Open Access Article
Open Access ArticleConstruction of a luminescent sensor based on a lanthanide complex for the highly efficient detection of methyl parathion†
Xuan Hua,
Fengyi Wanga,
Qianqian Penga,
Jing Hua,
Huaqiao Pengc,
Lin Lic,
Baozhan Zheng *ab,
Juan Du
*ab,
Juan Du *ab and
Dan Xiaoab
*ab and
Dan Xiaoab
aCollege of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu 610064, China. E-mail: dujuanchem@scu.edu.cn; zhengbaozhan@scu.edu.cn
bKey Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, SichuanUniversity, Chengdu, Sichuan 610064, China
cThe Second Research Institute of Civil Aviation Administration of China (CAAC), Chengdu 610041, China
First published on 29th April 2019
Abstract
A highly sensitive and selective luminescent sensor for the detection of methyl parathion (MP) pesticide was described in this study. The target molecule HL was synthesized by modifying the structure of 4-hydroxybenzlidene imidazolinone (HBI) with nitrogen-containing heterocyclic 1,10-phenanthroline. In the presence of Eu3+, a HL–Eu3+ complex was formed which could emit strong red fluorescence due to the removal of coordinated water molecules and an intramolecular energy transfer from HL to Eu3+. Addition of MP into the strongly fluorescent solution of HL–Eu3+ induced quenching of the complex's fluorescence, and this quenching behavior occurred because of the competition coordination of MP and HL for Eu3+. A calibration curve was developed that related the extent of fluorescence quenching to MP concentration, making the HL–Eu3+ system a sensitive and selective fluorescent sensor for MP. Under the experimental conditions, the detection limit for MP was down to 95 nM based on LOD = 3σ/S. Moreover, the fluorescence assay developed here allowed the detection of MP in two different types of real samples including pond water and pear juice, and satisfactory results demonstrate that this fluorescent sensor based on HL–Eu3+ has potential application in environment and food analysis.
Introduction
Organophosphorus pesticides (OPs) have served as the primary pesticides and insecticides in agriculture since organochlorine pesticides were banned.1,2 In fact, OPs can also enter the human body through polluted water, foods or transdermal absorption,3 resulting in several health issues such as neurological disorders, muscular paralysis tremors, language disorders and even death.4,5 Therefore, great concerns over the damage of OPs have resulted in the development of a variety of detection technologies including gas chromatography,6 high performance liquid chromatography (HPLC),7 mass spectrometry,8 immunoassays,9 spectrophotometry,10 electrochemical methods,11 flow injection analysis,12 and chemiluminescence methods.13 Despite the high sensitivity and selectivity of these methods, most of them have the disadvantages of being time-consuming and laborious and needing large instruments, which still limit their wide practical applications. Therefore, it is of great urgency and necessary to develop more rapid, simple and accurate methods for the determination of OPs with higher sensitivity and selectivity. As a widely used strategy, the fluorescent sensors have aroused considerable attention due to their advantages of excellent sensitivity and selectivity, short-time data acquisition, facile manipulations, low cost and visual detection properties. Therefore, a number of fluorescent sensors based on organic fluorophores, Au nanoparticles, quantum dots and carbon dots et al. have been constructed for the detection of OPs in real samples.14–18 Barba-Bon and co-workers designed two Eu3+ and Au3+ complexes based on commonly used organic fluorophore BODIPY. The BODIPY-containing complexes can effectively detect V-type nerve agent surrogates, demeton-S, by a colorimetric and fluorescent double-detection assay with high sensitivity and selectivity. The displacement of BODIPY ligand by demeton-S from the metallic centre resulted in obvious colour change and fluorescence quenching of the detecting system. The detection limit for demeton-S reached as low as 9 ppm.19 Wu and co-workers used carbon dots as fluorescent probe for the determination of paraoxon. The fluorescence emission intensity of CDs decreased significantly in the presence of AuNPs via fluorescence resonance energy transfer (FRET). Upon addition of acetylthiocholine (ATC) and butyrylcholinesterase (BChE), the fluorescence was recovered because the thiocholine generated by enzymatic catalysis reaction caused the aggregation of Au NPs. Furthermore, the fluorescence was