched. Then, solid dispersion systems were prepared by hot melt extrusion and the method combining ball milling and hot melt extrusion. The solubilization of RES into EPO was investigated by screening the operation parameters and physicochemical characterization through various analytical techniques. Six-month stability was performed according to the ICH guidelines.
Materials and methods
Materials
RES with a purity of 98% was obtained from Nanjing Zelang Pharmaceutical Technology Co., Ltd. (Nanjing, China). EPO (poly(butyl methacrylate, (2-dimethylaminoethyl) meth-acrylate, methyl methacrylate) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was kindly donated by Evonik Industries Ltd. (Darmstadt, Germany). The other reagents used were of analytical reagent grade.
1) was kindly donated by Evonik Industries Ltd. (Darmstadt, Germany). The other reagents used were of analytical reagent grade.
Thermogravimetric analysis (TGA)
Degradation temperatures of RES, EPO, PM and PM (ball-milling) were performed by a Pyris 1 thermogravimetric analyzer (PerKin Elmer, Waltham, USA). Approximately 10 mg of the sample was placed in a small aluminum pan. Samples were heated from 25 °C to 500 °C at a heating rate of 10 °C min−1.High-performance liquid chromatography (HPLC)
The content of RES in SD(HME) and SDs(co-process) samples and the dissolution samples were determined by HPLC in Waters 2695. The column used was Hedera ODS-2 (250 mm × 4.6 mm, 5 μm). A mobile phase comprising water and methanol in a volume ratio of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 was used. The wavelength of UV detection was 306 nm, and the isocratic flow rate was 1 ml min−1.
45 was used. The wavelength of UV detection was 306 nm, and the isocratic flow rate was 1 ml min−1.
Preparation of solid dispersion systems
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (w/w, 10 g
1 ratio (w/w, 10 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g) was mixed with mortar and pestle and subsequently fed manually into the hopper of the extruder at barrel temperature 160 °C and screw speed of 120 rpm. The extrudates were solidified in liquid nitrogen and collected, then ground softly in liquid nitrogen with pestle and mortar, passed through an 80 mesh sieve, and kept in a desiccator at room temperature for further analysis.
10 g) was mixed with mortar and pestle and subsequently fed manually into the hopper of the extruder at barrel temperature 160 °C and screw speed of 120 rpm. The extrudates were solidified in liquid nitrogen and collected, then ground softly in liquid nitrogen with pestle and mortar, passed through an 80 mesh sieve, and kept in a desiccator at room temperature for further analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (w/w, 10 g
1 ratio (w/w, 10 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 g) was blended in a XQM-4L vertical planetary ball milling prior to extrusion (Tianchuang Powder Technology Co. Ltd., Changsha, China). Each grinding tank (volume of 50 ml) was filled with agate grinding balls (8 mm φ, number: 12), the mass ratio of the ball and the mixture was nearly 1
10 g) was blended in a XQM-4L vertical planetary ball milling prior to extrusion (Tianchuang Powder Technology Co. Ltd., Changsha, China). Each grinding tank (volume of 50 ml) was filled with agate grinding balls (8 mm φ, number: 12), the mass ratio of the ball and the mixture was nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The effect of time and frequency of the ball milling process on the extrudates were determined. The ball milling frequency was set 20, 30, or 40 Hz and the milling time to 30 min. Then, the milling time was set to 30, 40, and 50 min at milling frequency of 30 Hz. The samples were fed into the extrusion system. The extruder was run at 160 °C and 120 rpm. The extrudates were milled and sieved after cooling in liquid nitrogen and used for in vitro dissolution testing.
