 Open Access Article
Open Access ArticleConstructing “breathing” dynamic skeletons with extra π-conjugated adsorption sites for iodine capture†
Lixin Xia a,
Dongqi Yanga,
Hongcui Zhanga,
Qian Zhanga,
Naishun Bub,
Peng Song
a,
Dongqi Yanga,
Hongcui Zhanga,
Qian Zhanga,
Naishun Bub,
Peng Song c,
Zhuojun Yan*a and
Ye Yuan*d
c,
Zhuojun Yan*a and
Ye Yuan*d
aCollege of Chemistry, Liaoning University, Shenyang 110036, P. R. China. E-mail: zjyan@lnu.edu.cn
bSchool of Environmental Science, Liaoning University, Shenyang 110036, P. R. China
cDepartment of Physics, Liaoning University, Shenyang 110036, P. R. China
dKey Laboratory of Polyoxometalate Science of the Ministry of Education, Faculty of Chemistry, Northeast Normal University, Changchun 130024, P. R. China. E-mail: Yuany101@nenu.edu.cn
First published on 3rd July 2019
Abstract
Radioiodine (129I and 131I) emission from the nuclear waste stream has aroused enormous apprehension because of its quick diffusion and radiological contamination. Conventional porous adsorbents such as zeolites and carbon with rigid skeletons and constant pore volumes reveal a limited performance for reliable storage. Here, a series of soft porous aromatic frameworks (PAFs) with additional π-conjugated fragments is disclosed to serve as physicochemical stable media. Due to the flexibility of the tertiary amine center, the PAF products provide sufficient space for the binding sites, and thus exhibit a considerable capability for iodine capture from both gaseous and soluble environments. The obtained capacity of PAFs is ca. 1.6 times higher than that of PAF-1 which possesses similar aromatic constituents featuring an ultra-large specific surface area (BET = 5600 m2 g−1). The novel paradigm of dynamic frameworks is of fundamental importance for designing adsorbents to treat environmental pollution issues.
With the rapid development of the nuclear industry, a large amount of volatile radionuclides (129I, 131I, 3H, 85Kr etc.) accompanying nuclear fission are effused into the atmosphere.1 Radioiodine with high volatility and long radioactive half-life (1.57 × 107 years) attracts particular attention because its sustained pernicious effects on human metabolism ultimately result in an increased incidence of thyroid cancer.2–4 In this regard, great efforts have been focused on adsorbents to effectively capture and store radioactive iodine for safety and accessibility in the use of nuclear energy.5,6 Typical nuclear fuel processing conditions are under ambient pressure and 350 K where iodine exists as a coagulative vapor (diameter 5.4 Å).7 Therefore, iodine uptake in porous materials mostly depends on the binding sites and pore volume.8 However, the traditional adsorbents including zeolites, carbon, and inorganic–organic hybrid porous materials (MOFs, PCPs etc.) possess a narrow pore size and finite volume in their rigid skeletons.9–19 Their settled pore space can be occupied by iodine as the maximum accessibility of 50%, which generates confined iodine capacity.7,20 To achieve zero emission of radio-active vapors, the development of high-performance porous materials is desperately urgent.
Organic porous frameworks constructed by organic building units through covalent bonds are emerging as novel porous solids.21–27 Due to the low density, high stability, and large surface area, these functional products have made a probable promise in the field of gas storage, energy conversion, catalysis, and optoelectronics.28–36 Porous aromatic frameworks (PAFs) as an amorphous representative are well-known for their stable skeletons and large surface areas.37 Based on the advanced organic chemistry, one is able to prepare versatile PAFs with tunable organic composition and tailored structure.38 Their salient characteristics motivate wide interests in the design and synthesis of unique porous skeletons to challenge the traditional rigid frameworks for radioiodine adsorption.
In this contribution, we prepared a class of tertiary amine centered PAF materials (LNU 1–4, LNU represents Liaoning University) via a Suzuki coupling reaction. Unlike the traditional porous adsorbents, the N linked aromatic fragments could be distorted and triggered into a “breathing” dynamic skeleton for fitting iodine storage.39–44 Besides, various π-conjugated units were incorporated into the architecture to serve as adsorption sites. Consequently, the soft PAFs with dynamic architectures render a significant adsorption for iodine molecules and enable cycle use for many times while retaining their high capacity.