quenched again by paraoxon through the inhibition effect on the activity of BChE. Therefore, a facile FRET sensing platform for OPs was constructed.20The ideal fluorescent probe should have the advantages of controllable fluorescence, good stability, fast response, high selectivity and sensitively. When applied to environmental analysis, it should also have favorable water solubility and non-polluting characteristics and function independently of environmental factors and interferences. Lanthanide elements have special luminescence features and excellent coordination properties.21,22 Compared to other fluorescent systems, lanthanide fluorescent complexes also have the advantages of high fluorescence quantum yield, large Stokes shift, adjustable photophysical and photochemical characteristics, good stability, narrow emission peaks and long fluorescence lifetime.23,24 Therefore, lanthanides has become an alternative to organic fluorophores especially in considering of background autofluorescence interference. If an analyte can act as a competitive binder for the lanthanide ion center in the luminescent Ln-containing complex, then it has the ability to ‘switch off’ the lanthanide emission. In particular, the excellent abilities of Eu3+ to selectively interact with the phosphate group in OPs coupled with favourable photophysical characteristics make the luminescent Eu-containing complexes attractive choices for the detection of organophosphorus compounds. Knapton and co-workers prepared some fluorescent organometallic sensors for the detection of chemical-warfare-agent (CWA) mimics by a displacement assay. In this work, several ligands based on a 2,6-bis(1′-methylbenzimidazolyl)pyridine (Mebip) structure was developed. The complexation of Mebip with Eu3+ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand/Eu3+ ratio resulted in a strong luminescence emission. The addition of triethyl phosphate (TEP) to the solution of Eu3+–Mebip complex led to the complete and immediate quenching of the characteristic red luminescence of Eu3+.25 Zhang and co-workers immobilized Mebip onto poly(ethylene-glycol)-grafted polystyrene micro-spheres, and the following incorporation of Eu3+ ions resulted in luminescent microspheres. Upon addition of diethyl chlorophosphate (DCP), the luminescence of the microspheres was immediately quenched. These results indicated that even be immobilized on a solid substrate, some ligands for Ln were still able to be effectively displaced by organophosphorus compounds.26 However, direct excitation of lanthanide ions to luminesce is difficult because the f–f transitions are Laporte-forbidden, thus some light-harvesting ligands called ‘antenna ligands’ are necessary to absorb and transfer the exciting energy to lanthanide ions.27,28 For light conversion systems based on Ln3+ ions, the main requirements are high ligand-absorption coefficients, efficient ligand-to-metal energy transfer and minimal non-radiative deactivation of the excited state of the metal.29 Encapsulation of the Ln3+ ion by polydentate/macrocyclic ligands,30 Schiff bases,31 organic acids,32 and even proteins is the most common strategy employed to satisfy these criteria.33 As one of the most toxic OPs, methyl parathion (O,O-dimethyl O-4-nitrophenyl phosphorothionate, MP, Scheme 1(a)) was classified as ‘Category Ia’ (extremely toxic) by the World Health Organization (WHO) and ‘Toxicity Category I’ (most toxic) by the United States Environmental Protection Agency (USEPA).34–36 However, its detection by fluorescent methods was seldom reported, and almost all of them utilized enzymes as catalyst,37,38 which made the analytical process complex and expensive. Based on the above analysis, herein, a luminescent lanthanide complex of HL–Eu3+ with red fluorescent emission was synthesized and chosen as the sensing and detecting units for MP. The aromatic cyclic polyamine ligand HL was designed and synthesized by modifying the structure of 4-hydroxybenzlidene imidazolinone (HBI) with nitrogen-containing heterocyclic 1,10-phenanthroline, as shown in Scheme 1(b). HL itself had very weak fluorescence in solution. With the addition of Eu3+, HL can act as an effective ‘antenna ligand’ to coordinate with Eu3+ and form a complex HL–Eu3+ with the characteristic emission of Eu3+. We foresaw that the Eu3+ emission of HL–Eu3+ could be ‘switched off’ through the displacement of the HL antenna in the presence of MP caused by the preferable interaction between Eu3+ and MP, therefore, an effective MP sensor can be successfully constructed.
