Open Access Article
Open Access ArticleCharacterization of biosurfactant lipopeptide and its performance evaluation for oil-spill remediation†
Jun-Qiao Feng a,
Hong-Ze Ganga,
Dong-Sheng Lia,
Jin-Feng Liua,
Shi-Zhong Yanga and
Bo-Zhong Mu
a,
Hong-Ze Ganga,
Dong-Sheng Lia,
Jin-Feng Liua,
Shi-Zhong Yanga and
Bo-Zhong Mu *ab
*ab
aState Key Laboratory of Bioreactor Engineering, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, P. R. China. E-mail: bzmu@ecust.edu.cn; Fax: +86 21 64252485; Tel: +86 21 64252063
bEngineering Research Center of Microbial Enhanced Oil Recovery, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China
First published on 26th March 2019
Abstract
Biosurfactant lipopeptide is a promising dispersant over varieties of chemical ones in oil-spill remediation. The toxicity, biodegradability and performance of the biosurfactant lipopeptide are studied in this paper.
Dispersants were globally applied to physico-chemically enhance the dispersion of oil in water and were assumed to stimulate oil biodegradation by indigenous microorganisms and to reduce the environmental impact of oil spills.1,2 Since the 1960s,3,4 chemical dispersants have been applied as an emergency response to oil spills in marine ecosystems,5 and have showed effectiveness at removing oil slicks from the coast.3,6,7 However, most of the chemically synthesized dispersants are inherently toxic to various aquatic species and hardly biodegradable in the natural environment.2,8 The application of chemical dispersants in the 2010 Gulf of Mexico oil spill also raised concerns regarding the toxicity and the potential environmental impact,9,10 and caused a debate about the effectiveness of chemical dispersants on the rates of oil biodegradation.11 Biosurfactants are promising dispersants in oil-spill remediation, owning to their environmentally friendly and biodegradable properties.12 Chemical surfactants could be replaced with biosurfactants and this change would diminish the environmental impact of traditional dispersants.8,13,14 Lipopeptide produced by microorganisms is one of the representative biosurfactants and has showed great potential applications in food,15 medicine,16 microbial enhanced oil recovery,17 and other fields.18 Nevertheless, the knowledge about the application of biosurfactant lipopeptide in marine oil-spill remediation is still limited.
In the present work, the dispersion effectiveness, aquatic toxicity, biodegradability and environmental compatibility of the biosurfactant lipopeptide were determined using recognized standardized methods,19–23 and the biosurfactant lipopeptide used as a bio-dispersant for marine oil-spill remediation were studied, which is, to the best of our knowledge, the first report about biosurfactant lipopeptide used in oil-spill remediation.
The lipopeptide samples were isolated from cell-free broth of B. subtilis HSO121 at our laboratory.24 The typical chemical structure of the lipopeptide used in the study was shown in Fig. 1 and its critical micelle concentration (CMC) was 8.69 × 10−5 mol L−1.
