Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of aminated indole-based porous organic polymer for highly selective capture of CO2 by the coefficient effect of π–π-stacking and hydrogen bonding†
Qiang He *,
Yi Xu and
Xiaoqiang Yang
*,
Yi Xu and
Xiaoqiang Yang
School of Aviation Engineering Institute, Civil Aviation Flight University of China, Guanghan, 618307, People's Republic of China. E-mail: heqiangxy@126.com
First published on 16th April 2019
Abstract
A new aromatic aminated indole-based porous organic polymer, PIN-NH2, has been successfully constructed, and it was demonstrated that the coefficient effect endows this porous material with outstanding CO2 absorption capacity (27.7 wt%, 1.0 bar, 273 K) and high CO2/N2 (137 at 273 K and 1 bar) and CO2/CH4 (34 at 273 K and 1 bar) selectivity.
Today, one of the most serious environmental problems is climate change, such as global warming and sea-level rises, which are caused by increased concentrations of carbon dioxide (CO2) in the atmosphere.1–3 As we all know, CO2 mainly arises from fossil-fuel combustion in power plants, and the flue gas is always mixed with other gases including nitrogen (N2), methane (CH4) and so on. Therefore, it is necessary to design materials for selectively separating and adsorbing CO2 from these industrial and energy-related sources to improve the environmental problems.4–6 Aqueous amine solutions are the most common adsorbents for CO2 separation and capture,7 however, not only do these adsorbents degrade over time and are corrosive, toxic, and volatile, but also the regeneration process is highly energy demanding for these systems due to the chemical capture mechanism. As alternatives, porous organic polymers (POPs)8–10 relying on physical adsorption have become the research focus due to their low density, large specific surface area, good thermal stability, and narrow pore size distribution, but the low uptake capacity, and especially, the poor selectivity are two urgent issues that need to be addressed that seriously restrict the commercialization of POP adsorbents.11 Hence, in the past few years, many methods have been developed to improve the POP performance including increasing the surface area and adjusting the pore size.12,13
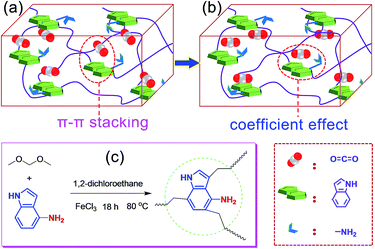
Recently, based on the rapid development of supramolecular interactions14,15 and the unique advantage of POP materials, i.e., the structure designability, researchers found that introducing special active sites into the framework, such as heteroatoms and diverse organic groups, is a simple and effective way to ameliorate the adsorption performance by the formation of some special non-covalent interactions and various functional groups have been explored.16–18 Recently, Chang et al.19 have designed and prepared an novel aerogel (PINAA) that contains both amide and indole groups and they demonstrated that the CO2 can be rapidly adsorbed on the heteroaromatic ring of indole because of its relatively large binding area via strong π–π-stacking interactions, and then, the desorbed CO2 molecule can be captured by an adjacent amide group because of “electrostatic in-plane” interaction. This synergistic effect of electrostatic in-plane and dispersive π–π-stacking interactions of amide and indole with CO2 endows the resulting aerogel enhanced CO2 adsorption capacity and CO2/CH4 and CO2/N2 selectivity. Inspired by this fascinating study, we hypothesized that when the indole group is aminated, the CO2 can be rapidly adsorbed on the heteroaromatic ring of indole because of its relatively large binding area via strong π–π-stacking interaction (Fig. 1a), after that, the hydrogen bonding interactions between the O of the CO2 and –NH of the aniline group would make the CO2 to further form a stable conformation with the aminated indole system (Fig. 1b), as a result, the coefficient effect of π–π-stacking interactions and hydrogen bonding interactions would ensure the high CO2 adsorption capacity and further enhanced CO2/CH4 and CO2/N2 selectivity.