1 ligand/Eu3+ ratio resulted in a strong luminescence emission. The addition of triethyl phosphate (TEP) to the solution of Eu3+–Mebip complex led to the complete and immediate quenching of the characteristic red luminescence of Eu3+.25 Zhang and co-workers immobilized Mebip onto poly(ethylene-glycol)-grafted polystyrene micro-spheres, and the following incorporation of Eu3+ ions resulted in luminescent microspheres. Upon addition of diethyl chlorophosphate (DCP), the luminescence of the microspheres was immediately quenched. These results indicated that even be immobilized on a solid substrate, some ligands for Ln were still able to be effectively displaced by organophosphorus compounds.26 However, direct excitation of lanthanide ions to luminesce is difficult because the f–f transitions are Laporte-forbidden, thus some light-harvesting ligands called ‘antenna ligands’ are necessary to absorb and transfer the exciting energy to lanthanide ions.27,28 For light conversion systems based on Ln3+ ions, the main requirements are high ligand-absorption coefficients, efficient ligand-to-metal energy transfer and minimal non-radiative deactivation of the excited state of the metal.29 Encapsulation of the Ln3+ ion by polydentate/macrocyclic ligands,30 Schiff bases,31 organic acids,32 and even proteins is the most common strategy employed to satisfy these criteria.33 As one of the most toxic OPs, methyl parathion (O,O-dimethyl O-4-nitrophenyl phosphorothionate, MP, Scheme 1(a)) was classified as ‘Category Ia’ (extremely toxic) by the World Health Organization (WHO) and ‘Toxicity Category I’ (most toxic) by the United States Environmental Protection Agency (USEPA).34–36 However, its detection by fluorescent methods was seldom reported, and almost all of them utilized enzymes as catalyst,37,38 which made the analytical process complex and expensive. Based on the above analysis, herein, a luminescent lanthanide complex of HL–Eu3+ with red fluorescent emission was synthesized and chosen as the sensing and detecting units for MP. The aromatic cyclic polyamine ligand HL was designed and synthesized by modifying the structure of 4-hydroxybenzlidene imidazolinone (HBI) with nitrogen-containing heterocyclic 1,10-phenanthroline, as shown in Scheme 1(b). HL itself had very weak fluorescence in solution. With the addition of Eu3+, HL can act as an effective ‘antenna ligand’ to coordinate with Eu3+ and form a complex HL–Eu3+ with the characteristic emission of Eu3+. We foresaw that the Eu3+ emission of HL–Eu3+ could be ‘switched off’ through the displacement of the HL antenna in the presence of MP caused by the preferable interaction between Eu3+ and MP, therefore, an effective MP sensor can be successfully constructed.
Experimental section
Apparatus
1H NMR and 13C NMR were measured on a BrukerAVII-400 MHz spectrometer with chemical shifts reported in ppm (in DMSO; TMS as internal standard). Mass spectra were obtained from a commercial ion trap mass spectrometer (Thermo Fisher Scientific, San Jose, CA). Thermo Scientific Nicolet 6700 FT-IR spectrometer (Sugar Land, TX, USA) was used to record the FT-IR of HL. Fluorescence spectra were determined on F-7000 spectrophotometer equipped with a 1 cm quartz cell (HITACHI, Japan). UV-visible measurements were measured on U-2900 spectrophotometer (HITACHI, Japan).Materials reagents
Europium(III) chloride hexahydrate (EuCl3·6H2O), 2,9-dimethyl-1,10-phenanthroline, N-acetylglycine (AR) were obtained from Alfa Aesar; selenium dioxide was purchased from Sigma-Aladdin; sodium acetate, acetic anhydride and ethanol were purchased from Kelong Chemical Reagent Co., Ltd. (Chengdu, China); methyl parathion (MP) was purchased from shanghai Macklin Biochemical Co., Ltd. All chemicals and reagents were of analytical grade and used without further purification. Double distilled water was employed for all experiments.Synthesis of Compound 1
In this paper, HL and its intermediates (Compound 1, Compound 2) were synthesized via simple steps as depicted in Scheme 1(b). Typically, 2,9-dimethyl-1,10-phenanthroline (1.0 g, 4.60 mmol), selenium dioxide (2.5 g, 22.5 mmol) were dissolved in 1,4-dioxane (35 mL) and stirred at 110 °C for 2 h. The formed precipitate was separated with diatomite thermal filtration and then recrystallized in DMF/H2O with vacuum dried at 40 °C for 12 h. Thus, 0.70 g (yield 67.6%) yellow solid Compound 1 could be obtained. Compound 1: 1H NMR (400 MHz, DMSO-d6) δH (ppm): 10.31 (CHO, s, 2H), 8.71 (Ar–H, d, J = 8.2 Hz, 2H), 8.40 (Ar–H, d, J = 8.4 Hz, 2H), 8.19 (Ar–H, d, J = 4.0 Hz, 2H). (Fig. S1†) 13C NMR (400 MHz, DMSO) δC (ppm): 194.01, 152.3, 148.6, 138.2, 129.7, 120.6. (Fig. S2†).Synthesis of Compound 2
For the synthesis of Compound 2, Compound 1 (0.67 g, 3 mmol), anhydrous sodium acetate (0.5 g, 6 mmol), and N-acetylglycine (0.73 g, 6 mmol) were dissolved in 30 mL acetic anhydride refluxed at 90 °C for 4 h. When the mixture was cooled down to room temperature, 250 mL cold water was added into the solution and yellow precipitate could be formed quickly. After the filtration, washing with cold ethanol and vacuum-drying at 60 °C for 12 h, a yellow solid compound of Compound 2 could be obtained (0.49 g, yield 41.9%). Compound 2: 1H NMR (400 MHz, DMSO-d6) δH (ppm): 8.89 (Ar–H, d, J = 8.1 Hz, 2H), 8.83 (Ar–H, d, J = 8.2 Hz, 2H), 8.38 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, s, 2H), 8.30 (Ar–H, m, 2H), 1.83 (CH3, s, 6H), (Fig. S3†).