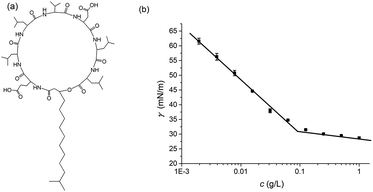 | ||
| Fig. 1 Typical chemical structure of lipopeptides (a) and the surface tensions of lipopeptides respect to concentrations (b). | ||
Dispersion effectiveness (DE) of lipopeptides was examined at different surfactant-to-oil ratios (SORs), temperatures, pH values, and salinities. It indicated in Fig. 2 that DE of lipopeptides reached 70.23% at SORs of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w) at 25 °C, pH 7 and the present of 3% NaCl (w/v). It should be noticed that the DE of lipopeptides was almost kept when SORs dropped to 1
10 (w/w) at 25 °C, pH 7 and the present of 3% NaCl (w/v). It should be noticed that the DE of lipopeptides was almost kept when SORs dropped to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 w/w. The increase in DE with increasing SORs can be attributed to the generation of emulsions with smaller droplets and lower rising velocity.2 Sharp drop off in DE was observed for lipopeptides when SORs below 1
250 w/w. The increase in DE with increasing SORs can be attributed to the generation of emulsions with smaller droplets and lower rising velocity.2 Sharp drop off in DE was observed for lipopeptides when SORs below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (w/w), and DE value was 36.45% at an extreme SORs of 1
500 (w/w), and DE value was 36.45% at an extreme SORs of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1250 (w/w). It had been reported that the abrupt decline for 80
1250 (w/w). It had been reported that the abrupt decline for 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 lecithin
20 lecithin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tween 80 (w/w) surfactant happened when SORs below 1
Tween 80 (w/w) surfactant happened when SORs below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 v/v, from 77% (SORs 1
100 v/v, from 77% (SORs 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 v/v) to 15% (SORs 1
100 v/v) to 15% (SORs 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 v/v),25 indicating a lower SOR in lipopeptides usage could reach its maximum effectiveness. Lipopeptides exhibited >70% DE values with temperatures ranged from 15 °C to 25 °C, and an increasing DE values when pH values raised, the largest DE was 77.45% at pH 11. DE of lipopeptides increased from 56.12% to 71.14% with increase in salinity. Higher DE at higher salinity was observed for anionic biosurfactants, which can be attributed to the electrostatic repulsion between polar head groups reduced by ions, and a close-packed arrangement of surfactant molecules at the oil–water interface were formed.2
200 v/v),25 indicating a lower SOR in lipopeptides usage could reach its maximum effectiveness. Lipopeptides exhibited >70% DE values with temperatures ranged from 15 °C to 25 °C, and an increasing DE values when pH values raised, the largest DE was 77.45% at pH 11. DE of lipopeptides increased from 56.12% to 71.14% with increase in salinity. Higher DE at higher salinity was observed for anionic biosurfactants, which can be attributed to the electrostatic repulsion between polar head groups reduced by ions, and a close-packed arrangement of surfactant molecules at the oil–water interface were formed.2
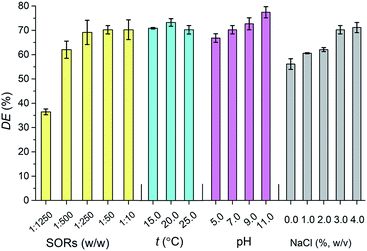 | ||
Fig. 2 The dispersion effectiveness (DE) of lipopeptides under different SORs ( ), temperatures ( ), temperatures ( ), pH values ( ), pH values ( ) and salinities ( ) and salinities ( ). ). | ||
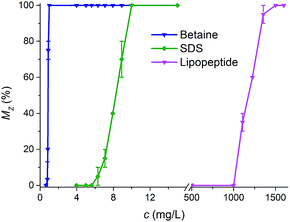