To verify our suppose, in this work, we tactfully designed and fabricated an aminated indole-based aerogel PIN-NH2 via Friedel–Crafts alkylation (Fig. 1c), and its CO2 adsorption capacity and CO2/CH4 and CO2/N2 selectivity were immediately investigated. The successful preparation of PIN-NH2 was confirmed by Fourier transform infrared spectroscopy (FT-IR) and 13C solid state cross-polarization magic-angle-spinning nuclear magnetic resonance (13C CP/MAS NMR) spectrometer, and the results are in good agreement with the proposed structures (Fig. S1 and S2, ESI†). In the 13C CP/MAS NMR spectrum of PIN-NH2, the peaks at 169–103 ppm are ascribed to the indole group carbons, and the signals located at 35–40 ppm are assigned to the methylene carbons (Fig. S1, ESI†). For FT-IR spectrum (Fig. S2, ESI†), the peak at 3438 cm−1 is attributed to the stretching vibrations of N–H in amine unit and indole amine. The peaks at 2999 cm−1 and 2927 cm−1 are assigned to the stretching vibration of –CH2– in the polymer network and the peaks at 1630 cm−1 and 1480 cm−1 are ascribed to the vibrations of the aromatic ring skeleton.
The porosity of PIN-NH2 was quantified by scanning electron microscopy (SEM), high-resolution transmission electron microscopy (TEM) and N2 adsorption–desorption isotherms at 77 K. As shown in Fig. 2a, the SEM image displays that the PIN-NH2 consists of aggregated particles with sub-micrometer sizes. And the microporous characteristic can be observed clearly from the TEM image as shown in Fig. 2b, the presence of porous structure provides the essential condition for CO2 capture and separation. As shown in Fig. 2c, at a low pressure (0–0.1 bar), there is a rapid raise in the N2 adsorption–desorption isotherm, indicating its microporous nature, and the increase in the N2 sorption at a relatively high pressure (∼0.9 bar) shows the presence of meso- and macrostructures of the PIN-NH2. The specific surface area calculated in the relative pressure (P/P0) range from 0.01 to 0.1 shows that the Brunauer–Emmett–Teller (BET) specific surface area of PIN-NH2 is up to 480 m2 g−1. Additionally, the pore-size distribution (PSD)20 calculation result was shown in Fig. 2d and S3 ESI,† which indicating the pore diameter is about 14 Å and further confirming the microporous feature of the PIN-NH2. To gain further insight into the microstructural information, the powder wide-angle X-ray diffraction (PXRD) was further performed on the PIN-NH2 polymer. As shown in Fig. S4, ESI,† only a broad peak at 17.8° 2θ in the PXRD pattern is present, which clearly suggests that the polymer is mainly amorphous in nature. Additional, the thermogravimetric analysis (TGA) show that the microporous material is stable up to 370 °C indicating its potential in post combustion processes operated at high temperatures (Fig. S5, ESI†).
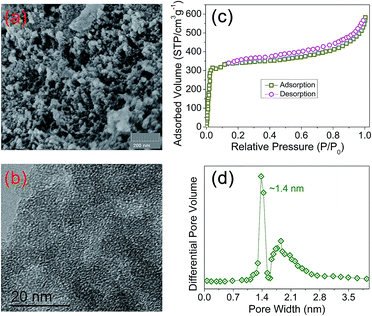 | ||
| Fig. 2 The microstructures of PIN-NH2 framework. (a) SEM, (b) TEM, (c) the nitrogen adsorption–desorption isotherms and (d) the pore size distribution of PIN-NH2 framework. | ||
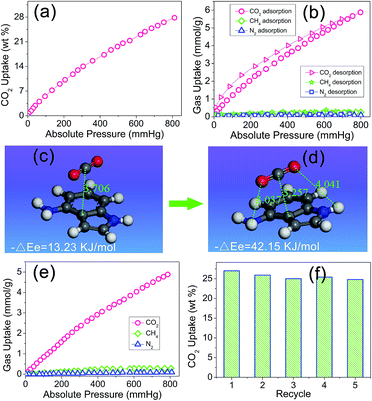
Owing to the artful structure design and particular preparation method, there is a reserved aniline group on the side of the indole group in the PIN-NH2 network. It was expected that after the rapidly capture of the CO2 molecule via the π–π-stacking interactions, the next aniline group would assist to further stabilize the CO2 molecule via hydrogen bonding interactions, in other words, the coefficient effect of π–π-stacking interactions and hydrogen bonding interactions would make this porous organic polymer more efficiently attract CO2 molecules, which inspires us to investigate the gas uptake capacity. Physisorption isotherms for CO2 (at 273 K) measured with a pressure more than 1.0 bar indicated that the resulting PIN-NH2 network exhibited a high carbon dioxide uptake of 27.7 wt% at 1.0 bar, as shown in Fig. 3a. Comparing with most of reported porous materials such as metal–organic frameworks,21 activated carbons,22 and microporous organic polymers,23,24 the porous organic polymer PIN-NH2 shows an enhanced CO2 uptake (Table S1, ESI†). The calculation of isosteric heat of adsorption of PIN-NH2 shows that the heat of adsorption is 35.7 kJ mol−1 (Fig. S6, ESI†), which is higher than that of the reported azo-linked polymers (27.9–29.6 kJ mol−1),25 the acid-functionalized porous polymers (32.6 kJ mol−1).26,27 and the indole-based porous polymers.28,29 The high value of the heat of adsorption indicated the strong physisorption effect owing to the coefficient effect of π–π-stacking interactions and hydrogen bonding interactions.
