Open Access Article
Open Access ArticleFacile anion-exchange reaction in mixed-cation lead bromide perovskite nanocrystals
Ambra Pisanu,
Paolo Quadrelli and
Lorenzo Malavasi
and
Lorenzo Malavasi *
*
Department of Chemistry, INSTM, Viale Taramelli 16, 27100, Pavia, Italy. E-mail: lorenzo.malavasi@unipv.it
First published on 30th April 2019
Abstract
The present paper reports a facile and fast anion exchange reaction approach for perovskite nanocrystals (NCs). The reaction has been applied to a mixed-cation sample, namely FA0.8Cs0.2PbBr3, and just employs lead halide precursors to achieve a significant shift in the nanocrystals emission. Within 30 minutes reaction time and a molar ratio between the NCs and the halide precursors of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, it was possible to cover more than 120 nm in the visible region. The exchanged NCs maintain their cubic-shaped morphology and narrow emission peak width for the whole compositional range explored. The present approach also allows a reduction in solvent use since it does not require any halide precursor synthesis nor complex post-synthesis treatments.
1, it was possible to cover more than 120 nm in the visible region. The exchanged NCs maintain their cubic-shaped morphology and narrow emission peak width for the whole compositional range explored. The present approach also allows a reduction in solvent use since it does not require any halide precursor synthesis nor complex post-synthesis treatments.
Introduction
Lead halide semiconductors with a perovskite crystal structure have been thoroughly investigated during the last several years as a new class of optoelectronic materials. Starting with photovoltaic applications, with power conversion efficiencies beyond 22%, they are now particularly attractive in applications such as light-emitting devices, lasers and photodetectors.1–5 The majority of research has focused on bulk, single crystals and thin films perovskites, while colloidal synthesis to hybrid or to fully inorganic perovskite nanocrystals is a completely new method firstly reported in 2015.6 Controlled size reduction to the nanoscale dimension is a challenging issue because it gives rise to brand new and unique properties which pave the way for different research directions and applications.Cesium and formamidinium lead halide nanocrystals have been shown to retain striking optical properties such as broadly tuneable photo-luminescence adjusting the nanocrystal size, small full-width at half-maxima and high photoluminescence (PL) quantum yields up to 90%. In the future, the most viable application of these nanocrystals will be in television displays and related devices.6,7 Mixed cations perovskites show a significant enhancement of the power conversion efficiency and improved stability, in particular Zhang et al. proved that the optimized composition FA0.8Cs0.2PbBr3 exhibit a superior behaviour than others compositions in terms of high quantum efficiency, proper energy level and air stability, which together contribute to its outstanding luminance performance (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 005 cd m−2) in the LEDs.8
005 cd m−2) in the LEDs.8
Beyond the optical properties and the charming applications, one of the most appealing aspect of perovskites nanocrystals is their wide band gap tunability. Up to now the modulation of the band gap has been carried out above all at the synthesis step, by preparing different compositions or solid solutions of the cations or the anions.7–15 However, appealing post-synthetic strategies rely on the anion exchange of the prepared colloidal nano-dispersions, as it has been previously demonstrated on CsPbBr3.16–22 One of the most common routes for anion exchange in lead halide nanocrystal was reported by Nedelcu et al. and makes use of Schlenk line where lead halogens and OAmX as anion sources were mixed with octadecene (ODE) in neck flask and kept under vacuum at 120 °C. The dried oleic acid (OA) and oleylamine (OLA) were injected at 120 °C under N2 flow.17 While this approach is highly suitable to provide a full anion exchange, it would be desirable to provide more easy and facile routes to perform such anion modification on lead halide nanocrystals and also provide evidence of such exchange on mixed cation compositions.
For these reasons, in this work we selected the FA0.8Cs0.2PbBr3 optimized composition to perform a halide exchange reaction with I and Cl providing a facile and fast method to perform it.