CH, s, 2H), 8.30 (Ar–H, m, 2H), 1.83 (CH3, s, 6H), (Fig. S3†).
Synthesis of HL
Compound 2 (0.49 g, 1.2 mmol), potassium carbonate (0.1 g, 0.72 mmol) and 1 mL 40% aqueous methylamine solution were dissolved in 20 mL ethanol and stirred at 90 °C for 4 h. After cooling down to room temperature, the mixture was diluted with 100 mL water, the pH of the solution was adjusted to 3.0 with concentrated HCl, and left overnight at 4 °C in refrigerator. The formed yellow precipitate was then filtered, washed and dried. The obtained crude compound was purified by thin layer chromatography with chloroform/ethylacetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v), thus, a pure compound of HL (yellow solid (25%)) was synthesized, which was then used for the characterization of FT-IR, ESI-MS and 1H NMR. HL: 1H NMR (400 MHz, DMSO-d6) δH (ppm): 8.64–8.38 (Ar–H, d, J = 7.2 Hz, 2H), 8.32–7.9 (Ar–H, d, J = 7.0 Hz, 2H), 7.68 (C
3, v/v), thus, a pure compound of HL (yellow solid (25%)) was synthesized, which was then used for the characterization of FT-IR, ESI-MS and 1H NMR. HL: 1H NMR (400 MHz, DMSO-d6) δH (ppm): 8.64–8.38 (Ar–H, d, J = 7.2 Hz, 2H), 8.32–7.9 (Ar–H, d, J = 7.0 Hz, 2H), 7.68 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, s, 2H), 7.54 (Ar–H, d, J = 8.2 Hz, 2H), 3.75 (N–CH3, s, 6H), 2.07 (N
CH, s, 2H), 7.54 (Ar–H, d, J = 8.2 Hz, 2H), 3.75 (N–CH3, s, 6H), 2.07 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3, s, 6H), (Fig. S4†). The ESI-MS data of HL showed a peak with m/z 463.0298 ([HL + K]+) (Fig. S5†). The addition of Eu3+ resulted in the formation of HL–Eu3+ complex, and the MALDI-TOF MS data of HL–Eu3+ showed a peak with m/z 629.1 ([HL–Eu3+ + 3H2O − 2H]+) cal.: 629.1 found 629.1 (Fig. S6†). The MALDI-TOF MS data of Eu3+ with the presence of MP has been added and the peak with m/z 581.3 in Fig. S7† is assigned to [Eu + MP + K + 7H2O]4+ (cal.: 581.0 found 581.3).
C–CH3, s, 6H), (Fig. S4†). The ESI-MS data of HL showed a peak with m/z 463.0298 ([HL + K]+) (Fig. S5†). The addition of Eu3+ resulted in the formation of HL–Eu3+ complex, and the MALDI-TOF MS data of HL–Eu3+ showed a peak with m/z 629.1 ([HL–Eu3+ + 3H2O − 2H]+) cal.: 629.1 found 629.1 (Fig. S6†). The MALDI-TOF MS data of Eu3+ with the presence of MP has been added and the peak with m/z 581.3 in Fig. S7† is assigned to [Eu + MP + K + 7H2O]4+ (cal.: 581.0 found 581.3).