Mortalities of zebrafish under different concentrations of different surfactants were shown in Fig. 3. It was evident that the toxicity of lipopeptides was far less than those of sodium dodecyl sulfate (SDS) and 3-(N,N-dimethyl palmityl ammonio) propane sulfonate (Betaine). The 24 h median lethal concentration (LC50) values were calculated and showed in Table 1. The 24 h LC50 values of lipopeptides, SDS, and Betaine for zebrafish were 1145 mg L−1, 8.25 mg L−1, and 0.872 mg L−1, respectively. The toxicity of SDS was comparable to the study by Edwards et al.,26 in which an 96 h LC50 of 1.9 mg L−1 for Cyprinodon variegatus was reported. Low toxicities of lipopeptides on whiteleg shrimp and copepods were also evaluated that the 96 h LC50 of lipopeptides from Bacillus sp. GY19 were 1050 mg L−1 and 1174 mg L−1, respectively.27
| Surfactant | LC50 (mg L−1) | 95% confidence intervals (mg L−1) | r |
|---|---|---|---|
| Lipopeptide | 1145 | 1090–1229 | 0.981 |
| SDS | 8.25 | 7.75–8.77 | 0.998 |
| Betaine | 0.872 | 0.853–0.890 | 0.988 |
Fig. 4 illustrated the evolution of Pseudokirchneriella subcapitata concentrations in the algal growth inhibition test of lipopeptides and SDS. Growth rates of P. subcapitata were decreased with the increase of surfactant concentrations. 72 h median effect concentration (EC50) values were calculated using linear regression analysis based on the dose–response curves,28 and the 72 h EC50 value of lipopeptide was 1703 mg L−1, which was about 45 times higher than that of SDS, 36.51 mg L−1. EC50 was in well accordance with LC50 mentioned above, indicating that lipopeptides showed a much lower toxicity than that of SDS. The 72 h median inhibitory concentration (IC50, equivalent to EC50) of SDS on Raphidocelis subcapitata was 36.58 mg L−1,29 which was relatively close to the result in this work. However, De Oliveira et al.28 showed that the EC50 of crude surfactin on Selenastrum capricornutum (named as well as P. subcapitata) from B. subtilis ICA56 was 49.3 mg L−1. The lower toxicity of lipopeptides in our study was probably because the lipopeptides from various Bacillus sp. strains might have different activity.27 The EC50 values for 9 types of surfactants including anionic surfactants, nonionic surfactants, and zwitterionic surfactants on P. subcapitata were range from 1.5 to 4.4 mg L−1.30 Hence, according to data mentioned, lipopeptides from HSO121 in the present showed less toxicity.
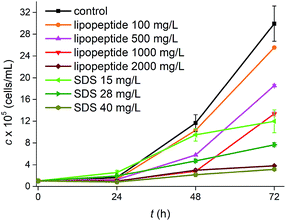 | ||
| Fig. 4 Effect of lipopeptides and SDS on growth concentrations (c) of Pseudokirchneriella subcapitata during 72 hours of incubation. | ||
Biodegradabilities of lipopeptides and sodium dodecyl benzene sulfonate (SDBS) versus time were shown in Fig. 5. With the same initial concentrations, 30 mg L−1, biodegradability values of biosurfactant lipopeptides and the synthetic surfactant SDS after 7 days incubation were 100% and 98.83%, respectively. Lipopeptides degraded much faster than SDBS that the degradability was nearly 100% after 3 days. Biodegradability of lipopeptides by P. putida CECT 324 strain, around 82% after 3 days, was reported, which was higher than that of amine oxides.29 Lipopeptides showed higher biodegradability as 94.01% even increasing the initial concentration to 300 mg L−1. Lima et al.31 studied the biodegradability of surfactants and observed the lowest decrease (24.8%) in SDS, the highest decrease in lipopeptides (69.1%) and glycolipid (73.4%). Biosurfactants were seemed to be more biodegradable than synthetic surfactants.
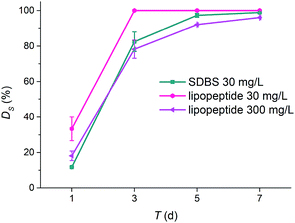 | ||
| Fig. 5 Degradation of surfactants (DS) including lipopeptides and SDBS during 7 d test in activated sludge systems. | ||