Experimental
Preparation of oleylammonium bromide (OAmBr)
Ethanol (100 mL, Aldrich, absolute) and oleylamine (OLA, 12.5 mL, Acros Organics) were combined in a 3-neck flask under vigorous stirring. The solution was cooled in an ice-water bath and 8.56 mL HBr (48% aqueous solution, Aldrich) were added very slowly and then the mixture was left to react overnight under N2 flow. The next day the reaction mixture was cooled again in an ice-water bath for 2 hours. The product (a white powder) was obtained after filtering, purifying by rinsing multiple times with diethylether and vacuum-drying at 60 °C in a Abderhalden's drying pistol overnight.Synthesis of FA0.8Cs0.2PbBr3
It follows the typical hot-injection method described by Protesescu et al. in their work7 on formamidinium nanocrystals: a precursor solution was prepared using Pb (0.2 mmol), formamidinium (FA) (0.6 mmol) and Cs (0.15 mmol) acetates (Sigma Aldrich) with dried oleic acid (2 mL, Acros Organics) and dried octadecene (8 mL, Acros Organics); at 130 °C, under N2 flow, oleyl ammonium bromide (0.6 mmol) dissolved in toluene (2 mL) was injected. After few seconds the nanocrystals were quenched in ice, to the crude solution were added 10 mL of toluene and 5 mL of acetonitrile and the mixture was centrifuged for 5 min at 12k rpm. The precipitate was dispersed in 5 mL of toluene.Exchange reactions
The anion exchange reactions reported were conducted at room temperature in toluene by mixing a specific ratio of the desired lead halide salt. In particular, 0.113 mmol of PbX2 were loaded in 4 mL of FA0.8Cs0.2PbBr3 NCs toluene solution 28.3 mM, followed by 1, 5, 10, 15 and 30 minutes of vigorous stirring. The transparent dispersion was separated from the solid phase by centrifugation and analysed.X-ray diffraction
The crystal structures of the samples were characterized by room-temperature Cu-radiation Powder X-ray diffraction (XRD) on a Bruker D8 diffractometer. Scans were performed in the 10–40° range, with a step size 0.04° and a counting time of 3 s per step.Absorbance
UV-Vis absorption spectra for colloidal solutions were run on Varian Cary 50 Scan spectrophotometer in absorbance mode with quartz cuvettes of 1 cm path length.Photoluminescence (PL)
A PerkinElmer LS 50B spectrofluorimeter was used to acquire steady-state PL spectra from colloidal solutions.Transmission electron microscopy (TEM)
TEM images were collected on a JEOL JEM-1200 EX II microscope operating at 100 kV (tungsten filament gun) and equipped with the TEM CCD camera Olympus Mega View G2 with 1376 × 1032 pixel format. Samples were prepared by drop-casting the solution on coated copper grids.Results and discussion
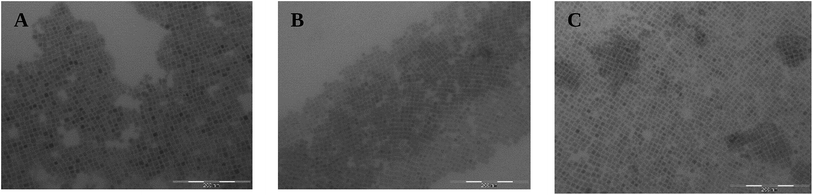