As illustrated in Scheme 1, LNU-1, LNU-2, LNU-3, and LNU-4 were synthesized by polycondensation reactions of 4-(tetramethyl-1,3,2-dioxaborolan-2-yl)-N,N-bis-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]aniline (BBA) with respective dibromo monomer (2,7-dibromofluorene, 1,4-dibromonaphthalene, 2,6-dibromonaphthalene and 9,10-dibromoanthracene, respectively). According to the Fourier transform infrared spectroscopy (FTIR) spectra (Fig. S1†), the disappearance of C–Br stretching vibration frequency (500 cm−1), C–B vibration band (1417 cm−1), and B–O vibration band (1351 cm−1) from the initial building blocks indicates the completion of cross-coupling reactions and suggests the formation of porous polymers. The structural details of LNU 1–4 networks were also characterized at the molecular level by solid-state 13C NMR spectroscopy (Fig. S2†). The major peaks observed at 135–150 ppm could be assigned to the C substituted aromatic carbons and signals in the range of 120–135 ppm are ascribed to the H coupled aromatic carbons, respectively. Powder X-ray diffraction (PXRD) pattern (Fig. S3†) shows that all PAF samples possess no long-range ordered structure. Scanning electron microscopy (SEM) indicates these resultants are composed of aggregated spheres in micrometer size (Fig. S4†). The worm-like patterns from transmission electron microscopy (TEM) manifest the amorphous textures of LNU 1–4 which is coincident with the PXRD conclusion (Fig. S5†).
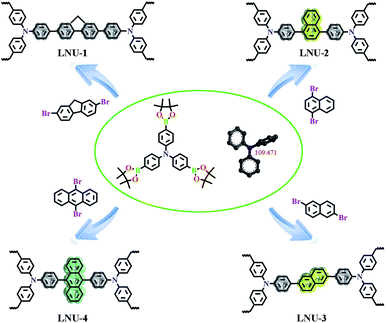 | ||
| Scheme 1 Synthetic routes for PAF materials LNU-1, LNU-2, LNU-3, and LNU-4 via a Suzuki coupling reaction. | ||
The stability and porosity of adsorbents are critical factors for the practical molecular storage application. The four polymers are stable up to 350 °C assessed by thermogravimetric analysis (TGA) in air atmosphere (Fig. S6†). Also there is no weight loss after immersing PAF powders into a variety of common organic solvents, such as DMF, THF, acetone, and CHCl3, respectively, proving the excellent chemical stability of PAFs. The porosities of LNU 1–4 products were evaluated by nitrogen gas adsorption and desorption experiments at 77 K. As shown in Fig. S7,† all resultants show almost no gas sorption at relatively low pressure (P/P0 < 0.9), and show Type-III isotherms indicating the weak adsorbate–adsorbent interactions.45,46 The specific surface areas calculated by the BET equation are 26, 20, 30, and 24 m2 g−1 for LNU-1, LNU-2, LNU-3, and LNU-4, respectively. Meanwhile, the CO2 isotherms at 273 K and 298 K reveal a typical CO2 adsorption behaviour (Fig. S8†). As illustrated from CO2 desorption, all four LUN materials show a hysteretic behavior, which together with the relatively large capacity is the typical character of “breathing” adsorbents. This result suggests that the adsorption mechanism of LNUs preceeds the guest-responsive structural rearrangements and realized the “dynamic” skeletons.47–49 This phenomenon is attributed to that the N linked aromatic fragments could be distorted leading to a soft skeleton which tends to collapse into a dense, non-porous solid.50,51