Detection procedure of methyl parathion
HL stock solution (0.3 mM) was prepared in Tris–HCl buffer (pH 7.0, 25 mM) and further diluted to 60 μM for the assay. The fluorescence spectra were then measured with an excitation wavelength (λex) of 356 nm, and the fluorescence spectrum of HL–Eu3+ solution was recorded between 600–650 nm with a maximum emission wavelength of 617 nm (λem). All of the fluorescence intensity measurements were conducted in triplicate. Three polluted water samples were collected from a pond nearby and filtered prior to analysis. All the stock solutions were stored at 4 °C in a refrigerator until use.Results and discussion
Design and characterization of HL–Eu3+
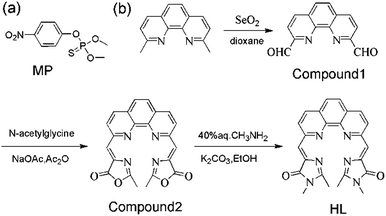
In order to obtain a fluorescent system with excellent performance, the fluorescence behavior of HL toward several lanthanide metal ions were firstly examined in Tris–HCl buffer (pH 7.0, 25 mM). As shown in Fig. 1(A), excepting the comparatively stable and weak fluorescence peak of HL at 460 nm, there was no characteristic fluorescence emission of any one of the lanthanide metal ions including La3+, Nd3+, Tb3+, Yb3+, Er3+ and Ce3+ that can be detected when they were introduced into HL solution. In comparison, the fluorescence spectrum changed immediately upon Eu3+ addition and the characteristic peaks of Eu3+ at 581 nm, 593 nm and 617 nm appeared. This result demonstrated that Eu3+ can coordinate with HL, which could transfer the absorbed energy to Eu3+ ions to excite them. The coordination process of Eu3+ with HL was displayed in Scheme 2(a). In order to explore the optimal concentration of Eu3+ in HL–Eu3+ system, we analyzed the fluorescence intensity of HL–Eu3+ with different amounts of Eu3+. As shown in Fig. S8,† the emission intensity at 617 nm increased gradually with continuous addition of Eu3+, then reached the highest level upon addition of 60 μM Eu3+. Nevertheless, an obvious fluorescence decrease was appeared when the concentration of Eu3+ is higher than 60 μM. Therefore, 60 μM Eu3+ was selected as the ideal concentration and used in following experiments. Importantly, the maximal fluorescence intensity of HL–Eu3+ was remained nearly steady with constant light irradiation (356 nm) for 2 h, and the unchanged intensity proved its excellent photo-stability and expectable application potential as an ideal sensor used in complex environment systems (Fig. S9†).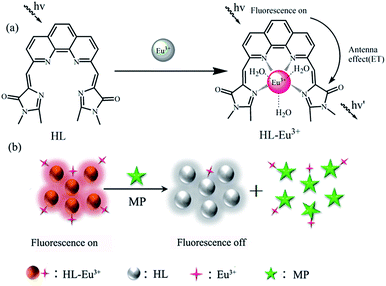 | ||
| Scheme 2 (a) The coordination process of Eu3+ with HL. (b) The schematic representation of the fluorescent sensing mechanism for MP determination based on HL–Eu3+. | ||
To understand the stoichiometric nature of HL–Eu3+, the Job's plot for the binding activity between HL and Eu3+ was studied, and the result exhibited 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry from the fluorescence titration (Fig. S10†). The FT-IR spectra of HL and HL–Eu3+ were reported in Fig. S11.† From which, the characteristic peaks at about 1630 cm−1, 1660 cm−1 and 3320 cm−1 should be attributed to the stretching vibration of C
1 stoichiometry from the fluorescence titration (Fig. S10†). The FT-IR spectra of HL and HL–Eu3+ were reported in Fig. S11.† From which, the characteristic peaks at about 1630 cm−1, 1660 cm−1 and 3320 cm−1 should be attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H of HL, respectively. Compared with the peaks of HL (1347 cm−1 and 1630 cm−1 for C–N and C
O and N–H of HL, respectively. Compared with the peaks of HL (1347 cm−1 and 1630 cm−1 for C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups, respectively), the slight peak shifts (1319 cm−1 and 1610 cm−1) in HL–Eu3+ suggested that the N atoms in both of the C–N and C
N groups, respectively), the slight peak shifts (1319 cm−1 and 1610 cm−1) in HL–Eu3+ suggested that the N atoms in both of the C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups were involved in the coordination with Eu3+ in the formation of HL–Eu3+ complex. In addition, the Eu–N stretching vibration was observed at 479 cm−1 in HL–Eu3+. The strong and wide stretching vibration peak of O–H bond at about 3349 cm−1 in HL–Eu3+ could be assigned to the comprised water molecules of Eu3+. On the basis of the above analysis, the conclusion can be reached that HL–Eu3+ has been formed.
N groups were involved in the coordination with Eu3+ in the formation of HL–Eu3+ complex. In addition, the Eu–N stretching vibration was observed at 479 cm−1 in HL–Eu3+. The strong and wide stretching vibration peak of O–H bond at about 3349 cm−1 in HL–Eu3+ could be assigned to the comprised water molecules of Eu3+. On the basis of the above analysis, the conclusion can be reached that HL–Eu3+ has been formed.