The synthesis of the optimized FA0.8Cs0.2PbBr3 follows the typical hot-injection method described by Protesescu et al. in their work on formamidinium nanocrystals: a precursor solution was prepared using Pb, FA and Cs acetates with dried oleic acid and dried octadecene; at 130 °C oleyl ammonium bromide dissolved in toluene was injected and after few seconds the nanocrystals were quenched in ice and purified.7 This simple synthesis allowed us to obtain luminescent cubic shaped nanocrystals (Fig. 1) with average size around 15 nm. Both nanocrystal size and emission properties agree with the results reported previously for FA0.8Cs0.2PbBr3.8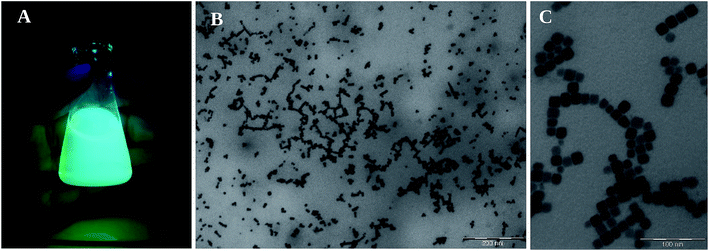 | ||
| Fig. 1 (A) Dispersion of FA0.8Cs0.2PbBr3 NCs in toluene under UV lamp; (B) and (C) TEM images of FA0.8Cs0.2PbBr3 NCs. | ||
In Fig. 2A are shown the XRD patterns of the FA0.8Cs0.2PbBr3 sample together with FAPbBr3 and CsPbBr3 prepared with the same method as well as the reference pattern of the orthorhombic cell found for these halides (space group, Pnma). Fig. 2B reports a selected region of the patterns in order to discuss better and highlight the effects of cation composition on lattice size. First of all, all the samples are single phase without detectable impurities. Moreover, from Fig. 2B, it can be appreciated that the (040) and (202) reflections of the reference pattern of CsPbBr3 (vertical red lines) are found in good agreement with the experimental data indicating the proper chemical composition of the sample. The same peaks are found at lower angle by replacing Cs with FA, indicating an increase of the cell size in agreement with the difference in the ionic radii between the two cations (rCs: 1.81 Å, rFA: 2.79 Å). Finally, the mixed sample, i.e. FA0.8Cs0.2PbBr3, is found at peak positions close to FAPbBr3 but at slightly higher angle, in agreement with the 20% Cs-content.
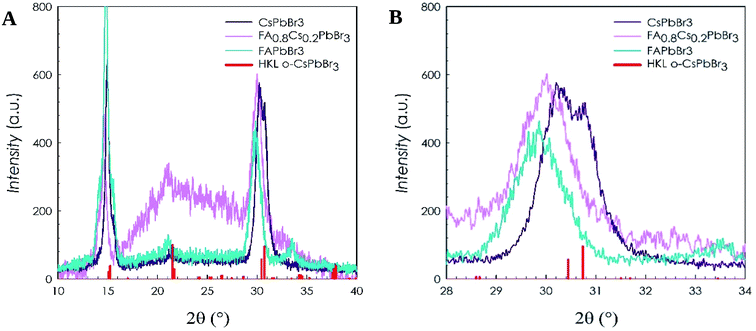 | ||
| Fig. 2 (A) XRD patterns of CsPbBr3, FAPbBr3 and FA0.8Cs0.2PbBr3 NCs; (B) same XRD patterns zoomed around 30°. | ||
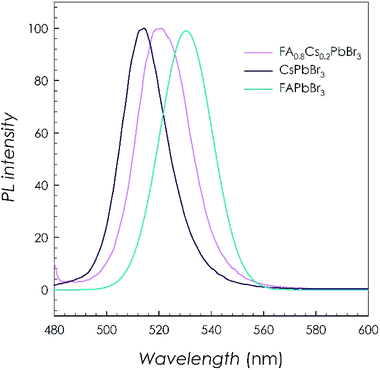
The optical properties of the FA0.8Cs0.2PbBr3 sample, together with the two end-members CsPbBr3 and FAPbBr3, have been measured by means of PL spectroscopy. The results are reported in Fig. 3.
For all the three samples reported in Fig. 3, the PL shows a single narrow peak (FWHM ca. 10 nm). The emission of pure CsPbBr3 is centred at about 513 nm in agreement with previous literature data for an analogous crystal size.6–8 On the other hand, the replacement of Cs with FA red-shift the emission peak initially to 2.38 eV (FA0.8Cs0.2PbBr3) and then to 2.33 eV (FAPbBr3 sample).
Now that the properties of the FA0.8Cs0.2PbBr3 starting sample have been properly characterized and compared to the data of the two end-members of the mixed compositions, we realized an anion–exchange reaction using lead halide precursors, with a simple dynamic solution process, a fast method to tune the optical properties in a post-synthetic reaction with the preservation of the crystal structure and morphology.