1. The effect of time and frequency of the ball milling process on the extrudates were determined. The ball milling frequency was set 20, 30, or 40 Hz and the milling time to 30 min. Then, the milling time was set to 30, 40, and 50 min at milling frequency of 30 Hz. The samples were fed into the extrusion system. The extruder was run at 160 °C and 120 rpm. The extrudates were milled and sieved after cooling in liquid nitrogen and used for in vitro dissolution testing.The mixture was ball-milled into power under the best operation conditions. The mixture power was manually fed into the extruder under elevated barrel temperature (120 °C, 140 °C, and 160 °C) and screw speed (100, 120, and 140 rpm). The extrudates were collected, cooled under different conditions, milled, and sieved (in the same manner as those prepared through hot-melt extrusion), then kept into a desiccator at room temperature for future analysis.
Differential scanning calorimetry (DSC)
Whether the samples contain crystals was determined with a NETZSCH 200F3 differential scanning calorimeter. A 5 mg powder sample was packed into an aluminum pan with a lid. A pinhole was made in the lid to allow moisture to escape. The experiments were conducted at a heating rate of 10 °C min−1 from 30 °C to 300 °C under nitrogen atmosphere. All the experiments were run in triplicate. The data were evaluated with NETZSCH Proteus Analysis Software (NETZSCH Group, Selb, Germany)X-ray powder diffraction (PXRD)
The molecular transformation of the drug from crystalline to amorphous state was determined. The samples were measured with a D/max 2500 X-ray powder diffractometer (Rigaku, Tokyo, Japan). A Cu Kα radiation was used at 40 kV and 100 mA. The samples were scanned in the reflection mode from 3° to 40° with a scanning step size of 0.02°.Fourier transform infrared spectroscopy (FTIR)
The presence of molecular interactions between the drug and polymer was determined with a NEXUS870 Fourier transform infrared spectrometer (NICOLET, Wisconsin, USA). The samples were gently mixed with dry potassium bromide and then compressed. Then, the samples were analyzed from 4000 cm−1 to 400 cm−1, and each disc was scanned 10 times at a resolution of 2 cm−1.Scanning electron microscopy (SEM)
The surface morphology of each sample was examined by an S-34000 II scanning electron microscope (Hitachi, Tokyo, Japan). A small amount of powder was sprinkled onto a double sided electrically conductive adhesive sheet mounted on an aluminum stub. The powder was subsequently coated with a layer of platinum and then examined under a vacuum. The scanning electron microscope was operated at an accelerating voltage of 5 kV.In vitro dissolution study
In vitro dissolution study was carried out with a ZRS-8 GD paddle dissolution apparatus (Tiandatianfa, Tianjin, China) with paddle rotation speed of 100 rpm at 37 ± 0.5 °C in 900 ml of pH 1.2 HCl solution according to Ch.P. Samples equivalent to 12 mg of RES were added to the dissolution apparatus. Then, 3 ml of each samples was withdrawn at predetermined time intervals, and an equal amount of fresh medium was added to the dissolution medium vessel. The collected samples were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, and the supernatant was collected and detected by HPLC. The quantitation of RES was determined, and each experiment was carried out in triplicate.
000 rpm for 10 min, and the supernatant was collected and detected by HPLC. The quantitation of RES was determined, and each experiment was carried out in triplicate.
Preparation of the drugs sample for DSC, PXRD, FTIR, SEM and dissolution study
The original drug RES was ground and sieved (same as Section “Hot-melt extrusion”) and then kept in desiccator at room temperature for future analysis.Phase solubility study of RES
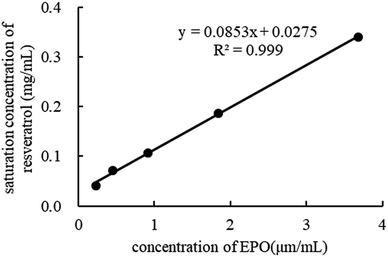
An excess amount of crystalline RES was dispersed in the pH 1.2 HCl solution in the presence or absence of EPO at 37 °C. Then, 5 ml from each sample was withdrawn from each vessel after 24 h and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant was determined by HPLC, and RES was quantified. Each experiment was carried out in triplicate.
000 rpm for 10 min. The supernatant was determined by HPLC, and RES was quantified. Each experiment was carried out in triplicate.