The traditional rigid adsorbents with a constant porosity exhibit the limited iodine capacity, such as porous silver-doped zeolite mordenite (Ag-MOR), carbon (Cg-5P), and zeolitic imidazolate framework-8 (ZIF-8) possess the slight iodine capacities of 0.07, 0.28, and 1.25 g g−1, respectively.7 For purposes of comparison, the novel PAF materials with excellent stability and soft architecture were carried out the iodine enrichment experiments by exposing the powder samples into iodine vapor. As shown in Fig. 1 and S9,† LNU-1 without accessible porosity for N2 molecules could adsorb 2.49 g g−1 iodine molecules. As a reference, PAF-1 possesses similar chemical constituents of multiple benzene rings as LNU-1, but a carbon center and high porosity (BET surface area = 5600 m2 g−1), which only adsorbs 1.86 g g−1 iodine.52 As known, the expanded conjugated network together with the electron-rich characteristic of triphenylamine units possesses strong affinity with I2 molecules.15,19,52 For non-porous PAF skeleton (LNU-1), this outstanding iodine uptake implies that the dense structure generates a specific dynamic configuration transformation and is triggered into a “breathing” state due to the flexibility of triphenylamine fragment, which favours the access and storage of iodine molecules.51,53,54
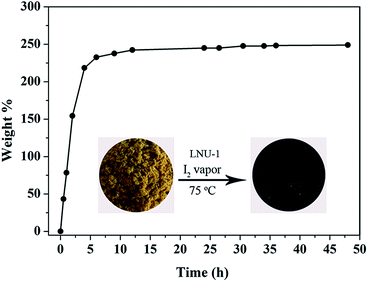 | ||
| Fig. 1 Iodine uptake of the LNU-1 as a function of exposure time. Photograph for PAF powder LNU-1 before and after iodine capture (inset). | ||
To confirm this speculation, all PAF samples after performed the iodine adsorption procedure were conducted by the TGA analysis. As shown in Fig. 2a, there is a gradual mass loss started from 90 °C and ended at 200 °C, which suggests the strong affinity of iodine with PAF architecture (I2 boiling point is 184 °C). In addition, we conducted FT-IR, PXRD, and Raman analyses for PAFs after it absorbed I2 molecules, with the resultant material named PAF-I2. As illustrated in Fig. 2b, the special bands of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H in the phenyl ring change from 1504 and 819 cm−1 to 1502 and 822 cm−1, respectively, demonstrating the interaction of aromatic fragments with I2 molecules.55 There are no characteristic peaks of elemental iodine in the PXRD patterns compared with I2 and PAF-I2 which demonstrates the uniform distribution of iodine molecules in LNU-1 framework (Fig. 2c). Furthermore, the strong peaks at 113 and 173 cm−1 recorded by Raman spectroscopy manifest the I5− state of adsorbed iodine molecules, which is attributed to the charge transfer between the electron-deficient guest iodine molecules and the electron-rich host network at high coverage (Fig. 2d).55,56 All these results demonstrate the adsorbed I2 molecules are uniformly distributed in the PAF architecture suggesting the dynamic configuration transformation of PAF skeleton for fitting the I2 storage.
C and C–H in the phenyl ring change from 1504 and 819 cm−1 to 1502 and 822 cm−1, respectively, demonstrating the interaction of aromatic fragments with I2 molecules.55 There are no characteristic peaks of elemental iodine in the PXRD patterns compared with I2 and PAF-I2 which demonstrates the uniform distribution of iodine molecules in LNU-1 framework (Fig. 2c). Furthermore, the strong peaks at 113 and 173 cm−1 recorded by Raman spectroscopy manifest the I5− state of adsorbed iodine molecules, which is attributed to the charge transfer between the electron-deficient guest iodine molecules and the electron-rich host network at high coverage (Fig. 2d).55,56 All these results demonstrate the adsorbed I2 molecules are uniformly distributed in the PAF architecture suggesting the dynamic configuration transformation of PAF skeleton for fitting the I2 storage.