The application in CO2 separation and adsorption field of the traditional POPs is limited in a great degree by the poor gas selectivity as the flue gas and natural gas are both mixed gas. Here, we believed that the CO2 can be easily attracted by the heteroaromatic ring via the π–π-stacking interactions and then stabled with the assist of hydrogen bonding interactions, which leading an enhanced gas selectivity. Therefore, we urgently evaluated the selective gas uptake of the PIN-NH2 network for small gases (CO2/CH4, CO2/N2). In the calculation, the ratio of CO2/N2 is 15/85 and the ratio of CO2/CH4 is 5/95, which is the typical composition of flue gas and natural gas, respectively, the test results were shown in Fig. 3b. It can be found there is a rapid increase for the CO2 uptake while there is a negligible increase for the CH4 and N2 uptake with the increase of the pressure, which maybe due to the unique local dipole–π interactions between the porous organic framework PIN-NH2 and CO2 molecule. The test results shows that the CO2 uptake of PIN-NH2 is up to 5.92 mmol g−1 at a pressure of 1.0 bar and a temperature of 273 K while the CH4 and N2 uptake of PIN-NH2 is only 0.18 and 0.04 mmol g−1, respectively. The estimated ideal CO2/CH4 and CO2/N2 adsorption selectivities are up to 34 and 137, respectively. Additionally, the selectivities of PIN-NH2 toward CO2 over CH4 and N2 at 291 and 303 K were also investigated, respectively, and the results indicated that the resulting polymer PIN-NH2 still exhibited good selectivity at higher temperatures (Fig. S7 and S8, ESI†).
The high gas selectivities of this microporous framework may attribute to the strong affinity for CO2 compared with N2 and CH4 arising from the coefficient effect of π–π-stacking interactions and hydrogen bonding interactions between the sorbent and CO2 guest molecule, to further attest the above surmise, we used density functional theory (DFT)30 at the M06-2X level with the aug-cc-pVDZ basis set to investigate the interaction of aminated indole system with CO2 and the details of the calculation is shown in the ESI.† Fig. 3c and d shows the snapshot for CO2 capture by a model compound. The calculation result shows that owing to the electron-rich and large binding area, the CO2 was very easily attracted by the indole plane at a distance of 3.706 Å, and the computational binding energy was 13.23 kJ mol−1 (Fig. 3c). Soon, the balance structure was changed, the CO2 molecular was moved towards the amino group till the distance between the amino group and CO2 molecular was 3.037 Å, indicating a hydrogen bonding interaction was formed in this system. As a result, the distance between the indole plane and CO2 molecular decreased to 3.257 Å from 3.706 Å, and the computational binding energy increased to 42.15 kJ mol−1, which meaning a more steady system was formed (Fig. 3d). In the sense of computational chemistry, the expected strong coefficient effect of π–π-stacking interactions and hydrogen bonding interactions would favor the uptake of CO2 of the PIN-NH2 network. Additional, The DFT result also indicated that the interaction energy between CO2 and the imine group of indole is relatively weak with a correlation distance at 4.041 Å.