Accelerate stability study
The physical stability of the extrudates was investigated by storing them in a Climacell 222 humidity chamber (MMM, Germany) at 40 °C and 75% relative humidity (RH) for six months. DSC and PXRD studies were utilized to determine the crystallinity of RES regularly.Results and discussion
Thermogravimetric analysis (TGA)
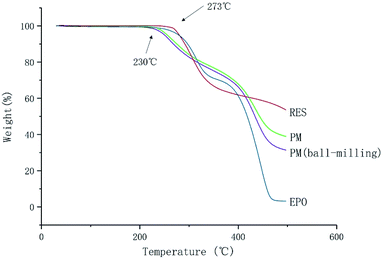
The melting point (Tm) of RES is 253 °C. It is observed that no apparent weight loss of RES and EPO under 273 °C in Fig. 3, indicating lack of significant decomposition under 273 °C. The result implied that no degradation occurred under the melting temperature of RES, and degradation occurred above 273 °C. However, significant decomposition happened at 230 °C for PM and PM (ball-milling), the degradation temperature decreased, reasons need further research and discovery. Lin et al. found that intramolecular ester condensation forms six-membered cyclic anhydrides when EPO is exposed to elevated temperatures (above 180 °C).26 The side chain cyclization of EPO under high temperatures may have an effect on the properties of EPO, thus affecting the ultimate product. Therefore, the process temperature for the preparation of chemically stable solid dispersion systems should not exceed 180 °C.Optimization of preparation of solid dispersion systems
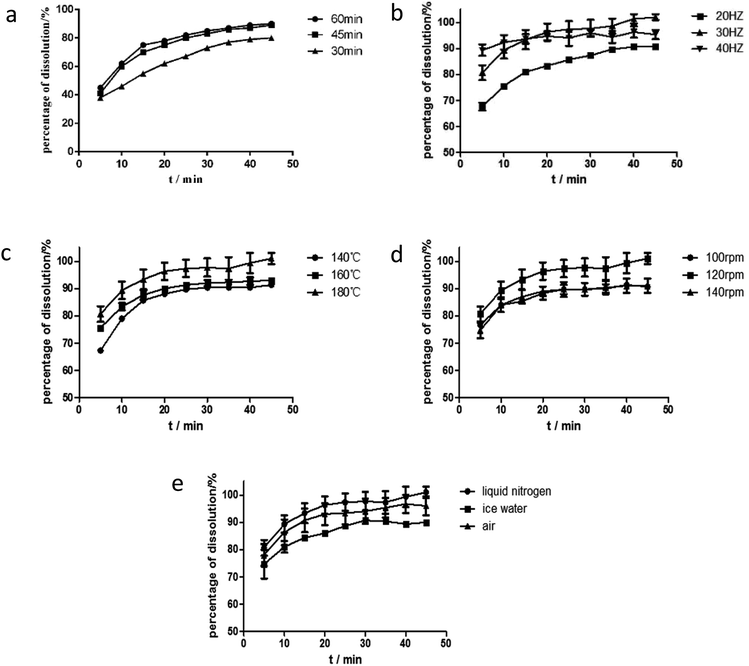
RES has a high melting point and large crystal lattice energy. A large amount of energy is required for the breaking of the crystal lattice of RES. Given that a considerably high temperature result in thermal degradation, preparing amorphous solid dispersion through hot melt extrusion is difficult. The method must be combined with other methods, such as ball milling, which is one of the preparation methods that do not require solvents and mainly act on the mixture through shear force. However, the energy provided by the shearing force is insufficient to break all the lattices of a drug, and the heat generated in the shearing process promotes the recrystallization of the drug. In the ball milling technique, shear force breaks part of the lattice and increases distributive mixing and dispersive mixing steps. These features may be conducive to the preparation of amorphous solid dispersion systems. The processing parameters for hot melt extrusion, as a continuous process, have an important role in controlling the quality of the extrudates.27 Several reports illustrated the significant impact of processing parameters on the physicochemical properties of solid dispersion systems.28,29 Among them, feed rate, barrel temperature, screw speed, and screw configuration are the primary and vital process parameters. Meanwhile, degass, torque, and cooling type have effects on the final products. In the study, the effects of preparation methods and operation parameters, such as barrel temperature, screw speed, and cooling rate were considered in the examination of the extrudate quality, and a dissolution test was used as an evaluation index. A DSC study was performed during the optimization of the solid dispersion. RES content in SD(HME) and SD(co-process) (dissolved in methanol) was assayed by HPLC, the quantitation of RES was determined, and each experiment was carried out in triplicate.Eventually, the optimum process parameter for the solid dispersion was as follows: the mixture was firstly mixed by ball milling for 45 min, then fed into the extruder at a barrel temperature of 160 °C and screw speed of 120 rpm. The extrudates were cooled in liquid nitrogen, then milled and sieved.