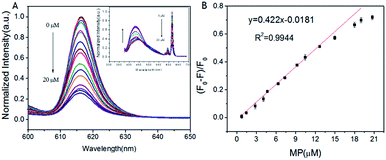
The fluorescence spectra of HL–Eu3+ in the presence of MP were measured in Tris–HCl buffer (pH 7.0, 25 mM). As illustrated in Fig. 1(B), comparing the fluorescent emission at 460 nm with HL alone, HL–Eu3+ revealed an extra strong characteristic emission center at 617 nm when excited at 356 nm with a fluorescent color change from weak blue to bright red (inset of Fig. 1(B)). With the addition of 20 μM MP into the solution of HL–Eu3+, the complex's fluorescence was obviously quenched and the change was easily discernible to the naked eye under UV light irradiation as Fig. 1(B) inset showed. Moreover, the fluorescent response time of HL–Eu3+ toward MP was also investigated in this work. The results showed that when MP was added to HL–Eu3+ solution, about 70% fluorescence intensity quench was detected at 617 nm within one minute and the fluorescent intensity remained almost constant in around 10 min (Fig. S12†). Therefore, all the fluorescent spectra measurement in this work are carried out after the addition of MP for 10 minutes. These facts made the sensor HL–Eu3+ the potential candidate for effectively detecting MP.
Investigation of the experimental conditions
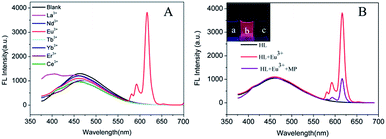
In order to explore the influence of pH on the performance of HL–Eu3+ towards MP determination, the response of constructed fluorescent assay pH condition was further optimized by the addition of various Tris–HCl buffer at pH 2.0–11.0 (Fig. 2(A)). The measured results revealed that the fluorescence intensity of HL–Eu3+ increased gradually to a maximum value with the increase of pH until pH 7.0 and then slowly decreased. With the presence of MP, it can be observed that the fluorescence quenching efficiency was affected by the pH value, and a maximum quenching rate was obtained at pH 7.0. Hence, pH 7.0 was employed as the favorable pH value. Furthermore, the fluorescence intensity of HL–Eu3+ with or without MP was comparatively satisfactory stability even in a salty medium with a NaCl concentration as high as 0.1 M (Fig. 2(B)), indicating its potential applicability in complex water environmental samples analysis.Specificity of HL–Eu3+ for MP detection
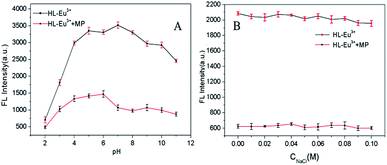
To examine the selectivity of HL–Eu3+ for MP detection, we investigated the fluorescence behavior of HL–Eu3+ to commonly existent organic pollute compounds (OPCs), anions and metal ions (Fig. 3 and S13†), including p-nitrotoluene, 2,4,6-trinitrotoluene, o-cresol, phenol, 2,5-dimethylphenol, resorcinol, carbaryl, phoxim, triethylphosphate, dimethoate, PO43−, HPO42−, H2PO4−, SO42−, F−, Br−, NO3−, Ac−, Ba2+, Mg2+, Ca2+, Hg2+, Na+, Fe3+, Pb2+, Cd2+, Zn2+ and MP. Negligible fluorescence intensity changes were observed upon the addition of the above mentioned substances except MP (Fig. 3 blank bars, Fig. S13A†). Another important feature of HL–Eu3+ was its high selectivity toward MP in a competitive environment. The competitive experiments were conducted in coexist of MP and excess amount of various other OPCs, metal ions and anions (Fig. 3 red bars, Fig. S13B†). There were no distinct variations of the fluorescence signal caused by the co-existence of these potential interrupting species. The absorption spectra of HL–Eu3+ with the addition of as-mentioned OPs (carbaryl, phoxim, triethyl phosphate, dimethoate and MP) helped to explain the observed selectivity of HL–Eu3+ toward MP. As displayed in Fig. S14,† HL–Eu3+ had two distinct absorption bands centred at 232 nm and 282 nm, respectively. The addition of MP induced an obvious disappearance of the absorption band centred at 232 nm and a minor blue-shift from 282 nm to 280 nm. Otherwise, there was no apparent change of the absorption bands of HL–Eu3+ with the presence of other OPs except MP. Therefore, it can be reasonably presumed that only the addition of MP can break the combination between HL and Eu3+ and induce the displacement of HL from HL–Eu3+ complex. These results clearly manifested that the constructed fluorescence sensor based on HL–Eu3+ has an excellent selective response to MP with good sensitivity.Detection of MP and the mechanism
Under the optimized condition, the changes of HL–Eu3+ fluorescent were monitored by fluorescence emission spectroscopy with different concentrations of MP (0.75–20 μM). As expected, the fluorescence intensity of HL–Eu3+ at 617 nm gradually decreased with increasing MP concentration (Fig. 4(A)), and accurate change monitoring of the fluorescence intensity was realized in the presence of MP as Fig. 4(B) showed. The HL–Eu3+ sensor exhibited a linear response toward the concentration of MP (0.75–20 μM), and the fitting linear equation was expressed as y = 0.422x − 0.0181 with a linear relative coefficient of R2 = 0.9944. Furthermore, we also compared our assay with other reported methods for the determination of MP (Table S1†), and the results showed that our sensor provided lower detection limit for the detection of MP.Considering the above analysis, the detection mechanism of the fluorescence sensor based on HL–Eu3+ toward MP was displayed in Scheme 2(b). After the addition of Eu3+ into HL solution, the coordination of HL with Eu3+ could occurred immediately through four nitrogen atoms and led to the formation of HL–Eu3+ quaternary complex, which can emit strong red fluorescence due to the removal of coordinated water molecules and an intramolecular energy transfer from HL to Eu3+ through the so-called ‘antenna effect’. When MP was added into the system of HL–Eu3+, it could bind with Eu3+ and form a more stable complex because of the stronger nucleophilicity, leading to the obvious decrease of the fluorescence intensity at 617 nm. This selective binding behavior of Eu3+ also resulted in the release of free HL ligand, and the fluorescence of HL at 460 nm increased. By employing the fluorescent HL–Eu3+ as the sensor, the specific and sensitive detection for MP was realized and the detection limit reached down to 95 nM with LOD = 3σ/S.