As we all know that the CO2 adsorption property will be affected in a great degree for porous polymers in the presence of water.31 In real industrial applications, the flue gas from a power plant is a mixture of CO2, water vapor, and others. As a result, it has very important practical significance to study the CO2 capture performance under humid condition. Here, the CO2 capture property of PIN-NH2 was studied at a relative humidity of 3% RH, as shown in Fig. 3e, the CO2 adsorption capacity of PIN-NH2 decreased from 5.92 to 4.88 mmol g−1 (1.0 bar, 273 K), however, the uptake of CH4 and N2 does not affected by the water. These results indicate that adsorption of water diminishes the CO2 capture. Although the selectivity (CO2/N2 = 104, CO2/CH4 = 21) is decreased under humid condition, PIN-NH2, to the best of our knowledge, still has very good CO2 selectivity over other CO2 capture materials in similar conditions.32 Moreover, the CO2 adsorption process is fully reversible (Fig. 3f). Herein, the new aminated indole-based aromatic porous organic polymer PIN-NH2 synthesized from easily available starting materials demonstrated not only remarkable CO2 capture capacity, but also prominent CO2/N2 and CO2/CH4 selectivities. Further, the dynamic breakthrough separation experiments of gas mixture at 298 K using a fixed-bed column packed with PIN-NH2 was carried out to evaluate the performances of PIN-NH2 aerogel in an actual adsorption-based separation process. The details of the experiment process were described in ESI.† As shown in Fig. S9 and S10, ESI,† the CH4 and N2 penetrated through the bed firstly with a retention time for only 6.5 and 3.4 min, respectively, while PIN-NH2 column can retain CO2 until above 23 min, which means the high CO2 adsorption capacity and selectivity of the PIN-NH2 adsorbent in actual application.
Conclusions
In this work, we have designed and synthesized a novel aromatic aminated indole-based porous organic polymer PIN-NH2 via Friedel–Crafts alkylation of 4-aminoindole with formaldehyde dimethyl acetal. FTIR and 13C CP/MAS NMR characterizations were performed to study the structural information and confirmed the successful formation of the resulting porous organic polymer PIN-NH2. The nitrogen adsorption–desorption test shows that the Brunauer–Emmett–Teller (BET) specific surface area of PIN-NH2 is up to 480 m2 g−1 and the thermal analysis indicates that the PIN-NH2 possesses good thermal stability. More interestingly, we proved that the CO2 adsorption capacity (27.7 wt%, 1.0 bar, 273 K) and selectivities (CO2/N2 = 137, CO2/CH4 = 34) could be significantly improved may owing to the presence of coefficient effect of π–π-stacking interactions and hydrogen bonding interactions between the sorbent and CO2 guest molecule, making it a promising material for potential application in gas separation. In addition, upon exposure to moisture (RH = 3%), CO2 capture of the PIN-NH2 is still highly efficient and selective, with only minor decreases in the CO2 adsorption capacity and selectivity. Moreover, we demonstrated that the CO2 adsorption process is fully reversible. The above advantages make the porous organic polymer PIN-NH2 a outstanding candidate for CO2 separation material, more importantly, the proposed coefficient effect is expected to be a new rationale for the design and fabrication of CO2 capture materials for applications in natural gas purification, greenhouse gas reduction, etc.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. U1233202 and No. 51175434). And the Youth Research Foundation of the Civil Aviation Flight University of China (No. Q2019-106), the Laboratory Research Foundation for the State Key Laboratory of Environment-friendly Energy Materials (No. 17kffk03).Notes and references
- T. Gasser, M. Kechiar, P. Ciais, E. J. Burke, T. Kleinen, D. Zhu, Y. Huang, A. Ekici and M. Obersteiner, Nat. Geosci., 2018, 11, 830 CrossRef CAS.
- A. I. Cooper, Nature, 2015, 519, 294 CrossRef CAS PubMed.
- A. Dani, V. Crocellà, C. Magistris, V. Santoro, J. Yuana and S. Bordiga, J. Mater. Chem. A, 2017, 5, 372 RSC.
- M. A. Naeem, A. Armutlulu, Q. Imtiaz, F. Donat, R. Schäublin, A. Kierzkowska and C. R. Müller, Nat. Commun., 2018, 9, 2408 CrossRef PubMed.
- L. P. Cavalcanti, G. N. Kalantzopoulos, J. Eckert, K. D. Knudsen and J. O. Fossum, Sci. Rep., 2018, 8, 11827 CrossRef PubMed.
- B. Ghalei, K. Sakurai, Y. Kinoshita, K. Wakimoto, A. P. Isfahani, Q. Song, K. Doitomi, S. Furukawa, H. Hirao, H. Kusuda, S. Kitagawa and E. Sivaniah, Nat. Energy, 2017, 2, 17086 CrossRef.
- G. Rochelle, Science, 2009, 325, 1652 CrossRef CAS PubMed.
- G. Chang, Y. Wang, C. Wang, Y. Li, Y. Xu and Li. Yang, Chem. Commun., 2018, 54, 9785 RSC.
- J. Wang, P. Zhang, L. Liu, Y. Zhang, J. Yang, Z. Zeng and S. Deng, Chem. Eng. J., 2018, 348, 57 CrossRef CAS.