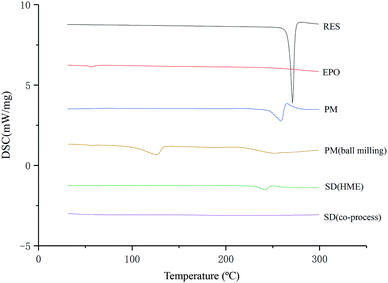
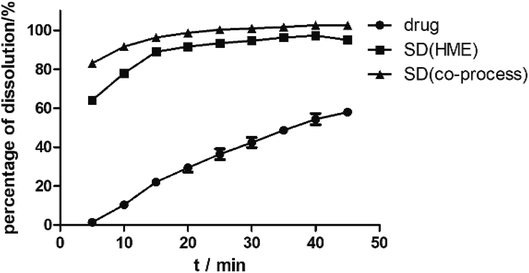
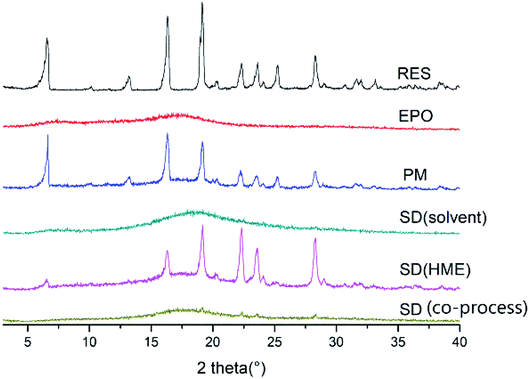
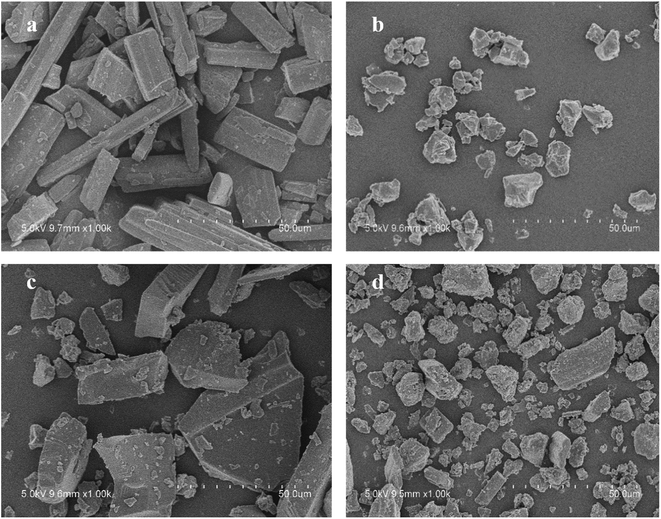
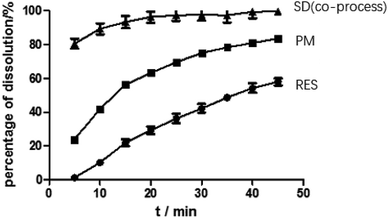
As illustrated in Fig. 4, the physical mixture possessed an offset and smaller crystallization peak than a pure a drug. Compared with the physical mixture, the mixture powder prepared through ball milling had a smaller crystallization peak. Moreover, the molten crystal peak of the drug moved toward the lower temperature region. The crystal lattice of the drug was partly destroyed, and some drugs were converted into an amorphous form. An interaction existed between the drug and polymer. As shown in Fig. 4, the solid dispersion prepared by hot melt extrusion alone had a smaller crystal melting peak, which indicated that the crystallinity of the drug in the solid dispersion was reduced. However, the drug was incompletely transformed into an amorphous form, partly in the form of microcrystalline, whereas the melting peak of the drug almost disappeared in the solid dispersion prepared by the co-processing method, indicating few drug existed in crystal form. As shown in Fig. 5, the release rate of the extrudates prepared by the co-processing method was higher than that of the extrudates prepared by hot melt extrusion alone. Thus, the amorphous drug is easier to dissolve than the crystal one, and the co-process is the best preparation method for the RES solid dispersion.