The degradation rates of aliphatic fractions (n-C11–C29) in crude oil (Xinjiang oilfield, P. R. China) were analysis using GC-MS and the results were illustrated in Fig. 6. Significant degradation rates of n-alkanes were observed in presence of biosurfactant lipopeptides, while n-alkanes dispersed by commercial dispersant degraded similar to that in control group (in absence of any surfactant). It was well known that the degradation rates of alkanes decreased and finally vanished with increase in the chain length of hydrocarbon. In the present study, lipopeptides showed excellent activity in accelerating degradation of long-chain hydrocarbons after 2 days. The alkanes degradation rates treated by lipopeptides after 1 day and 2 days were 38.78% (Fig. 6a) and 71.45% (Fig. 6b), respectively, which were much higher than those of commercial dispersant-treated group (9.16% and 34.16% after 1 day and 2 days treatment) and control group (13.26% and 33.55% after 1 day and 2 days treatment). It was reported that commercial chemical dispersants such as Corexit 9500A and GM-2 made no enhancement to the degradation of the petroleum hydrocarbons, whereas biosurfactants such as rhamnolipids enhanced the degradation.32,33 Degradation stimulation by lipopeptides could be attributed to their good dispersion activities and biocompatible. Dispersed oil droplets formed and considerable interfacial area was available to the microorganisms followed by microbial bioremediation. In addition, microorganisms could utilize the nutrients derived from culture broth as an excellent substrate for growth.34
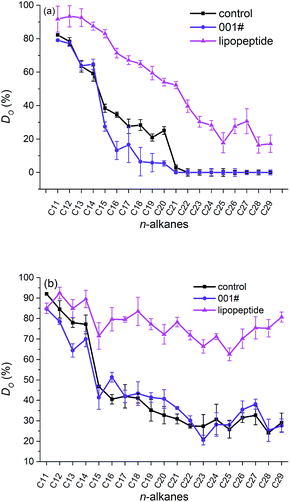 | ||
| Fig. 6 Degradation of n-alkanes in crude oil (DO) either non-dispersed (control) or dispersed by commercial dispersant (001#) and lipopeptides at day 1 (a), day 2 (b). | ||
Conclusions
In the present study, lipopeptides secreted by Bacillus subtilis HSO121 showed good dispersion effectiveness on crude oil (Xinjiang oilfield, P. R. China) at low SOR and kept this activity against temperature, pH values, and salinity. Comparing with SDS and Betaine, lipopeptides exhibited much lower toxicity, significant biocompatible, and higher activity in stimulating in microbial biodegradation of crude oil. In conclusion, lipopeptides acted effectively at dispersing oil and performed excellently at stimulating microbial oil biodegradation, which indicated its application in oil spill cleaning.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by the National High Technology Research and Development Program of China (2013AA064403), the Fundamental Research Funds for the Central Universities of China (222201717017, 22221818014), and Research Program of State Key Laboratory of Bioreactor Engineering.Notes and references
- S. Kleindienst, J. H. Paul and S. B. Joye, Nat. Rev. Microbiol., 2015, 13, 388–396 CrossRef CAS PubMed.
- E. Nyankson, M. J. DeCuir and R. B. Gupta, ACS Sustainable Chem. Eng., 2015, 3, 920–931 CrossRef CAS.
- R. R. Lessard and G. Demarco, Spill Sci. Technol. Bull., 2000, 6, 59–68 CrossRef CAS.
- EMSA, Manual on the applicability of oil spill dispersants, version 2, 2009 Search PubMed.
- O. G. Brakstad, T. R. Storseth, A. Brunsvik, K. Bonaunet and L. G. Faksness, Chemosphere, 2018, 204, 290–293 CrossRef CAS PubMed.
- H. Chapman, K. Purnell, R. J. Law and M. F. Kirby, Mar. Pollut. Bull., 2007, 54, 827–838 CrossRef CAS PubMed.
- L. S. Stoermer and L. G. Butler, International Oil Spill Conference, Tampa, Florida, 2001 Search PubMed.
- R. S. Makkar and S. S. Cameotra, Appl. Microbiol. Biotechnol., 2002, 58, 428–434 CrossRef CAS PubMed.
- R. Almeda, C. Hyatt and E. J. Buskey, Ecotoxicol. Environ. Saf., 2014, 106, 76–85 CrossRef CAS PubMed.
- R. C. Prince, Environ. Sci. Technol., 2015, 49, 6376–6384 CrossRef CAS PubMed.
- S. M. Techtmann, M. B. Zhuang, P. Campo, E. Holder, M. Elk, T. C. Hazen, R. Conmy and J. W. S. Domingo, Appl. Microbiol. Biotechnol., 2017, 83, e03462-16 Search PubMed.
- H. Y. Gong, Y. M. Li, M. T. Bao, D. Lv and Z. N. Wang, RSC Adv., 2015, 5, 37640–37647 RSC.