The anion exchange reactions reported were conducted at room temperature in toluene by mixing a specific ratio of the desired lead halide salt. In particular, 0.113 mmol of PbX2 were loaded in 4 mL of FA0.8Cs0.2PbBr3 NCs toluene solution 28.3 mM, followed by 10 seconds of vigorous stirring which were found to be enough to appreciate a first colour change suggesting the occurrence of anion exchange. After a minute the transparent solution was separated from the solid phase by centrifugation and analysed.
Compared to most of the reported methods, this one is facile, fast and environmentally friendly.16–24 The halide precursors do not need any solvent to be dissolved in, they can be separated by a simple filtration and, most relevant, the reaction can be carried out at room temperature and under ambient conditions. The products were characterized by means of X-ray diffraction, UV absorbance, photoluminescence and high resolution TEM.
Fig. 4 shows the appearance of mixed Br/Cl, pure Br and mixed Br/I nanocrystals solutions under daylight and UV light illumination after 1 minute of exchange reaction. Already from this simple visual inspection, a blue-shift of FA0.8Cs0.2PbBr3 NCs is observed after the exchange with Cl ions while a red-shift of the emission is clear for the sample exchanged with I anions.
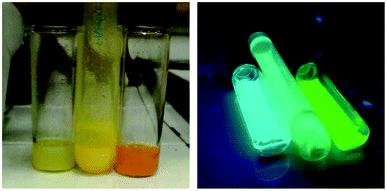 | ||
| Fig. 4 FA0.8Cs0.2Pb(Br/Cl)3, FA0.8Cs0.2PbBr3 and FA0.8Cs0.2Pb(Br/I)3 NC's under daylight (left panel) and under UV light (right panel). | ||
The XRD patterns reported in Fig. 5 for the FA0.8Cs0.2PbBr3, FA0.8Cs0.2Pb(Br/I)3 and FA0.8Cs0.2Pb(Br/Cl)3 NCs display the typical reflections of the orthorhombic perovskite structure of CsPbBr3. All the samples are single phase, anion exchange did not alter the crystal phase of the initial NCs. In Fig. 5B is reported a selected region to better analyse the effects of halide substitution: as expected the incorporation of I− cause an expansion of the cell and the peaks shift at lower angles, while upon the incorporation of Cl−, the cell shrunk, and the peaks shift to higher angles.
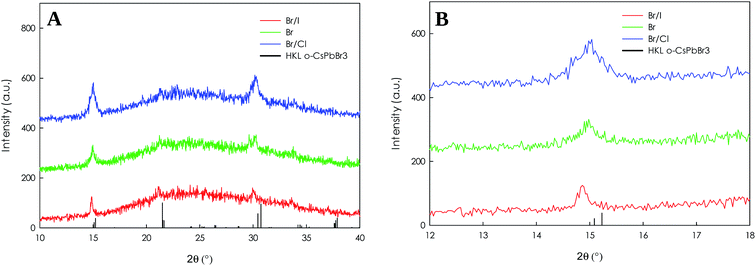 | ||
| Fig. 5 (A) XRD patterns of FA0.8Cs0.2PbBr3, FA0.8Cs0.2Pb(Br/I)3 and FA0.8Cs0.2Pb(Br/Cl)3 NCs; (B) same XRD patterns zoomed around 15°. | ||
The optical properties of mixed Br/Cl, pure Br and mixed Br/I nanocrystals have been measured by means of UV absorbance and PL spectroscopy. The results are reported in Fig. 6. In the absorption spectra, with respect to Br-based NCs, the sample exchanged with Cl-ions shows a blue shift, while a red shift is observed for the iodide one: the composition tuning of the samples enabled the band gap to display absorption over the range of 500–540 nm for a very short time of exchange reaction.