Physical characteristics
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
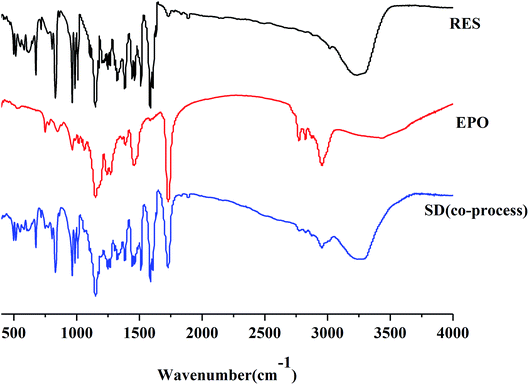
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 drug–carrier interactions. This finding is in agreement with the FTIR results.39–41
1 drug–carrier interactions. This finding is in agreement with the FTIR results.39–41
Stability study
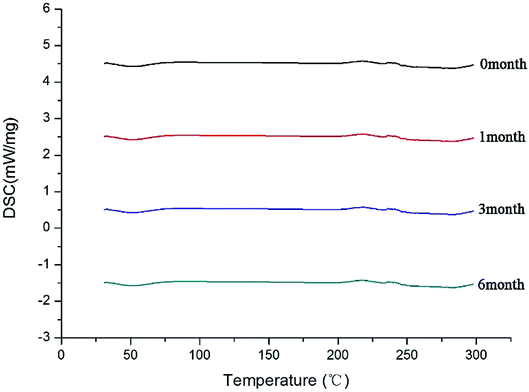
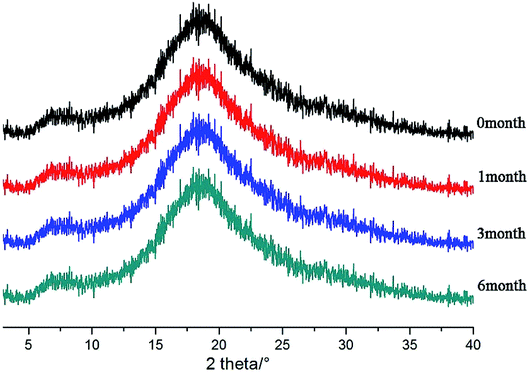
After the SDs were subjected to high temperature and humidity, the stability of the SDs were determined by DSC and PXRD. The results are shown in Fig. 12 and 13. The DSC and PXRD results on aged samples confirmed that no recrystallization of the amorphous drug occurred in the SDs, suggesting good physical stability. This condition can be ascribed to the molecular interaction and good miscibility between the drug and polymer.Conclusions
The amorphous solid dispersion was developed for the enhancement of the solubility and dissolution rate of RES. In the study, the solid dispersion systems prepared by hot melt extrusion alone and those by ball milling and hot melt extrusion were compared. Due to the process temperature limitation of hot melt extrusion, the amorphous solid dispersion cannot be prepared by hot melt extrusion alone and can be prepared by the co-processing method. The process parameters include barrel temperature, screw speed, and cooling rate, which affect the dissolution of extrudates and are critical to the optimization of process parameters. The RES in the optimum solid dispersion appeared as an amorphous form and interacted with EPO and showed higher dissolution property than pure RES. Thus, EPO is a promising carrier for RES, and the processing parameters of HME can be adjusted for the successful release of RES.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was funded by National Natural Science Fundation of China (No. 30801552 & No. 81274095), the third key project funded by Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization (No. ZDXM-3-10), the 55th Postdoctoral Project (No. 021062001001), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX18_1629).References
- A. Haser, S. Huang, T. Listro, D. White and F. Zhang, Int. J. Pharm., 2017, 524, 55 CrossRef CAS.