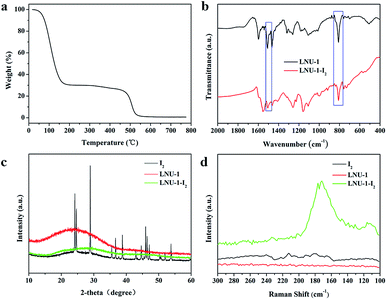 | ||
| Fig. 2 (a) TGA curve of the iodine adsorbed LNU-1. FT-IR spectra (b), PXRD profiles (c), and Raman spectra (d) of the LNU-1 and iodine adsorbed LNU-1. | ||
This “breathing” dynamic skeleton of LNU-1 prompted us a further investigate to achieve a better performance for radioiodine capture. Various π-conjugated groups including naphthalene and anthracene rings are introduced into the soft framework. In accordance with analysis above, naphthalene based LNU-2 and LNU-3 and anthracene based LNU-4 exhibit a similar “breathing” state which is demonstrated by the TGA, FT-IR, PXRD, and Raman spectra (Fig. S10–S13†). The PAF resultants reveal increased iodine uptake capacities of 2.95 g g−1 for LNU-2 and 2.76 g g−1 for LNU-3, respectively from 2.49 g g−1 for LNU-1. This increased capacity is attributed to the flexible skeleton provides sufficient space for the accessibility of iodine to π-conjugated groups which possess a strong affinity for guest binding through charge transfer.55–57 Furthermore, two increased FT-IR peaks of conjugated group located at 1258 and 1159 cm−1 and the emerging Raman signals at 110 and 175 cm−1 demonstrate this charge transfer from the highest occupied molecular orbital (HOMO) of π-conjugated group to the lowest unoccupied molecular orbital (LUMO) of iodine.45 As to LNU-4 (2.04 g g−1 iodine uptake), the anthracene constituent with a similar binding structure as naphthalene group increases the weight of structural unit resulting in the reduced maximum I2 capacity compared with LNU-2 and LNU-3. Due to the elaborate design, the tertiary amine centered PAF materials express a better performance than the classical microporous polymers including PAF-21 (1.52 g g−1), PAF-23 (2.71 g g−1), pha-HcoP-1 (1.31 g g−1), NAPOP-1 (2.06 g g−1), NIP-CMP (2.02 g g−1), CMPN-3 (2.08 g g−1), and SCMP-1 (1.88 g g−1).18,19,58–61
Additionally, the iodine sorption experiments of PAF products were exploited in the iodine containing solution. When PAFs were immersed in a hexane solution of iodine (C = 1000 mg L−1) at room temperature, the dark purple solutions fade gradually to colourless status (Fig. 3a and S14†). This phenomenon indicates the porous frameworks with strong affinity could be utilized to extract iodine molecules from the liquid environments. Notably, the LNU PAFs could be activated upon the thermal treatment at 398 K for 120 min of iodine-captured PAF samples. Also the iodine adsorbed porous skeletons release the guest molecules by immersing the resultants in organic solvents at room temperature (Fig. 3b and S15†). The soft PAF materials are recyclable after the activation at 398 K and 120 min, while retaining a considerable uptake capacity after five cycles (Fig. S16†). All these results demonstrate the tertiary amine centered PAF materials possess tremendous potential for iodine capture application compared to the conventional adsorbents.
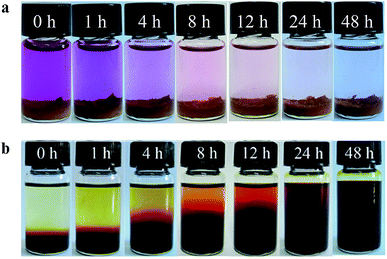 | ||
| Fig. 3 Photographs indicate the gradual changes in iodine adsorption (a) and desorption (b) processes of LNU-1. | ||
Conclusions
In summary, we constructed a series of N centered PAF materials with π-conjugated groups for radioiodine sorption. After exposed into iodine vapor, the specific dynamic skeleton is triggered into a “breathing” state for fitting the iodine storage and provides sufficient space for the iodine access into the π-conjugated adsorption sites. As a consequence, the soft PAFs possess a significant adsorption capability for iodine molecules. Apart from the remarkable adsorption capacity, the success of constructing PAFs with “breathing” dynamic skeleton opens a novel strategy for preparation of novel radioiodine adsorbents.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21704037, 21671089, and 21604008), the Shenyang Natural Science Foundation of China (F16-103-4-00), Scientific Research Fund of Liaoning Province (LT2017010, 20170540409), Startup Foundation for Doctors of Liaoning Province (20170520069), the Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (ES201809), the Open Research Fund of State Key Laboratory of Estuarine and Coastal Research (SKLEC-KF201713), and Natural Science Foundation of Jilin Province of China (20180520144JH).Notes and references
- Z. J. Yin, S. Q. Xu, T. G. Zhan, Q. Y. Qi, Z. Q. Wu and X. Zhao, Chem. Commun., 2017, 53, 7266–7269 RSC
.