Detection of MP in real samples
To demonstrate the feasibility of the present method for selective probing MP in real samples, different concentrations of MP in the spiked pond water and pear juice samples were determined and the results were listed in Table 1. It can be seen that good recoveries ranging from 96.23% to 106.62% were obtained and the RSD were less than 4.0% for all indicating that the present method is reliable and applicable for MP assay in environmental and food samples.| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (n = 3%) |
|---|---|---|---|---|
| Pond water | 2.00 | 2.13 | 106.62 | 3.28 |
| 4.00 | 3.96 | 99.15 | 2.53 | |
| 6.00 | 5.84 | 97.37 | 1.78 | |
| Pear juice | 2.00 | 2.06 | 102.82 | 2.97 |
| 4.00 | 4.06 | 101.51 | 1.08 | |
| 6.00 | 5.95 | 96.23 | 2.68 |
Conclusions
In summary, a lanthanide complex sensor HL–Eu3+ based on aromatic cyclic polyamine ligand was constructed by modifying the structure of 4-hydroxybenzlidene imidazolinone (HBI) with nitrogen-containing heterocyclic 1,10-phenanthroline. In this system, the competition coordination of MP and HL for Eu3+ transformed the fluorescence and established a fluorescence sensor for MP. HL–Eu3+ displayed excellent selectivity and sensitivity toward MP detection, and the detection limit reached 95 nM. At the same time, it showed good recovery performance in actual pond water and pear juice samples, indicating that the sensor has the potential application for detecting MP in complex real samples.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from National Natural Science Foundation of China (No. 21876117, U1833124 and U1833202). We also appreciate Analytical & Testing Centre of Sichuan University for the characterizations.References
- F. Yang, J. R. Wild and A. J. Russell, Biotechnol. Prog., 1995, 11, 471–474 CrossRef CAS PubMed.
- G. Yao, R. Liang, C. Huang, Y. Wang and J. Qiu, Anal. Chem., 2013, 85, 11944–11951 CrossRef CAS PubMed.
- X. Lian and B. Yan, ACS Appl. Mater. Interfaces, 2018, 10, 14869–14876 CrossRef CAS PubMed.
- N. Fahimi-Kashani and M. R. Hormozi-Nezhad, Anal. Chem., 2016, 88, 8099–8106 CrossRef CAS PubMed.
- X. Gao, Y. Zhao, B. Zhang, Y. W. Tang, X. Y. Liu and J. Li, Analyst, 2016, 88, 1105–1111 Search PubMed.
- Q. Tang, X. Wang, F. Yu, X. Qiao and Z. Xu, J. Sep. Sci., 2014, 37, 820–827 CrossRef CAS PubMed.
- L. Tan, W. Li, H. Li and Y. Tang, J. Chromatogr. A, 2014, 1336, 59–66 CrossRef CAS PubMed.
- S. D. Da, F. E. Paiva Silva, F. G. Silva, G. S. Nunes and M. Badea, Pest Manage. Sci., 2015, 71, 1497–1502 CrossRef PubMed.
- H. Ouyang, L. Wang, S. Yang, W. Wang, L. Wang, F. Liu and Z. Fu, Anal. Chem., 2015, 87, 2952–2958 CrossRef CAS PubMed.
- X. Yan, H. Li, Y. Yan and X. Su, Food Chem., 2015, 173, 179–184 CrossRef CAS PubMed.
- X. C. Fu, J. Zhang, Y. Y. Tao, J. Wu, C. G. Xie and L. T. Kong, Electrochim. Acta, 2015, 153, 12–18 CrossRef CAS.