- C. Leuner and J. Dressman, Eur. J. Pharm. Biopharm., 2000, 50, 47–60 CrossRef CAS.
- J. H. Seo, J. B. Park, W. K. Choi, S. Park, J. S. Yun, E. Oh and S. K. Bae, Drug Des., Dev. Ther., 2015, 3961–3968 CAS.
- A. Sarkar and S. Rohani, J. Pharm. Biomed. Anal., 2015, 110, 93–99 CrossRef CAS.
- W. S. Cheow and K. Hadinoto, J. Colloid Interface Sci., 2012, 367, 518–526 CrossRef CAS.
- B. Lang and J. W. Mcginity, Mol. Pharm., 2014, 11, 186 CrossRef CAS.
- J. Guan, Q. Liu, X. Zhang, Y. Zhang, R. Chokshi, H. Wu and S. Mao, Eur. J. Pharm. Sci., 2018, 114, 346 CrossRef CAS.
- P. Kanaujia, P. Poovizhi, W. K. Ng and R. B. H. Tan, Powder Technol., 2015, 285, 2–15 CrossRef CAS.
- S. Baghel, H. Cathcart and N. J. O'Reilly, J. Pharm. Sci., 2016, 105, 2527–2544 CrossRef CAS.
- T. Vasconcelos, S. Marques, J. D. Neves and B. Sarmento, Adv. Drug Delivery Rev., 2016, 100, 85–101 CrossRef CAS.
- M. Zhang, H. Li, B. Lang, K. O'Donnell, H. Zhang, Z. Wang, Y. Dong and C. Wuab, Eur. J. Pharm. Biopharm., 2012, 82, 534–544 CrossRef CAS.
- H. Patil, R. V. Tiwari and M. A. Repka, AAPS PharmSciTech, 2016, 17, 20–42 CrossRef CAS.
- J. P. Lakshman, Y. Cao, J. Kowalski and A. T. Serajuddin, Mol. Pharm., 2008, 5, 994–1002 CrossRef CAS.
- J. R. Hughey, J. M. Keen, C. Brough, S. Saeger and J. W. Mcginity, Int. J. Pharm., 2011, 419, 222–230 CrossRef CAS.
- Y. Zhang, R. Luo, Y. Chen, X. Ke, D. Hu and M. Han, AAPS PharmSciTech, 2014, 15, 560–568 CrossRef CAS.
- K. Robinson, C. Mock and D. Liang, Drug Dev. Ind. Pharm., 2015, 41, 1–6 CrossRef.
- L. Pei-Chi, N. Lean-Teik, L. Liang-Tzung, C. D. Richardson, W. Guey-Horng and L. Chun-Ching, J. Med. Food, 2010, 13, 1415–1423 CrossRef.
- L. Guo, Y. J. Peng, L. Sui, A. Gu and J. Wang, Cancer Biother. Radiopharm., 2010, 25, 471 CrossRef CAS.
- D. Dominique, L. O. Allan, C. Didier, J. Brigitte and L. Norbert, Curr. Drug Targets, 2006, 7, 423–442 CrossRef.