- R. Marchant and I. M. Banat, Biotechnol. Lett., 2012, 34, 1597–1605 CrossRef CAS PubMed.
- E. Nyankson, D. Rodene and R. B. Gupta, Water, Air, Soil Pollut., 2015, 227, 29 CrossRef.
- G. S. Kiran, S. Priyadharsini, A. Sajayan, G. B. Priyadharsini, N. Poulose and J. Selvin, Front. Microbiol., 2017, 8, 1138 CrossRef PubMed.
- F. Rivardo, R. J. Turner, G. Allegrone, H. Ceri and M. G. Martinotti, Appl. Microbiol. Biotechnol., 2009, 83, 541–553 CrossRef CAS PubMed.
- T. de Sousa and S. Bhosle, Bioresour. Technol., 2012, 123, 256–262 CrossRef CAS PubMed.
- I. Mnif and D. Ghribi, Biopolymers, 2015, 104, 129–147 CrossRef CAS PubMed.
- OECD, OECD guidelines for testing of chemicals: algal growth inhibition test, test guideline no. 201, 1984 Search PubMed.
- ISO, Water quality—determination of the acute lethal toxicity of substances to a freshwater fish, 1996, ISO 7346-7341 Search PubMed.
- SBQTS, Test method for biodegradability of surfactants, GB 15818-12006, 2006 Search PubMed.
- SBQTS, Oil Spill Dispersant-Technical Regulations, GB 18188.18181-12000, 2000 Search PubMed.
- EPA, EPA NCP Product Schedule 40 CFR Part 300, 1996, appendix C Search PubMed.
- X. Y. Liu, S. Z. Yang and B. Z. Mu, J. Pept. Sci., 2008, 14, 864–875 CrossRef CAS PubMed.
- D. A. Riehm, J. E. Neilsen, G. D. Bothun, V. T. John, S. R. Raghavan and A. V. McCormick, Mar. Pollut. Bull., 2015, 101, 92–97 CrossRef CAS PubMed.
- K. R. Edwards, J. E. Lepo and M. A. Lewis, Mar. Pollut. Bull., 2003, 46, 1309–1316 CrossRef CAS.
- W. Rongsayamanont, S. Soonglerdsongpha, N. Khondee, O. Pinyakong, C. Tongcumpou, D. A. Sabatini and E. Luepromchai, J. Hazard. Mater., 2017, 334, 168–177 CrossRef CAS PubMed.
- D. W. De Oliveira, A. B. Cara, M. Lechuga-Villena, M. Garcia-Roman, V. M. Melo, L. R. Goncalves and D. A. Vaz, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2017, 52, 174–181 CrossRef CAS PubMed.
- E. Liwarska-Bizukojc, K. Miksch, A. Malachowska-Jutsz and J. Kalka, Chemosphere, 2005, 58, 1249–1253 CrossRef CAS PubMed.
- Z. Pavlic, Z. Vidakovic-Cifrek and D. Puntaric, Chemosphere, 2005, 61, 1061–1068 CrossRef CAS PubMed.
- T. M. S. Lima, L. C. Procópio, F. D. Brandão, A. M. X. Carvalho, M. R. Tótola and A. C. Borges, Biodegradation, 2011, 22, 585–592 CrossRef CAS PubMed.
- Y. R. Pi, M. T. Bao, Y. Q. Liu, T. Y. Lu and R. He, J. Cleaner Prod., 2017, 153, 74–82 CrossRef CAS.
- Y. R. Pi, B. Chen, M. T. Bao, F. Q. Fan, Q. H. Cai, L. Ze and B. Y. Zhang, Bioresour. Technol., 2017, 232, 263–269 CrossRef CAS PubMed.
- H. Saeki, M. Sasaki, K. Komatsu, A. Miura and H. Matsuda, Bioresour. Technol., 2009, 100, 572–577 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01430f |
| This journal is © The Royal Society of Chemistry 2019 |