- J. Gong, Z. Guan and D. Song, Biosens. Bioelectron., 2013, 39, 320–323 CrossRef CAS PubMed.
- R. S. Chouhan, A. C. Vinayaka and M. S. Thakur, Anal. Methods, 2010, 2, 924–928 RSC.
- X. Li, D. Zhang, Z. Liu, Y. Xu and D. Wang, Inorg. Chim. Acta, 2018, 471, 280–289 CrossRef CAS.
- T. Kim, S. B. Maity, J. Bouffard and Y. Kim, Anal. Chem., 2016, 88, 9259–9263 CrossRef CAS PubMed.
- N. Fahimi-Kashani and M. R. Hormozi-Nezhad, Anal. Chem., 2016, 88, 8099–8106 CrossRef CAS PubMed.
- X. Gao, G. Tang and X. Su, Biosens. Bioelectron., 2012, 36, 75–80 CrossRef CAS PubMed.
- J. Chang, H. Li, T. Hou and F. Li, Biosens. Bioelectron., 2016, 86, 971–977 CrossRef CAS PubMed.
- A. Barba-Bon, A. M. Costero, S. Gil, F. Sancenón and R. Martínez-Máñez, Chem. Commun., 2014, 50, 13289–13291 RSC.
- X. Wu, Y. Song, X. Yan, C. Zhu, Y. Ma, D. Du and Y. Lin, Biosens. Bioelectron., 2017, 94, 292–297 CrossRef CAS PubMed.
- S. V. Eliseeva and J. C. BãNzli, Chem. Soc. Rev., 2009, 39, 189–227 RSC.
- K. Mikami, M. Terada and H. Matsuzawa, Angew. Chem., 2002, 114, 3704–3722 CrossRef.
- A. T. Bui, M. Beyler, A. Grichine, A. Duperray, J. C. Mulatier, Y. Guyot, C. Andraud, R. Tripier, S. Brasselet and O. Maury, Chem. Commun., 2017, 53, 6005–6008 RSC.
- K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- D. Knapton, M. Burnworth, S. J. Rowan and C. Weder, Angew. Chem. Int. Ed., 2006, 45, 5825–5829 (Angew. Chem., 2006, 118, 5957–5961) CrossRef CAS PubMed.
- H. Zhang, X. Hua, X. Tuo, C. Chen and X. Wang, J. Rare Earths, 2012, 30, 1203–1207 CrossRef CAS.
- J. C. G. Bünzli and S. V. Eliseeva, Lanthanide Luminescence, Springer Series on Fluorescence (Methods and Applications), Berlin, Heidelberg, 2010 Search PubMed.
- C. Piguet, J. C. G. Bünzli, G. Bernardinelli, G. Hopfgartner and A. F. Williams, J. Alloys Compd., 1995, 225, 324–330 CrossRef CAS.
- S. W. Magennis, S. Parsons and Z. Pikramenou, Chem.–Eur. J., 2002, 8, 5761–5771 CrossRef CAS.
- A. Casnati, F. Sansone, J. F. Dozol, H. Rouquette, F. O. Arnaud-Neu, D. Byrne, S. Fuangswasdi, M. J. Schwing-Weill and R. Ungaro, J. Inclusion Phenom. Macrocyclic Chem., 2001, 41, 193–200 CrossRef CAS.
- W. J. Wu, H. X. Huang, M. Chen and D. J. Qian, Chin. Chem. Lett., 2015, 26, 343–347 CrossRef CAS.
- X. B. Sun, X. Z. Jin, W. Pan and J. P. Wang, Carbohydr. Polym., 2014, 113, 194–199 CrossRef CAS PubMed.
- X. B. Gao, J. Yu, N. Li, H. Y. Yin and J. H. Yang, Chin. Chem. Lett., 2007, 18, 1289–1292 CrossRef CAS.
- D. Mababa, O. Nihal and M. A. Oturan, Chemosphere, 2007, 66, 841–848 CrossRef PubMed.
- F. L. Edwards and P. B. Tchounwou, Int. J. Environ. Res. Public Health, 2005, 2, 430–441 CrossRef CAS PubMed.
- B. Kmellár, P. Fodor, L. Pareja, C. Ferrer, M. A. Martínezuroz, A. Valverde and A. R. Fernandezalba, J. Chromatogr. A, 2008, 1215, 37–50 CrossRef PubMed.
- L. Chen, D. Wu and J. Yoon, ACS Sens., 2017, 3, 27–43 CrossRef PubMed.
- X. Tian, L. Liu, Y. Li, C. Yang, Z. Zhou, Y. Nie and Y. Wang, Sens. Actuators, B, 2018, 256, 135–142 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01748h |
| This journal is © The Royal Society of Chemistry 2019 |