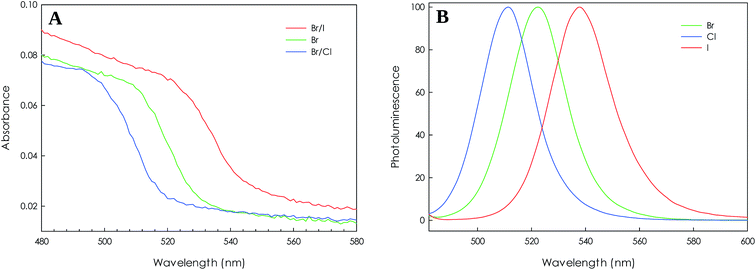 | ||
| Fig. 6 (A) UV spectra and (B) normalized PL spectra of FA0.8Cs0.2PbBr3, FA0.8Cs0.2Pb(Br/I)3 and FA0.8Cs0.2Pb(Br/Cl)3 NCs. | ||
Along with the orthorhombic crystal structure, bright PL is retained, and the spectra are in good agreement with literature. For all the three samples reported the PL shows a single narrow peak (FWHM ca. 10 nm). The emission of pure Br is centred at about 523 nm; the replacement of Br with I red-shift the emission peak to 538 nm while the replacement with Cl blue-shift the peak to 511 nm.
From the TEM images reported in Fig. 7, it is possible to observe that the morphology of the nanocrystals does not change, the cubic shape of the NC's whit bromide (Fig. 7A) is retained adding PbI2 and PbCl2 (Fig. 7B and C).
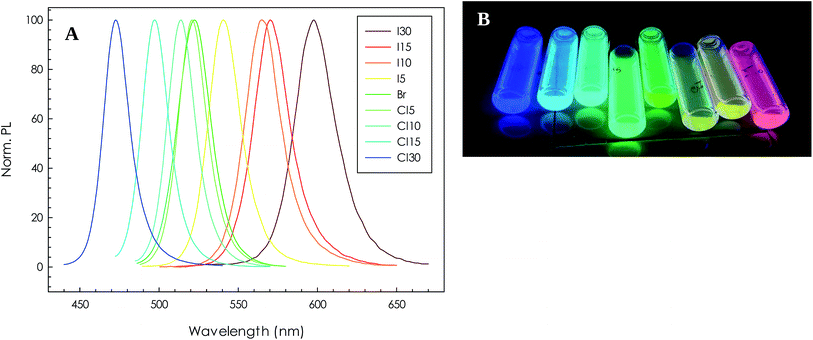
After assessing that this exchange methodology is effective in inducing an anion compositions modulation already for very short times (1 minute exchange), without changing the stoichiometry ratios between NCs and lead halide salts, the exchange was further investigated by increasing the reaction time to 5, 10, 15 and 30 minutes. The PL spectra of the samples for the corresponding reaction times and exchanged anions are reported in Fig. 8A, while Fig. 8B shows the appearance of the colloidal dispersions to which the PL spectra refer.
As can be appreciated, by simply increasing the stirring time the emission peaks significantly blue- and red-shift. Details about the PL peak maximum and FWHM are reported in Table 1. Overall, a 120 nm shift is achieved with this procedure and this mixing times. In addition, the peak widths remain quite narrow with a slight increase for the mixed Br/I samples by increasing the exchange reaction time which however is around 13 nm. An increase of the peak width for mixed Br/I samples has been previously observed but with FWHM reaching values around 45 nm for mixed Br/I samples.6 By comparing the actual PL emission values for the most exchanged samples with current literature for analogous crystal size, we may conclude that the maximum exchange level reached is around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Br
2 Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X (X = Cl, I).16 This result is in agreement with the data reported by Akkerman and co-workers where, however, they employed for the exchange, octadecylammonium halides (ODA-X) precursors which requires a not trivial separate reaction step and a 20-fold halide excess to achieve results analogous to those reported here.16 Still with reference to this last paper, the authors attempted as well the use of PbX2 salts for the exchange but concluded that the reaction was to slow and required more than 1 day to achieve a significant exchange and for this reason was not investigated.16 The reasons for this significant disagreement between the data reported here and previous literature is not clear and might be due to the different quality of the reagents employed.
X (X = Cl, I).16 This result is in agreement with the data reported by Akkerman and co-workers where, however, they employed for the exchange, octadecylammonium halides (ODA-X) precursors which requires a not trivial separate reaction step and a 20-fold halide excess to achieve results analogous to those reported here.16 Still with reference to this last paper, the authors attempted as well the use of PbX2 salts for the exchange but concluded that the reaction was to slow and required more than 1 day to achieve a significant exchange and for this reason was not investigated.16 The reasons for this significant disagreement between the data reported here and previous literature is not clear and might be due to the different quality of the reagents employed.
| Sample label | Emission (nm) | Emission (eV) | FWMH (nm) |
|---|---|---|---|
| Cl30 | 473.3 | 2.62 | 8.8 |
| Cl15 | 497.7 | 2.49 | 9.1 |
| Cl10 | 514.2 | 2.41 | 9.4 |
| Cl5 | 521.4 | 2.38 | 9.6 |
| Br | 522.3 | 2.37 | 11.0 |
| I5 | 541.5 | 2.29 | 10.9 |
| I10 | 565.8 | 2.19 | 12.3 |
| I15 | 571.3 | 2.17 | 12.7 |
| I30 | 605.7 | 2.04 | 13.9 |
It is clear that a lot of space if left to further investigate the simple and effective anion exchange reaction proposed here, such as, for example, by further increasing the reaction time or by changing the molar ratio between the precursor NCs and the lead halide source, which in the present case was fixed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Conclusions
In summary, cubic shaped FA0.8Cs0.2PbBr3 NCs were synthesized by solution reaction with the hot-injection method. These NCs were transformed in FA0.8Cs0.2Pb(Br/I)3 and FA0.8Cs0.2Pb(Br/Cl)3 by a fast and simple post-synthetic halide exchange reaction using PbI2 and PbCl2 directly in the form of powders in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ration with respect to the NCs with the preservation of shape and crystal structure of the starting sample.
1 molar ration with respect to the NCs with the preservation of shape and crystal structure of the starting sample.
The innovative aspects of this work are the conditions of the halide exchange reactions reported: they were conducted in toluene at room temperature, without the necessity of N2 atmosphere, with the simple use of lead halides thus not requiring the synthesis of complex halide precursors; in addition such precursors do not require the use of any solvent to be dissolved and the exchanged nanocrystals can be separated by a simple filtration or centrifugation.
By using the present NCs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) halide precursors molar ratio and extending the reaction time to 30 min the exchanged nanocrystals covered a large part of the visible region of the spectrum, with a fine tuning of the emission with reaction time and by preserving a high quality of the final crystals with very narrow FWHM.
halide precursors molar ratio and extending the reaction time to 30 min the exchanged nanocrystals covered a large part of the visible region of the spectrum, with a fine tuning of the emission with reaction time and by preserving a high quality of the final crystals with very narrow FWHM.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the Centro Grandi Strumenti (CGS) of the University of Pavia for TEM measurements.References
- https://www.nrel.gov/pv/assets/pdfs/pv-efficiencies-07-17-2018.pdf.
- J. P. C. Baena, L. Steier, W. Tress, M. Saliba, S. Neutzner, T. Matsui, F. Giordano, T. J. Jacobsson, A. R. S. Kandada, S. M. Zakeeruddin, A. Petrozza, A. Abate, M. Khaja Nazeeruddin, M. Gratzel and A. Hagfeldt, Energy Environ. Sci., 2015, 8, 2928 RSC.
- Z. K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Nat. Nanotechnol., 2014, 9, 687 CrossRef CAS PubMed.
- M. Saliba, S. M. Wood, J. B. Patel, P. K. Nayak, J. Huang, J. A. Alexander-Webber, B. Wenger, S. D. Stranks, M. T. Hörantner, J. Tse-Wei Wang, R. J. Nicholas, L. M. Herz, M. B. Johnston, S. M. Morris, H. J. Snaith and M. K. Riede, Adv. Mater., 2016, 28, 923 CrossRef CAS PubMed.