- G. Steinhauser, Environ. Sci. Technol., 2014, 48, 4649–4663 CrossRef CAS PubMed
.
- S. Xu, S. Freeman, X. L. Hou, A. Watanabe, K. Yamaguchi and L. Y. Zhang, Environ. Sci. Technol., 2013, 47, 10851–10859 CrossRef CAS PubMed
.
- Y. Chen, F. G. Zhang and Z. M. Xue, J. Mol. Liq., 2016, 223, 202–208 CrossRef CAS
.
- B. J. Riley, J. D. Vienna, D. M. Strachan, J. S. McCloy and J. L. Jerden, J. Nucl. Mater., 2016, 470, 307–312 CrossRef CAS
.
- K. W. Chapman, P. J. Chupas and T. M. Nenoff, J. Am. Chem. Soc., 2010, 132, 8897–8899 CrossRef CAS PubMed
.
- P. Wang, Q. Xu, Z. Li, W. Jiang, Q. Jiang and D. Jiang, Adv. Mater., 2018, 1801991–1801997 CrossRef PubMed
.
- R. Krishna, J. Phys. Chem. C, 2009, 113, 19756–19781 CrossRef CAS
.
- J. T. Hughes, D. F. Sava, T. M. Nenoff and A. Navrotsky, J. Am. Chem. Soc., 2013, 135, 16256–16259 CrossRef CAS PubMed
.
- B. J. Riley, J. Chun, J. V. Ryan, J. Matyas, X. S. Li, D. W. Matson, S. K. Sundaram, D. M. Strachana and J. D. Vienna, RSC Adv., 2011, 1, 1704–1715 RSC
.
- K. S. Subrahmanyam, C. D. Malliakas, D. Sarma, G. S. Armatas, J. Wu and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 13943–13948 CrossRef CAS PubMed
.
- X. R. Zhang, I. D. Silva, H. G. W. Godfrey, S. K. Callear and S. A. Sapchenko, J. Am. Chem. Soc., 2017, 139, 16289–16296 CrossRef CAS PubMed
.
- D. F. Sava, K. W. Chapman, M. A. Rodriguez, J. A. Greathouse, P. S. Crozier, H. Zhao, P. J. Chupas and T. M. Nenoff, Chem. Mater., 2013, 25, 2591–2596 CrossRef CAS
.
- N. X. Zhu, C. W. Zhao, J. C. Wang, Y. A. Li and Y. B. Dong, Chem. Commun., 2016, 52, 12702–12705 RSC
.
- Q. Q. Dang, X. M. Wang, Y. F. Zhang and X. M. Zhang, Polym. Chem., 2016, 7, 643–647 RSC
.
- H. Li, X. S. Ding and B. H. Han, Chem.–Eur. J., 2016, 22, 11863–11868 CrossRef CAS PubMed
.
- Y. F. Chen, H. X. Sun, R. X. Yang, T. T. Wang, C. J. Pei, Z. T. Xiang, Z. Q. Zhu, W. D. Liang, A. Li and W. Q. Deng, J. Mater. Chem. A, 2015, 3, 87–91 RSC
.
- Z. J. Yan, Y. Yuan, Y. Y. Tian, D. Zhang and G. S. Zhu, Angew. Chem., Int. Ed., 2015, 54, 12733–12737 CrossRef CAS PubMed
.
- X. Qian, Z. Q. Zhu, H. X. Sun, F. Ren, P. Mu, W. D. Liang, L. H. Chen and A. Li, ACS Appl. Mater. Interfaces, 2016, 8, 21063–21069 CrossRef CAS PubMed
.