- A. Amri, J. C. Chaumeil, S. Sfar and C. Charrueau, J. Controlled Release, 2012, 158, 182–193 CrossRef CAS.
- R. Kumar, K. Kaur, S. Uppal and S. K. Mehta, Ultrason. Sonochem., 2017, 37, 478–489 CrossRef CAS.
- L. Jian, X. Miao, T. Chen, D. Ouyang and Z. Ying, Asian J. Pharm. Sci., 2016, 11, 528–535 CrossRef.
- M. R. Vijayakumar, K. Y. Vajanthri, S. K. Mahto, N. Mishra, M. S. Muthu and S. Singh, Colloids Surf., B, 2016, 145, 479–491 CrossRef CAS.
- B. Li, L. A. Wegiel, L. S. Taylor and K. J. Edgar, Cellulose, 2013, 20, 1249–1260 CrossRef CAS.
- A. B. Gangurde, H. S. Kundaikar, S. D. Javeer, D. R. Jaiswar, M. S. Degani and P. D. Amin, J. Drug Delivery Sci. Technol., 2015, 29, 226–237 CrossRef CAS.
- S. Y. Lin, H. L. Yu and M. J. Li, Polymer, 1999, 40, 3589–3593 CrossRef CAS.
- S. M. Alshahrani, J. T. Morott, A. S. Alshetaili, R. V. Tiwari, S. Majumdar and M. A. Repka, Eur. J. Pharm. Sci., 2015, 80, 43–52 CrossRef CAS.
- G. Verreck, K. Six, G. V. D. Mooter, L. Baert, J. Peeters and M. E. Brewster, Int. J. Pharm., 2003, 251, 165–174 CrossRef CAS.
- H. Liu, W. Peng, X. Zhang, S. Fei and C. G. Gogos, Int. J. Pharm., 2010, 383, 161–169 CrossRef CAS PubMed.
- L. Yongcheng, P. Huishi, G. Zhefei, L. Ling, D. Yixuan, L. Ge, L. Ming and W. Chuangbin, J. Pharm. Pharmacol., 2014, 66, 148–166 CrossRef.
- M. Maniruzzaman, A. Nair, N. Scoutaris, M. S. A. Bradley, M. J. Snowden and D. Douroumis, Int. J. Pharm., 2015, 496, 42–51 CrossRef CAS.
- E. Reitz, H. Podhaisky, D. Ely and M. Thommes, Eur. J. Pharm. Biopharm., 2013, 85, 1200–1205 CrossRef CAS.
- B. Chamsai and P. Sriamornsak, Asian J. Pharm. Sci., 2016, 11, 193–194 CrossRef.
- C. Francesco, T. Joseph, V. E. Adriel and R. Miriam, J. Agric. Food Chem., 2004, 52, 7279 CrossRef PubMed.
- W. Wang, X. Zhou, L. E. Shengfeng, D. U. Ning, N. Lin and J. Zhang, China Meas. Test, 2015, 4, 39–42 Search PubMed.
- J. Li, I. W. Lee, G. H. Shin, X. Chen and H. J. Park, Eur. J. Pharm. Biopharm., 2015, 94, 322–332 CrossRef CAS.
- A. L. Sarode, H. Sandhu, N. Shah, W. Malick and H. Zia, Eur. J. Pharm. Sci., 2013, 48, 371–384 CrossRef CAS.
- M. Maghsoodi, Adv. Pharm. Bull., 2015, 5, 13 CAS.
- M. Xu, C. Zhang, Y. Luo, L. Xu, X. Tao, Y. Wang, H. He and X. Tang, Drug Dev. Ind. Pharm., 2013, 40, 208–213 Search PubMed.
- M. T. França, R. P. Nicolay, M. K. Riekes, J. Pinto and H. K. Stulzer, Eur. J. Pharm. Sci., 2018, 533, 266 Search PubMed.
- A. Tambe and N. Pandita, J. Drug Delivery Sci. Technol., 2018, 44, 172–180 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |