Open Access Article
Open Access ArticleNon-alkaloidal constituents from the genus Aconitum: a review
Tianpeng Yinab,
Hao Zhoub,
Le Cai*b and
Zhongtao Ding *b
*b
aZhuhai Key Laboratory of Fundamental and Applied Research in Traditional Chinese Medicine, Department of Bioengineering, Zunyi Medical University Zhuhai Campus, Zhuhai 519041, China
bFunctional Molecules Analysis and Biotransformation Key Laboratory of Universities in Yunnan Province, School of Chemical Science and Technology, Yunnan University, Kunming 650091, China. E-mail: ztding@ynu.edu.cn; caile@ynu.edu.cn
First published on 2nd April 2019
Abstract
Species of the genus Aconitum are widely distributed in the north temperate region and have been used in traditional medicine since antiquity due to their biological activities. Phytochemical investigations of Aconitum species have revealed the presence of multiple active ingredients, including flavonoids, phenylpropanoids, phenolics and acids, terpenoids, and polysaccharides in addition to their diterpenoid alkaloids. These non-alkaloidal constituents show great research significance for their novel structures, broad bioactivities, and chemotaxonomical significance. This review addresses, for the first time, the non-alkaloidal constituents and their biological properties and their taxonomic significance for Aconitum plants to facilitate future research.
1. Introduction
Aconitum L. is a large genus of the Ranunculaceae family comprising approximately 400 species that are distributed mainly in the north temperate region, such as in Asia, Europe, and North America, and more than 200 species of this genus have been found in China.1 Plants from this genus have been widely used as herbal medicines in traditional medicine for the treatment of various diseases, such as fainting, rheumatic fever, painful joints, gastroenteritis, diarrhoea, oedema, bronchial asthma, various tumours, and some endocrine disorders, such as irregular menstruation.2,3 Especially in China, the first description of the utilization of Aconitum plants in traditional Chinese medicine (TCM) can be traced back to the Han dynasty, approximately two thousand years ago, and it was recorded in the earliest book of Chinese Materia Shennong Bencao Jing. Currently, at least 76 species of Aconitum are used as herbal medicines in China because of their unique and proven therapeutic effects. Several Aconitum-derived herbal drugs, such as Fuzi (processed lateral roots of A. carmichaelii), Chuanwu (processed mother roots of A. carmichaelii) and Caowu (processed roots of A. kusnezoffii), have become popular and common drugs in TCM.4Aconitum plants are well known for their characteristic diterpenoid alkaloid (DA) components, which have complex chemical structures and remarkable pharmacological activities, especially the prominent anti-inflammatory, antalgic and antiarrhythmic effects.5 More than one thousand DAs, which have been structurally classified into four categories, C18-, C19-, C20-, and bis-subtypes, have been isolated from this genus. The DAs in Aconitum plants have drawn scientists' lasting attention, and most of the studies on the Aconitum plants were devoted to their DA components.6–8 To date, a number of reviews considering various aspects of DAs in Aconitum plants have been published.9,10 Nevertheless, the non-alkaloidal natural constituents in Aconitum plants have also received researchers' strong interests. Recently, various kinds of non-alkaloidal constituents, including flavonoids, phenylpropanoids, phenolics and acids, terpenoids, steroids, free fatty acids (FFAs) and polysaccharides, have been reported to be isolated from the Aconitum plants.
It also shows great significance to study the non-alkaloidal constituents in Aconitum plants. Firstly, the preliminary screening tests showed that the non-alkaloidal constituents from Aconitum plants possess broad and impressive biological activities, including antioxidant, antiparasitic, antiphlogistic, antineoplastic, and immunoregulatory effects, which indicated that these constituents could also serve as a potential medicinal resource for drug discovery. In addition, unlike highly toxic DAs, these non-alkaloidal ingredients are generally less toxic, which is advantageous for the food and pharmaceutical industry. Some kinds of non-alkaloidal constituents from Aconitum plants possess new activities different from DAs, which implied novel potential applications for Aconitum plants in turn. On the other side, it is well known that TCM usually apply a multi-component approach in the treatment of diseases. Certain non-alkaloidal constituents such as phenylpropanoids and polysaccharides that displayed antiphlogistic, antineoplastic and immunore-gulatory effects in accordance with DAs, might also make contribution to the therapeutic effects of Aconitum-derived herbal drugs. Hence, it is beneficial for elucidating the pharmacological material basis and mechanism of Aconitum herbs to perform more extensive and in-depth studies on their non-alkaloidal constituents.
Secondly, studies on the non-alkaloidal constituents are conducive to the taxonomy of Aconitum plants. Aconitum is a taxonomically complex genus consisting of many species that are easily confused because their morphological characteristics are indistinguishable. The assistance and supplement from chemotaxonomy is of great importance in the identification of species in Aconitum genus. Research have revealed that, in addition to the DA components, non-alkaloidal constituents for example flavonoids also demonstrated taxonomical significance. These constituents might also be considered as chemo-taxonomic markers of this genus to facilitate taxonomy of the Aconitum plants.
Finally, the studies on the non-alkaloidal constituents in Aconitum plants might play a positive role in illuminating the biosynthetic pathway of DAs. A persuasive case was made by the non-alkaloidal diterpenoid atropurpuran from A. hemsleyanum var. atropurpureum, which demonstrated strong relevance in the biosynthetic of hetidine-type DAs.11 The studies on the non-alkaloidal constituents might open a new path to explain the construction of some types of DAs whose biosynthetic pathway remains undefined.
Therefore, based on above aspects, this review was conducted to summarize the structural features, biological activities and taxonomical significance of the non-alkaloidal constituents from the Aconitum species for the first time. The aim of this review is to provide a complete overview of the information currently available on the non-alkaloidal constituents of plants from the Aconitum genus, which will facilitate further research and exploitation of this genus.
2. Non-alkaloidal constituents of the Aconitum genus
2.1. Flavonoid glycosides
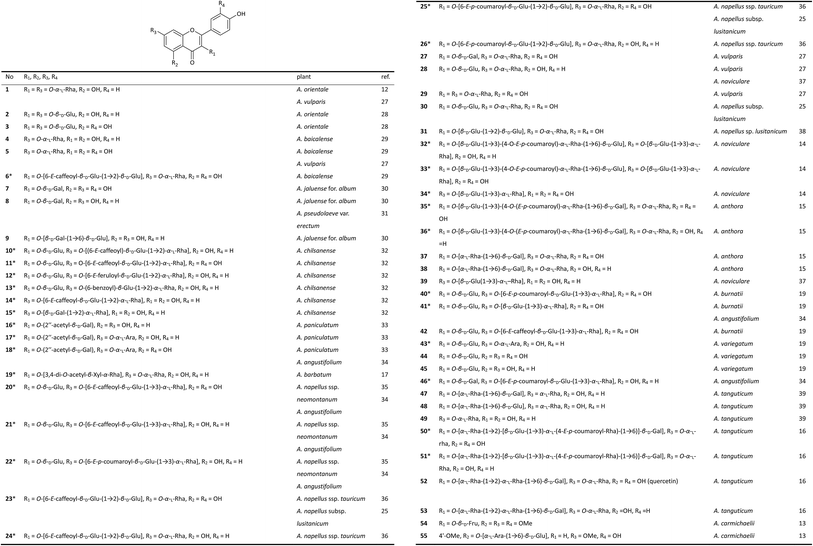
Studies on the flavonoid components of Aconitum plants can be traced back to 1969. Russian scientists Samokish et al. first reported the isolation of a flavonoid diglycoside (1) from the leaves of A. orientale (Caucasian monkshood) by paper chromatography.12 Flavonoid glycosides are widely distributed in the aerial parts of Aconitum plants, especially in the flowers. However, sample collection is very difficult due to the small size of the petals and the short blooming period, which hinders related research. To date, the flavonoid components of only a few species have been studied, and approximately 55 flavonoid glycosides have been isolated (Fig. 1).The flavonoids found in Aconitum plants are exclusively glycosides of flavones, with the most common aglycones being kaempferol and quercetin. Compounds 54 and 55, isolated from A. carmichaelii from Vietnam, are exceptions to this trend, as they possess aglycones of 5,7,4′-tri-methoxylquercetin and 5-methoxylluteolin, respectively.13 Notably, of the 55 flavonoids presented in Fig. 1, 29 are new glycosides (labelled with *). The novelty of these flavonoid glycosides is determined by the structures of their carbohydrate chains, which are normally located at C-3 or/and C-7, except for compound 55, which has its carbohydrate chain at C-5, a rare substitution pattern. The carbohydrate chain generally appears as a monosaccharide or disaccharide. Among isolated glycosides, new compounds 32, 33, 35, 36, 50, and 51 have trisaccharide chains at C-3.14–16 Most of the sugar moieties are glucose, rhamnose, galactose, arabinose or their ethanoyl, benzoyl, caffeoyl, coumaroyl, or feruloyl ester derivatives. New glycoside 19 from A. barbatum and glycoside 54 from A. carmichaeli have rare xylose and fructopyranose substituents, respectively.13,17
Natural flavonoid glycosides show broad biological activity, including antioxidant, antibacterial, antiphlogistic and immuno-regulation, antineoplastic, and antiviral effects as well as significant effects on metabolism and on the cardiovascular system of mammals. Among the flavonoid glycosides that have been isolated from Aconitum plants, a series of compounds (1, 16–18, 20–26, 28, 29, 35–38 and 40–45) have demonstrated high antioxidant activity, most of which displayed DPPH radical scavenging activity.15,18,19 In particular, glycosides 20 and 42 exhibited impressive antiradical activities (IC50 values of 1.9 and 2.0 μM, respectively), and they were more potent than the positive controls, quercetin and rutin (IC50 values of 2.5 and 3.9 μM, respectively). Quercetin glycosides possess better antioxidant activities than kaempferol glycosides, and the caffeoyl derivatives have a higher radical scavenging abilities than the p-coumaroyl esters, which could be attributed to the vital effect of the ortho-dihydroxy groups in their structures.20 Since most of DAs are ineffective in scavenging radicals,21,22 and the content of DAs in the flowers of Aconitum plants is pretty low, it suggests that flavonoid glycosides could be responsible for the antioxidant activities of the Aconitum flowers.15
Moreover, new compounds 23 and 24, isolated from A. napellus subsp. lusitanicum, along with other flavonoid glycosides from several Delphinium species, were tested for their antiparasitic activities against three Leishmania spp. and Trypanosoma cruzi.23 Compound 23 showed a higher trypanocidal activity with an IC50 value of 11.0 μM against the intracellular form of T. cruzi than was observed with the positive control BZN, which exerted an IC50 value of 23.3 μM. Compound 24 showed higher leishmanicidal activities against both the extra- and intracellular forms of L. infantum (IC50 values of 16.7 and 12.8 μM, respectively) and L. donovani (IC50 values of 12.5 and 15.5 μM, respectively) than the reference drug glucantime. Notably, compounds 23 and 24 also showed less toxicity to the corresponding host cells, highlighting their potential in the treatment of leishmaniasis and Chagas disease. Normally, the roots are the only officinal parts of Aconitum plants. The reseach presented above suggest that the flowers with abundant bioactive flavonoid glycosides might serve as a new officinal part of the Aconitum plants.
On the other side, similar to DAs, flavonoid components have also been proposed as chemotaxonomic markers of Aconitum plants to facilitate taxonomic discrimination. Fico et al. demonstrated that flavonoid profiles can be used to differentiate two A. napellus subspecies and that in turn, their flavonoid profiles differed from the profiles of A. paniculatum and A. vulparia. As a result, they suggested that species recognition within this large genus can be approached by using flavonoids as chemical molecular markers.24,25 Lim et al. also proved the utility of flavonoid analysis for the chemotaxonomy of Aconitum in their study on the flavonoid variations in members of the Korean A. jaluense complex.26 However, the relationship between the flavonoid composition and the degree of evolution of the Aconitum species remains unclear and requires more extensive and in-depth studies. Flavonoids and DAs are all common constituents in Aconitum plants distributed in different parts. Hence, we suggest that the chemotaxonomical studies on Aconitum plants should consider the flavonoid and DA components together, through analysis not only the structural diversity of flavonoid and DA components severally, but also their correlations in taxonomy of Aconitum genus.
2.2. Phenylpropanoids
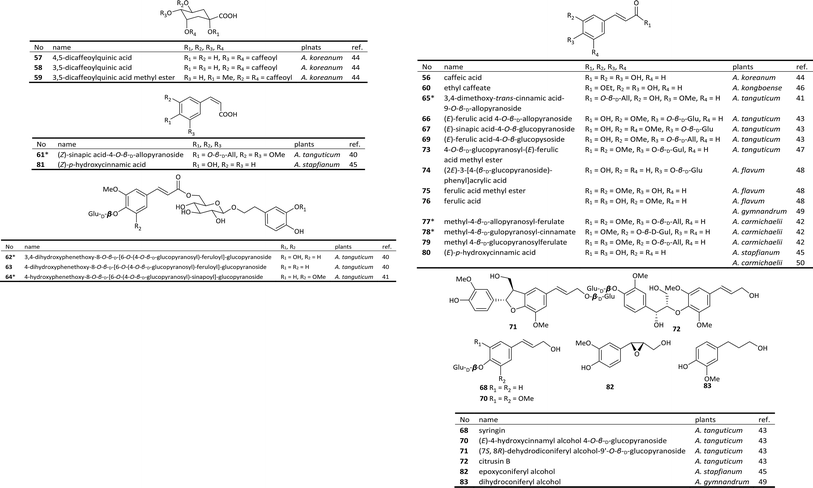
Phenylpropanoids, perhaps the most widespread type of natural products found in the plant kingdom, are composed of C6–C3 structural units biosynthesized from shikimic acid. Phenylpropanoids can be divided into three categories: phenylpropionic acids, coumarins and lignans. A series of phenylpropanoids isolated from Aconitum plants have been reported.As shown in Fig. 2, 22 phenylpropionic acids, including 6 new compounds (labelled with *), have been isolated from 8 species of the Aconitum genus.40–42 Phenylpropionic acids from Aconitum plants are generally glycosides of caffeic acid (56), ferulic acid (76), or p-hydroxycinnamic acid (80). Most of the double bonds in those acids are in the trans form. (Z)-Sinapic acid-4-O-β-D-allopyranoside (61), a new compound isolated from A. tanguticum, possesses a cis double bond. The glycosyl derivatives are generally monosaccharides composed of glucose, with only a small amount of gulose or allose, such as the new compounds 77 and 78.42 In addition to phenylpropionic acids, four phenylpropanols (68, 70–72) have also been isolated from A. tanguticum.43
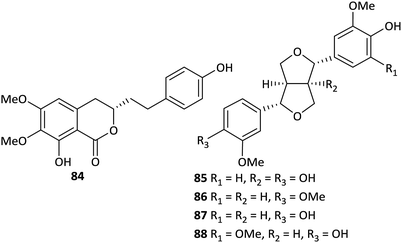
There are few reports on the lignan and coumarin components of Aconitum plants. Four furofuran-type lignans, namely (+)-1-hydroxy-pinoresinol (85) from A. gymnandrum,49 phillygenin (86) from A. stapfianum,45 (+)-pinoresinol (87) from A. tanguticum,47 and (+)-medioresinol (88) from A. kongboense,46 have been obtained to date (Fig. 3). In addition, a new coumarin derivative, 6,7-dimethoxy-8-hydroxy-3-[β-(p-hydroxyphenyl)ethyl]-3,4-dihydroisocoumarin (84), was obtained from A. gymnandrum.49
Several phenylpropanoids have displayed anti-inflammatory properties. Caffeic acid (56) and its derivatives 57–59 from A. koreanum inhibited NO production in LPS-stimulated RAW 264.7 mouse macrophages (IC50 values of 2.07, 1.20, 0.76, and 2.37 μM, respectively) significantly more effectively than was achieved by the positive control L-NMMA (IC50 value of 7.83 μM) by suppressing both the mRNA and protein expressions of iNOS and COX-2.44 New compound 78 from A. carmichaelii also inhibited NO production in LPS-stimulated RAW 264.7 cells with an IC50 value of 61.3 μg ml−1.42 Ferulic acid (76) inhibited TNF-α production in LPS-stimulated RAW 264.7 cells with an IC50 value of 27.6 μg ml−1.47 The consistency of anti-inflammatory activities between the phenylpropanoids and DAs suggests that they all jointly contribute to the antiphlogistic effects of the medicinal Aconitum plants.
Besides, studies showed that phenylpropanoids also possess antineoplastic activities. Phenylpropionic acid 73 displayed selective cytotoxicity against gastric cancer MGC80 cells with an IC50 value of 38.1 μg ml−1,49 and lignan 85 showed selective cytotoxicity against liver cancer HepG2 cells with an IC50 value of 48.7 μg ml−1. The Aconitum-derived drugs are also used in the treatment of various cancers. While a great many of DAs have been proved to possess certain cytotoxicity,10 these phenylpropanoids could also play a positive role in the antineoplastic effect of Acontim plants in conjunction with DAs.
2.3. Other phenolics and acids
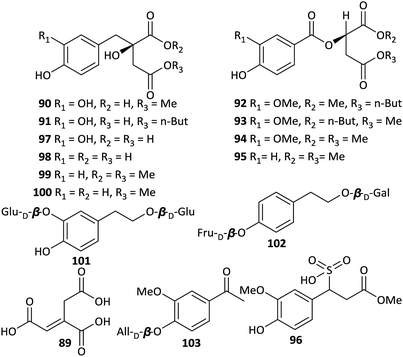
In addition to the phenylpropionic acids described above, other types of phenolics and acids have been isolated from Aconitum plants as well. The first representative was aconitic acid (89) (Fig. 4), which was first found in A. napellus in the 19th century and named after the Aconitum genus. A number of studies have revealed that aconitic acid is not restricted to Aconitum plants but plays a vital role in the tricarboxylic acid cycle of aerobes. In addition, Jiang et al. reported the isolation of seven new aromatic acid derivatives (90–96) and five known analogues (97–100) from the Fuzi.51 Compounds 90 and 91 are 2-benzylmalates (eucomate derivatives), 92–95 are 2-O-benzoylmalates, and 96 is a rare phenylpropionate containing a sulfonic acid group. The absolute configurations of eucomate derivatives were confirmed by X-ray crystallographic analysis of 4-methyl eucomate (100).Many benzoic or phenylacetic acid derivatives with hydroxyl, oxygen alkyl, or glycosyl substituents have been obtained from Aconitum plants. Most of these compounds are common, structurally simple and widely distributed in the plant kingdom; new structures are rarely discovered. Only three new glycosides, namely, 2-(3-O-β-D-glucopyranosyl-4-hydroxy-phenyl) ethanol 1-O-β-D-glucopyranoside (101), 2-(4-O-β-D-fructopyranosylphenyl)ethanol 1-O-β-D-galactopyranoside (102), and 3-methoxy-4-O-β-D-allopyranosyl acetophenone (103), were isolated from A. tanguticum.47 Compounds 101 and 103 also possess anti-inflammatory activities, which inhibited TNF-α production on LPS-stimulated RAW 264.7 macrophages with IC50 values of 38.2 and 27.6 μg ml−1, respectively.
2.4. Terpenoids and their glycosides
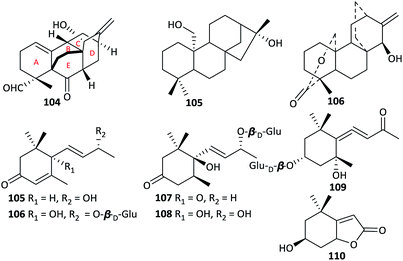
In contrast to the wide variety of DAs present in Aconitum plants, terpenoids are rare. To the best of our knowledge, only three non-alkaloidal diterpenoids from the genus Aconitum have been reported, namely, atropurpuran (104) from A. hemsleyanum var. atropurpureum,11 atisenol (105) from A. heterophyllum,52 and Guan fu diterpenoid A (106) from A. coreanum (Fig. 5).53 All of them were isolated as new compounds. These non-alkaloidal diterpenoids are of great interest for their biogenetic pathway; they may be the earlier intermediates in the DA biogenesis.54 Notably, atropurpuran (104) is the first example of a diterpenoid with a novel 6/6/6/6/6 carbocyclic framework, which features an unprecedented cage-like skeleton containing an unusual tetracyclo[5.3.3.04,9.04,12]-tridecane unit (rings B–E) fused to a highly functionalized cyclohexene fragment (ring A) with two adjacent chiral quaternary centres. Atropurpuran has drawn researchers' great attention for its intriguing structure, which has been introduced as “Hot off the Press” and synthesized successfully by outstanding chemists after report.55–57In addition, six megastigmane sesquiterpenoids and their glycosides, including (6R,7E,9R)-9-hydroxy-4,7-megastigma-dien-3-one (105) from A. gymnandrum,49 vomifoliol-O-β-D-glucopyranoside (106), dihydrovomifoliol-O-β-D-glucopyranoside (107), icariside B1 (108), (3R,5S,6S,7E,9R)-megastig-man-7-ene-3,5,6,9-tetrol-9-O-β-D-glucopy-ranoside (109) and loliolide (110) from A. tanguticum,39,43 have been reported. These compounds mainly vary in the degree of oxidation of their cyclohexene ring and the number and type of substituents on the side chain.
2.5. Steroids
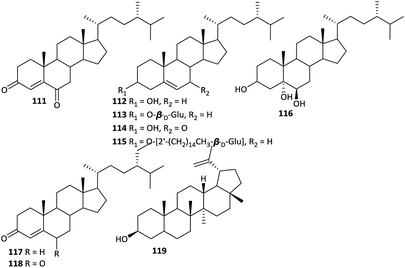
While little attention has been paid to the steroids in Aconitum plants, approximately nine steroids have been reported from various Aconitum plants (Fig. 6). Most of these steroids are common phytosterols, including β-sitosterol (112), daucosterol (113), 7-oxo-β-sitosterol (114), β-sitosteryl-3-O-β-D-glucopyranoside-2′-O-palmitate (115), sitostanetriol (116), β-sitost-4-en-3-one (117), 22-dihydro-stigmast-4-en-3,6-dione (118) and lupeol (119),31,45,58 which are common in many plants. 24S-ergost-4-en-3,6-dione (111) is the only new steroid isolated from Aconitum plants.592.6. Free fatty acids (FFAs)
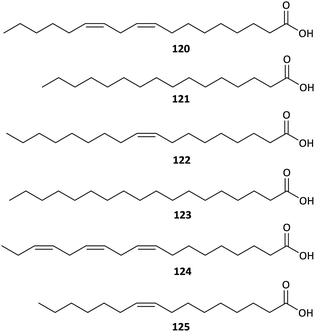
Fatty acids have been attracting increased interest from the food and pharmacy industries due to their nutritional and biological importance. But until now, there have been few studies on the FFA components of Aconitum plants, and these studies were performed using GC-MS techniques. A GC-MS-based analysis of the FFAs in A. taipeicum performed by Yue et al. revealed that the major FFAs in this plant are linoleic acid (120, 46.24%), palmitic acid (121, 28.24%), and oleic acid (122, 15.93%), followed by stearic acid (123, 4.35%), linolenic acid (124, 2.87%), and palmitoleic acid (125, 2.38%) (Fig. 7).60 Similar results were found in A. carmichaelii, in which linoleic acid (35.97%), palmitic acid (14.24%), oleic acid (7.42%) and linolenic acid (7.29%) are the major FFA components.61 In addition, Qu et al. reported that A. szechenyianum is remarkably rich in linoleic acid (124, 41.32%), palmitic acid (121, 29.64%) and linolenic acid (120, 23.77%).62 These FFAs could be bonded to C19-DAs through ester exchange reaction to generate lipo-alkaloids, a kind of DAs consist of a C19-DAs skeleton and one or two fatty acid side chains substituted at C-8 or C-14, which have been reported to exhibited impressive bioactivities.10,632.7. Polysaccharides
The discovery of bioactive homogeneous polysaccharides from Aconitum plants occurred much later than the identification of the small-molecule compounds described above due to the difficulties associated with both purification and identification of polysaccharides. In 1985, Chohachi et al. isolated the first four homogeneous polysaccharides, named aconitans A–D, from Fuzi using an activity-guided separation strategy, and the compounds showed strong hypoglycaemic effects in mice. However, their structures have not been clarified.64 Later, in 2006, Zhao et al. separated a water-soluble homogeneous polysaccharide, FPS-1, from the same herb, and the compound was determined to be an α-(1→6)-D-glucan with a weight-average molecular weight (m.w.) of approximately 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da. This glucan is highly branched with a single glucose at C-3 of every fourth residue along the main chain. FPS-1 displays various biological activities. In immunopharmacological studies, FPS-1 stimulated murine lymphocyte proliferation induced by concanavalin A or LPS in a potent manner both in vitro and in vivo in addition to stimulating splenocyte antibody production, giving this compound potential as a natural immunomodulator.65 FPS-1 also produces antidepressant-like and neurogenic effects with rapid onset in mice.66 In addition, FPS-1 has been shown to increase autophagy activity to protect against starvation-induced cytotoxicity in H9c2 cells through the AMPK/mTOR pathway activation, which could be exploited for drug discovery related to coronary heart disease and heart failure.67
000 Da. This glucan is highly branched with a single glucose at C-3 of every fourth residue along the main chain. FPS-1 displays various biological activities. In immunopharmacological studies, FPS-1 stimulated murine lymphocyte proliferation induced by concanavalin A or LPS in a potent manner both in vitro and in vivo in addition to stimulating splenocyte antibody production, giving this compound potential as a natural immunomodulator.65 FPS-1 also produces antidepressant-like and neurogenic effects with rapid onset in mice.66 In addition, FPS-1 has been shown to increase autophagy activity to protect against starvation-induced cytotoxicity in H9c2 cells through the AMPK/mTOR pathway activation, which could be exploited for drug discovery related to coronary heart disease and heart failure.67
Gao et al. reported a hot alkali extracted polysaccharide, AKP, from another important Aconitum herb Caowu, which was determined to be a linear α-(1→3),(1→4)-D-glucan with a m.w. of approximately 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, and the (1→3)-linked and (1→4)-linked α-D-Glup residues were in a ratio of 1
000 Da, and the (1→3)-linked and (1→4)-linked α-D-Glup residues were in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.68 AKP had significant in vitro antioxidant activities; it showed a high FRAP value and noticeable effects on the scavenging of free radicals and chelating ferrous ions.
7.68 AKP had significant in vitro antioxidant activities; it showed a high FRAP value and noticeable effects on the scavenging of free radicals and chelating ferrous ions.
Several studies have evaluated the bioactive polysaccharides present in Korean monkshood (A. coreanum), which is also called Guanbaifu in TCM. The homogeneous polysaccharide KMPS-2A consisted of a [α-1,6-D-Glc]n repeating structure with a m.w. of 67 6000 Da, and its sulfated derivatives exhibited better anticomplementary activity than heparin, which indicated its potential as a complement inhibitor.69 Another polysaccharide, KMPS-2E, consisted of [→6)-β-D-Galp (1→3)-β-L-Rhap-(1→4)-β-D-GalpA-(1→3)-β-D-Galp-(1→] units with→5)-β-D-Arap(1→3,5)-β-D-Arap(1→side chains attached to the backbone through O-4 of (1→3,4)-L-Rhap. T-β-D-Galp is attached to the backbone through O-6 of the (1→3,6)-β-D-Galp residues, and T-β-D-Ara is connected to the end group of each chain.70 KMPS-2E showed significant anti-inflammatory effects both in LPS-stimulated RAW 264.7 macrophages and carrageenan-induced hind paw oedema models. KMPS-2E could inhibit the expression of iNOS and inflammatory cytokines mediated by the NF-kB signalling pathways in macrophages. Moreover, KMPS-2E has no cytotoxicity up to 200 mg ml−1, which highlights its potential in the development of novel, natural anti-inflammatory agents.
Plant polysaccharides are well-known for their biocompatible and non-toxic properties, which have been applied to clinical side and the market of health products widely. As summarized above, polysaccharides from Aconitum plants have displayed broad bioactivities, including antioxidant, anti-tumour, antiphlogistic and immunoregulatory activities, as well as beneficial effects on the cardio-cerebrovascular system. Hence, polysaccharides from Aconitum plants may be considered to serve as a new individual effective part in the application of functional foods and drugs.
3. Conclusions
Aconitum is an important genus that is widely used in TCM and other Asian medical traditions. The data summarized above revealed that there are abundant non-alkaloidal components, including flavonoids, phenylpropanoids, phenolic acids, terpenoids, steroids, FFAs and polysaccharides, with broad bioactivities in Aconitum plants. In view of the chemical diversity described, the major classes of non-alkaloidal constituents found in the Aconitum plants were flavonoids, followed by phenylpropanoids and other phenolic acids. Among these compounds, several are new compounds, and their various biological activities have been reported. These findings underscore the large chemical and biological diversity of non-alkaloidal constituents in Aconitum plants, which could not only serve as a vast resource for drug discovery separately, but also contribute to the therapeutic effects of Aconitum-derived drugs.Although phytochemical and biological studies on non-alkaloidal constituents of Aconitum species have received considerable interest, some deficiencies remain. Firstly, most of the current studies are still aimed at illuminating the alkaloids in Aconitum species and not at characterizing the non-alkaloidal constituents. And investigations of non-alkaloidal constituents are restricted to the widespread Aconitum species, such as A. carmichaelii, A. tanguticum in China, while most less-common species are still largely unexplored. The potential role of non-alkaloidal constituents in the pharmacological activities of Aconitum plants remains ignored, as well as their chemotaxonomical significance. An extensive investigation of other species, especially those species used medicinally, is necessary. Secondly, various biological activities of the extracts and pure compounds were mainly investigated by using in vitro tests and less were carried out by in vivo models. Therefore, there are few reported data focused on toxicity, side effects, and clinical efficiency of these non-alkaloidal constituents. The few pharmacological studies are still insufficient to validate the effects of the non-alkaloidal constituents in Aconitum species, which hinders its application and promotion. It is necessary to evaluate the biological activities of the non-alkaloidal constituents using both in vitro and in vivo models.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by a grant from the National Natural Science Foundation of China (No. 31860095) and a grant from Guizhou Science and Technology Foundation of China (No. QKHJC[2018]1193).References
- L. Q. Li and K. Yuichi, Flora of China, 2001, vol. 6, pp. 149–222 Search PubMed.
- G. Zhou, L. Tang, X. Zhou, T. Wang, Z. Kou and Z. Wang, J. Ethnopharmacol., 2015, 160, 173–193 CrossRef CAS.
- E. Nyirimigabo, Y. Xu, Y. Li, Y. Wang, K. Agyemang and Y. Zhang, J. Pharm. Pharmacol., 2015, 67, 1–19 CrossRef CAS.
- P. G. Xiao, F. P. Wang, F. Gao, L. P. Yan, D. L. Chen and Y. Liu, Acta Phytotaxon. Sin., 2006, 44, 1–46 CrossRef.
- F. P. Wang, Q. H. Chen and X. Y. Liu, Nat. Prod. Rep., 2009, 27, 529–570 RSC.
- Q. Guo, H. Xia, G. Shi, T. Zhang and J. Shi, Org. Lett., 2018, 20, 816–819 CrossRef CAS PubMed.
- N. H. Chen, Y. B. Zhang, W. Li, P. Li, L. F. Chen, Y. L. Li, G. Q. Li and G. C. Wang, RSC Adv., 2017, 7, 24129–24132 RSC.
- Y. Shu, T. P. Yin, J. P. Wang, D. Gan, Q. Y. Zhang, L. Cai and Z. T. Ding, Chin. J. Nat. Med., 2018, 16, 866–870 Search PubMed.
- M. Reina and A. González-Coloma, Phytochem. Rev., 2007, 6, 81–95 CrossRef CAS.
- X. X. Liang, Y. Y. Gao and S. X. Luan, RSC Adv., 2018, 8, 23937–23946 RSC.
- P. Tang, Q. H. Chen and F. P. Wang, Tetrahedron Lett., 2009, 50, 460–462 CrossRef CAS.
- I. I. Samokish, A. L. Shinkarenko and V. A. Makarov, Chem. Nat. Compd., 1969, 5, 364–365 CrossRef.
- V. D. Loi, B. T. Tung, N. T. Hai and N. T. Vung, J. Chem. Pharm. Res., 2015, 7, 228–234 Search PubMed.
- B. B. Shrestha, S. Dall'Acqua, M. B. Gewali, P. K. Jha and G. Innocenti, Carbohydr. Res., 2006, 341, 2161–2165 CrossRef CAS PubMed.
- C. Mariani, A. Braca, S. Vitalini, N. De Tommasi, F. Visioli and G. Fico, Phytochemistry, 2008, 69, 1220–1226 CrossRef CAS PubMed.
- L. Xu, X. Zhang, L. M. Lin, C. Li, Z. M. Wang and Y. M. Luo, J. Asian Nat. Prod. Res., 2013, 15, 737–742 CrossRef CAS PubMed.
- N. N. Pogodaeva, S. V. Fedorov, L. V. Kanitskaya and A. A. Semenov, Russ. Chem. Bull., 2000, 49, 1905–1909 CrossRef CAS.
- A. Braca, G. Fico, I. Morelli, F. De Simone, F. Tomè and N. De Tommasi, J. Ethnopharmacol., 2003, 86, 63–67 CrossRef CAS.
- S. Vitalini, A. Braca, D. Passarella and G. Fico, Fitoterapia, 2010, 81, 940–947 CrossRef CAS PubMed.
- J. M. He, T. P. Yin, Y. Chen, L. Cai, Z. G. Tai, Z. J. Li, C. S. Liu, Y. R. Wang and Z. T. Ding, J. Funct. Foods, 2015, 17, 371–379 CrossRef CAS.
- T. P. Yin, L. Cai, Y. Xing, J. Yu, X. J. Li, R. F. Mei and Z. T. Ding, J. Asian Nat. Prod. Res., 2016, 18, 603–610 CrossRef CAS.
- H. Ahmad, S. Ahmad, S. A. A. Shah, A. Latif, M. Ali, F. A. Khan, M. N. Tahir, F. Shaheen, A. Wadood and M. Ahmad, Bioorg. Med. Chem., 2017, 25, 3368–3376 CrossRef CAS.
- C. Marín, J. G. Díaz, D. Irure Maiques, I. Ramírez-Macías, M. J. Rosales, R. Guitierrez-Sánchez, R. Cañas and M. Sánchez-Moreno, Phytochem. Lett., 2017, 19, 196–209 CrossRef.
- F. Gelsomina, S. Alberto, B. Alessandra, A. Elisabetta, M. Ivano and T. Franca, Biochem. Syst. Ecol., 2003, 31, 293–301 CrossRef.
- J. G. Díaz, J. G. Ruiz, B. R. Días, J. A. G. Sazatornil and W. Herz, Biochem. Syst. Ecol., 2005, 33, 201–205 CrossRef.
- C. E. Lim, J. H. Park and C. W. Park, Plant Syst. Evol., 1999, 218, 125–131 CrossRef CAS.
- G. Fico, A. Braca, I. Morelli and F. Tomè, Fitoterapia, 2003, 74, 420–422 CrossRef CAS.
- I. I. Samokish, Chem. Nat. Compd., 1974, 10, 809 CrossRef.
- T. Zhapova, L. D. Modonova, N. N. Pogodaeva, A. L. Vereshchagin, A. G. Gorshkov, S. V. Zinchenko and A. A. Semenov, Chem. Nat. Compd., 1992, 28, 421–429 CrossRef.
- W. K. Whang, I. S. Oh, M. T. Lee and I. H. Kim, Korean J. Pharmacogn., 1994, 25, 336–341 CAS.
- D. K. Kim, J. H. Kwak, K. W. Song, H. C. Kwon, O. P. Zee and K. R. Lee, Korean J. Pharmacogn., 1996, 27, 75–79 CAS.
- H. J. Jeong, W. K. Whang and I. H. Kim, Planta Med., 1997, 63, 329–334 CrossRef CAS PubMed.
- G. Fico, A. Braca, A. R. Bilia, F. Tomè and I. Morelli, J. Nat. Prod., 2000, 63, 1563–1565 CrossRef CAS.
- S. Vitalini, A. Braca and G. Fico, Phytochem. Lett., 2012, 5, 476–479 CrossRef CAS.
- G. Fico, A. Braca, N. De Tommasi, F. Tomè and I. Morelli, Phytochemistry, 2001, 57, 543–546 CrossRef CAS.
- G. Fico, A. Braca, A. R. Bilia, F. Tomè and I. Morelli, Planta Med., 2001, 67, 287–290 CrossRef CAS PubMed.
- S. Dall'Acqua, B. B. Shrestha, M. B. Gewali, P. K. Jha, M. Carrara and G. Innocenti, Nat. Prod. Commun., 2008, 3, 1985–1989 CrossRef.
- J. C. Luis, F. Valdes, R. Martin, A. J. Carmona and J. G. Diaz, Fitoterapia, 2006, 77, 469–471 CrossRef CAS PubMed.
- M. Luo, L. M. Lin, C. Li, Z. M. Wang and W. B. Guo, China J. Chin. Mater. Med., 2012, 37, 1245–1248 CAS.
- L. Xu, M. Luo, L. M. Lin, X. Zhang, C. Li, Z. M. Wang and Y. M. Luo, J. Asian Nat. Prod. Res., 2013, 15, 743–749 CrossRef CAS.
- Y. R. Li, L. Xu, C. Li, Z. M. Wang and L. X. Yang, J. Asian Nat. Prod. Res., 2014, 16, 730–734 CrossRef CAS.
- T. Q. Do, B. N. Truong, H. D. T. Mai, T. L. Nguyen, V. H. Nguyen, H. D. Nguyen, T. D. Nguyen, T. C. Nguyen, T. V. Luong, L. T. Giang, V. M. Chau and V. C. Pham, J. Asian Nat. Prod. Res., 2018, 1–9 CrossRef.
- L. Xu, Y. R. Li, C. Li, L. M. Lin, Z. M. Wang and Y. M. Luo, China J. Chin. Mater. Med., 2013, 38, 2018–2825 Search PubMed.
- K. H. Park, M. Park, S. E. Choi, M. S. Jeong, J. H. Kwon, M. H. Oh and M. W. Lee, Biol. Pharm. Bull., 2009, 32, 2029–2033 CrossRef CAS.
- T. P. Yin, Y. Chen, Y. Shu, L. Cai and Z. T. Ding, Yunnan Daxue Xuebao, 2018, 40, 148–153 Search PubMed.
- J. J. Fu, J. J. Qin, Q. Zeng, H. Z. Jin and W. D. Zhang, Chem. Nat. Compd., 2011, 47, 854–855 CrossRef CAS.
- Y. R. Li, T. Liu, R. Y. Yan, L. Q. Hui, L. M. Lin, C. Y. Cao, S. S. Guo, L. X. Yang, W. H. Feng, C. Li and Z. M. Wang, Phytochem. Lett., 2015, 11, 311–315 CrossRef CAS.
- X. F. Wang, L. Yu, W. J. Hao, L. Ma, L. Yin and X. Y. Fu, Chem. Nat. Compd., 2016, 52, 769–770 CrossRef CAS.
- J. Q. Zhao, Y. M. Wang, S. Wang, J. Dang, Y. P. Shi, L. J. Mei and Y. D. Tao, Nat. Prod. Res., 2016, 30, 1746–1752 CrossRef CAS PubMed.
- J. Zhang, G. B. Sun, Q. F. Lei, G. Z. Li, J. C. Wang and J. Y. Si, Acta Pharmacol. Sin., 2014, 49, 1150–1154 CAS.
- Z. B. Jiang, B. Y. Jiang, C. G. Zhu, Q. L. Guo, Y. Peng, X. L. Wang, S. Lin and J. G. Shi, J. Asian Nat. Prod. Res., 2014, 16, 891–900 CrossRef CAS.
- S. W. Pelletier, A. M. M. Ateya, J. Finer-Moore, N. V. Mody and L. C. Schramm, J. Nat. Prod., 1982, 45, 779–781 CrossRef CAS.
- C. H. Yang, X. C. Wang, Q. F. Tang, W. Y. Liu and J. H. Liu, Helv. Chim. Acta, 2008, 91, 759–765 CrossRef CAS.
- M. Weber, K. Owens and R. Sarpong, Tetrahedron Lett., 2015, 56, 3600–3603 CrossRef CAS PubMed.
- A. H. Robert and A. Sutherland, Nat. Prod. Rep., 2009, 26, 151 RSC.
- J. Gong, H. Chen, X. Y. Liu, Z. X. Wang, W. Nie and Y. Qin, Nat. Commun., 2016, 7, 12183 CrossRef CAS PubMed.
- T. Suzuki, A. Sasaki, N. Egashira and S. Kobayashi, Angew. Chem., Int. Ed. Engl., 2011, 50, 9177–9179 CrossRef CAS PubMed.
- H. Y. Wang, A. X. Zuo, Y. Sun and G. X. Rao, J. Chin. Med. Mater., 2014, 37, 1391–1395 CAS.
- K. A. Eshbakova, B. Tashkhodzhaev, Z. I. Tursunov, K. K. Turgunov, K. M. Bobakulov and N. D. Abdullaev, Chem. Nat. Compd., 2011, 47, 73–75 CrossRef CAS.
- X. F. Yue, Y. N. Zhang, J. Zhang and Z. Q. Zhang, Anal. Methods, 2010, 2, 668 RSC.
- Y. Liang, G. Y. Yan, J. L. Wu, X. Zong, Z. Liu, H. Zhou, L. Liu and N. Li, Phytochem. Anal., 2018, 29, 398–405 CrossRef CAS PubMed.
- W. X. Qu, Z. L. Mou, H. Y. Cui and Z. Q. Zhang, Phytochem. Anal., 2011, 22, 199–204 CrossRef CAS PubMed.
- T. Kiss, B. Borcsa, P. Orvos, L. Tálosi, J. Hohmann and D. Csupor, Planta Med., 2017, 83, 1321–1328 CrossRef CAS PubMed.
- C. Konno, M. Murayama, K. Sugiyama, M. Arai, M. Murakami, M. Takahashi and H. Hikino, Planta Med., 1985, 51, 160–161 CrossRef PubMed.
- C. Zhao, M. Li, Y. Luo and W. Wu, Carbohydr. Res., 2006, 341, 485–491 CrossRef CAS PubMed.
- H. C. Yan, H. D. Qu, L. R. Sun, S. J. Li, X. Cao, Y. Y. Fang, W. Jie, J. C. Bean, W. K. Wu, X. H. Zhu and T. M. Gao, Int. J. Neuropsychopharmacol., 2010, 13, 623–633 CrossRef CAS PubMed.
- L. Z. Liao, Y. L. Chen, L. H. Lu, Y. H. Zhao, H. L. Guo and W. K. Wu, Am. J. Chin. Med., 2013, 41, 353–367 CrossRef CAS PubMed.
- T. Gao, H. Bi, S. Ma and J. Lu, Int. J. Biol. Macromol., 2010, 46, 85–90 CrossRef CAS PubMed.
- X. J. Li, J. Y. Jiang, S. S. Shi, Y. Li, Y. B. Jiang, Y. Ke and S. C. Wang, Chem. J. Chin. Univ., 2014, 35, 1423–1426 CAS.
- X. J. Li, J. Y. Jiang, S. S. Shi, S. W. A. Bligh, Y. Li, Y. B. Jiang, D. Huang, Y. Ke and S. C. Wang, PLoS One, 2014, 9, e99697 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2019 |