- P. Zhang, Y. Zhong, J. Ding, J. Wang, M. Xu, Q. Deng, Z. Zeng and S. Deng, Chem. Eng. J., 2019, 355, 963 CrossRef CAS.
- T. Islamoglu, T. Kim, Z. Kahveci, O. M. El-Kadri and H. M. El-Kaderi, J. Phys. Chem. C, 2016, 120, 2592 CrossRef CAS.
- S. Kim and Y. M. Lee, Prog. Polym. Sci., 2015, 43, 1 CrossRef CAS.
- Z. Xiang, R. Mercado, J. M. Huck, H. Wang, Z. Guo, W. Wang, D. Cao, M. Haranczyk and B. Smit, J. Am. Chem. Soc., 2015, 137, 13301 CrossRef CAS PubMed.
- G. Chang, L. Yang, J. Yang, M. P. Stoykovich, X. Deng, J. Cui and D. Wang, Adv. Mater., 2018, 30, 1704234 CrossRef PubMed.
- P. Yang, L. Yang, Y. Wang, L. Song, J. Yang and G. Chang, J. Mater. Chem. A, 2019, 7, 531 RSC.
- R. W. Flaig, T. M. Osborn Popp, A. M. Fracaroli, E. A. Kapustin, M. J. Kalmutzki, R. M. Altamimi, F. Fathieh, J. A. Reimer and O. M. Yaghi, J. Am. Chem. Soc., 2017, 139, 12125 CrossRef CAS PubMed.
- H. Thakkar, S. Eastman, A. Al-Mamoori, A. Hajari, A. A. Rownaghi and F. Rezaei, ACS Appl. Mater. Interfaces, 2017, 9, 7489 CrossRef CAS PubMed.
- A. Alabadi, H. A. Abbood, Q. Li, N. Jing and B. Tan, Nature, 2016, 6, 38614 CAS.
- L. Yang, G. Chang and D. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 15213 CrossRef CAS PubMed.
- A. Vishnyakov, P. I. Ravikovitch and A. V. Neimark, Langmuir, 1999, 15, 8736 CrossRef CAS.
- J. Liu, P. K. Thallapally, B. P. McGrail and D. R. Brown, Chem. Soc. Rev., 2012, 41, 2308 RSC.
- B. S. Ghanem, M. Hashem, D. M. Harris, K. J. Msayib, M. Xu, P. M. Budd, N. Chaukura, D. Book, S. Tedds, A. Walton and N. B. McKeown, Macromolecules, 2010, 43, 5287 CrossRef CAS.
- X. S. Ding, H. Li, Y. C. Zhao and B. H. Han, Polym. Chem., 2015, 6, 5305 RSC.
- C. Zhang, P. C. Zhu, L. X. Tan, L. N. Luo, Y. Liu, J. M. Liu, S. Y. Ding, B. X. Tan, L. Yang and H. B. Xu, Polymer, 2016, 82, 100 CrossRef CAS.
- J. Lu and J. Zhang, J. Mater. Chem. A, 2014, 2, 13831 RSC.
- R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173 RSC.
- W. Lu, D. Yuan, J. Sculley, D. Zhao, R. Krishna and H. C. Zhou, J. Am. Chem. Soc., 2011, 133, 18126 CrossRef CAS PubMed.
- G. Chang, Z. Shang, Y. Tao and L. Yang, J. Mater. Chem. A, 2016, 4, 2517 RSC.
- G. Chang, L. Yang, J. Yang, Y. Huang, K. Cao, J. Ma and D. Wang, Polym. Chem., 2016, 7, 5768 RSC.
- C. Balzer, R. T. Cimino, G. Y. Gor, A. V. Neimark and G. Reichenauer, Langmuir, 2016, 32, 8265 CrossRef CAS PubMed.
- A. C. Kizzie, A. G. Wong-Foy and A. J. Matzger, Langmuir, 2011, 27, 6368 CrossRef CAS PubMed.
- J. Liu, J. Tian, P. K. Thallapally and B. P. McGrail, J. Phys. Chem. C, 2012, 116, 9575 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of materials, measurements and gas adsorption tests; synthesis and characterizations of PIN-NH2 aerogel; thermal properties, XRD and gas selectivities at 291 and 303 K and isosteric heat of CO2 adsorption of PIN-NH2 aerogel; the dynamic breakthrough separation curves; the details of the simulation calculation. See DOI: 10.1039/c9ra01532a |
| This journal is © The Royal Society of Chemistry 2019 |