- Q. Sun, B. Aguila and S. Q. Ma, Trends in Chemistry, 2019, 1, 292–303 CrossRef
.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC
.
- Q. Xu, S. Dalapati and D. Jiang, ACS Cent. Sci., 2016, 2, 586–587 CrossRef CAS PubMed
.
- C. R. Mulzer, L. Shen, R. P. Bisbey, J. R. McKone, N. Zhang, H. D. Abruña and W. R. Dichtel, ACS Cent. Sci., 2016, 2, 667–673 CrossRef CAS PubMed
.
- N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675–683 RSC
.
- A. I. Cooper, ACS Cent. Sci., 2017, 3, 544–553 CrossRef CAS PubMed
.
- Y. G. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083–2094 RSC
.
- B. J. Smith, L. R. Parent, A. C. Overholts, P. A. Beaucage, R. P. Bisbey, A. D. Chavez, N. Hwang, C. Park, A. M. Evans, N. C. Gianneschi and W. R. Dichtel, ACS Cent. Sci., 2017, 3, 58–65 CrossRef CAS PubMed
.
- M. Carta, R. Malpass-Evans, M. Croad, Y. Rogan, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Science, 2013, 339, 303–307 CrossRef CAS PubMed
.
- C. J. Doonan, D. J. Tranchemontagne, T. G. Glover, J. R. Hunt and O. M. Yaghi, Nat. Chem., 2010, 2, 235–238 CrossRef CAS PubMed
.
- J. Weber and A. Thomas, J. Am. Chem. Soc., 2008, 130, 6334–6335 CrossRef CAS PubMed
.
- R. W. Tilford, S. J. Mugavero, P. J. Pellechia and J. J. Lavigne, Adv. Mater., 2008, 20, 2741–2746 CrossRef CAS PubMed
.
- R. Schmid and M. Tafipolsky, J. Am. Chem. Soc., 2008, 130, 12600–12601 CrossRef CAS PubMed
.
- S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed
.
- S. Demir, N. Brune, J. F. V. K. Humbeck, J. A. Mason, T. V. Plakhova, S. Wang, G. Tian, S. G. Minasian, T. Tyliszczak, T. Yaita, T. Kobayashi, S. N. Kalmykov, H. Shiwaku, D. K. Shuh and J. R. Long, ACS Cent. Sci., 2016, 2, 253–265 CrossRef CAS PubMed
.
- A. G. Slater, P. S. Reiss, A. Pulido, M. A. Little, D. L. Holden, L. Chen, S. Y. Chong, B. M. Alston, R. Clowes, M. Haranczyk, M. E. Briggs, T. Hasell, G. M. Day and A. I. Cooper, ACS Cent. Sci., 2017, 3, 734–742 CrossRef CAS PubMed
.
- Q. Sun, S. Ma, Z. Dai, X. Meng and F. S. Xiao, J. Mater. Chem. A, 2015, 3, 23871–23875 RSC
.
- Y. Yuan and G. Zhu, ACS Cent. Sci., 2019, 5, 409–419 CrossRef CAS PubMed
.
- S. Das, P. Heasman, T. Ben and S. L. Qiu, Chem. Rev., 2017, 117, 1515–1563 CrossRef CAS PubMed
.
- Y. Yuan, Y. J. Yang, X. J. Ma, Q. H. Meng, L. L. Wang, S. Zhao and G. S. Zhu, Adv. Mater., 2018, 30, 1706507 CrossRef PubMed
.
- Y. Yuan, Y. J. Yang, M. Faheem, X. Q. Zou, X. J. Ma, Z. Y. Wang, Q. H. Meng, L. L. Wang, S. Zhao and G. S. Zhu, Adv. Mater., 2018, 30, 1800069 CrossRef PubMed
.
- Y. J. Sheng, Q. B. Chen, J. Y. Yao, Y. X. Lu, H. L. Liu and S. Dai, Angew. Chem., Int. Ed., 2016, 55, 3378–3381 CrossRef CAS PubMed
.
- A. D. G. Firmino, R. F. Mendes, M. M. Antunes, P. C. Barbosa, S. M. F. Vilela, A. A. Valente, F. M. L. Figueiredo, J. P. C. Tomé and F. A. A. Paz, Inorg. Chem., 2017, 56, 1193–1208 CrossRef CAS PubMed
.
- T. Tahier and C. L. Oliver, CrystEngComm, 2017, 19, 3607–3618 RSC
.
- B. Mu, F. Li, Y. G. Huang and K. S. Walton, J. Mater. Chem., 2012, 22, 10172–10178 RSC
.
- F. L. Zhao, A. J. Dong, L. D. Deng, R. W. Guo and J. H. Zhang, Polym. Chem., 2019, 10, 2436–2446 RSC
.
- M. Alhamami, H. Doan and C. H. Cheng, Materials, 2014, 7, 3198–3250 CrossRef PubMed
.
- C. H. Lee, M. R. Tsai, Y. T Chang, L. L. Lai, K. L. Lu and K. L. Cheng, Chem.–Eur. J., 2013, 19, 10573–10579 CrossRef CAS PubMed
.
- A. Schneemann, P. Vervoorts, I. Hante, M. Tu, S. P. Wannapaiboon, C. Sternemann, M. Paulus, D. C. Florian Wieland, S. Henke and R. A. Fischer, Chem. Mater., 2018, 30, 1667–1676 CrossRef CAS
.
- X. L. Wu, F. Luo, G. M. Sun, A. M. Zheng, J. Zhang, M. B. Luo, W. Y. Xu, Y. Zhu, X. M. Zhang and S. Y. Huang, ChemPhysChem, 2013, 14, 3594–3599 CrossRef CAS PubMed
.
- A. K. Mandal, M. Suresh, P. Das and A. Das, Chem.–Eur. J., 2012, 18, 3906–3917 CrossRef CAS PubMed
.
- V. A. Davankov and M. P. Tsyurupa, React. Polym., 1990, 13, 27–42 CrossRef CAS
.
- F. Ren, Z. Q. Zhu, X. Qian, W. D. Liang, P. Mu, H. X. Sun, J. H. Liu and A. Li, Chem. Commun., 2016, 52, 9797–9800 RSC
.
- C. Zhao, C. S. Diercks, C. Zhu, N. Hanikel, X. Pei and O. M. Yaghi, J. Am. Chem. Soc., 2018, 140, 16438–16441 CrossRef CAS PubMed
.
- C. Wang, Y. Wang, R. Ge, X. Song, X. Xing, Q. Jiang, H. Lu, C. Hao, X. Guo, Y. Gao and D. Jiang, Chem.–Eur. J., 2018, 24, 585–589 CrossRef CAS PubMed
.
- C. Y. Pei, T. Ben, S. X. Xu and S. L. Qiu, J. Mater. Chem. A, 2014, 2, 7179–7187 RSC
.
- L. Lin, H. D. Guan, D. L. Zou, Z. J. Dong, Z. Liu, F. F. Xu, Z. G. Xie and Y. X. Li, RSC Adv., 2017, 7, 54407–54415 RSC
.
- H. Xu, S. S. Tao and D. L. Jiang, Nat. Mater., 2016, 15, 722–726 CrossRef CAS PubMed
.
- Y. Z. Liao, J. Weber, B. M. Mills, Z. H. Ren and C. F. J. Fau, Macromolecules, 2016, 49, 6322–6333 CrossRef CAS
.
- J. Y. Weng, Y. L. Xu, W. C. Song and Y. H. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2016, 15, 1724–1730 CrossRef
.
- L. Q. Xu, L. P. Guo, G. S. Hu, J. H. Chen, X. Hu, S. L. Wang, W. Dai and M. H. Fan, RSC Adv., 2015, 5, 37964–37969 RSC
.
- X. Qian, B. Wang, Z. Q. Zhu, H. X. Sun, F. Ren, P. Mu, C. H. Ma, W. D. Liang and A. Li, J. Hazard. Mater., 2017, 338, 224–232 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01904a |
| This journal is © The Royal Society of Chemistry 2019 |