- R. Dong, Y. Fang, J. Chae, J. Dai, Z. Xiao, Q. Dong, Y. Yuan, A. Centrone, X. C. Zeng and J. Huang, Adv. Mater., 2015, 27, 1912 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Bertolotti, N. Masciocchi, A. Guagliardi and M. V. Kovalenko, J. Am. Chem. Soc., 2016, 138, 14202 CrossRef CAS PubMed.
- X. Zhang, H. Liu, W. Wang, J. Zhang, B. Xu, K. L. Karen, Y. Zheng, S. Liu, S. Chen, K. Wang and X. W. Sun, Adv. Mater., 2017, 29, 1606405 CrossRef PubMed.
- A. Amat, E. Mosconi, E. Ronc, C. Quarti, P. Umari, M. K. Nazeeruddin, M. Grätzel and F. De Angelis, Nano Lett., 2014, 14, 3608 CrossRef CAS PubMed.
- M. Mittal, A. Jana, S. Sarkar, P. Mahadevan and S. Sapra, J. Phys. Chem. Lett., 2016, 7, 3270 CrossRef CAS PubMed.
- W. Van der Stam, J. J. Geuchies, T. Altantzis, K. H. W. van den Bos, J. D. Meeldijk, S. Van Aert, S. Bals, D. Vanmaekelbergh and C. de Mello Donega, J. Am. Chem. Soc., 2017, 139, 4087 CrossRef CAS PubMed.
- M. R. Linaburg, E. T. McClure, J. D. Majher and P. M. Woodward, Chem. Mater., 2017, 29(8), 3507 CrossRef CAS.
- T. C. Jellicoe, J. M. Richter, H. F. J. Glass, M. Tabachnyk, R. Brady, S. E. Dutton, A. Rao, R. H. Friend, D. Credgington, N. C. Greenham and M. L. Böhm, J. Am. Chem. Soc., 2016, 138, 2941 CrossRef CAS PubMed.
- Q. A. Akkerman, G. Rainò, M. V. Kovalenko and L. Manna, Nat. Mater., 2018, 17, 394 CrossRef CAS PubMed.
- L. Wang, N. E. Williams, E. W. Malachosky, J. P. Otto, D. Hayes, R. E. Wood, P. Guyot-Sionnest and G. S. Engel, ACS Nano, 2017, 11, 2689 CrossRef CAS PubMed.
- Q. A. Akkerman, V. D'Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2015, 137(32), 10276 CrossRef CAS PubMed.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15(8), 5635 CrossRef CAS PubMed.
- Y. Bekenstein, B. A. Koscher, S. W. Eaton, P. Yang and A. P. Alivisatos, J. Am. Chem. Soc., 2015, 137(51), 16008 CrossRef CAS PubMed.
- S. E. Creutz, E. N. Crites, M. C. De Siena and D. R. Gamelin, Chem. Mater., 2018, 30, 4887 CrossRef CAS.
- D. Parobek, Y. Dong, T. Qiao, D. Rossi and D. H. Son, J. Am. Chem. Soc., 2017, 139(12), 4358 CrossRef CAS PubMed.
- M. Li, X. Zhang, K. Matras-Postolek, H.-S. Chen and P. Yang, J. Mater. Chem. C, 2018, 6, 5506–5513 RSC.
- M. Li, X. Zhang, T. Dong, P. Wang, K. Matras-Postolek and P. Yang, J. Phys. Chem. C, 2018, 122, 28968–28976 CrossRef CAS.
- D. Zhang, Y. Yang, Y. Bekenstein, Y. Yu, N. A. Gibson, A. B. Wong, S. W. Eaton, N. Kornienko, Q. Kong, M. Lai, A. P. Alivisatos, S. R. Leone and P. Yang, J. Am. Chem. Soc., 2016, 138(23), 7236 CrossRef CAS PubMed.
- D. M. Jang, K. Park, D. H. Kim, J. Park, F. Shojaei, H. S. Kang, J. P. Ahn, J. W. Lee and J. K. Song, Nano Lett., 2015, 15, 5191–5199 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |