Open Access Article
Open Access ArticleSynthesis of poly-functionalized benzofurans via one-pot domino oxidation/[3+2] cyclization reactions of a hydroquinone ester and ynamides†
Dongxin Zhang *,
Jingjing Man,
Yan Chen,
Lei Yin,
Junchao Zhong and
Qian-Feng Zhang*
*,
Jingjing Man,
Yan Chen,
Lei Yin,
Junchao Zhong and
Qian-Feng Zhang*
Institute of Molecular Engineering and Applied Chemistry, Anhui University of Technology, No. 59 Hudong Road, Ma'anshan 243002, China. E-mail: dxzhang@ahut.edu.cn; zhangqf@ahut.edu.cn
First published on 25th April 2019
Abstract
Densely substituted amino-functionalized benzofurans were concisely accessed via the first one-pot domino oxidation/[3+2] cyclization of a hydroquinone ester and easily accessible ynamides under mild conditions in a short time. The complex benzofurans were able to be efficiently synthesized all from simple and inexpensive starting materials in two steps.
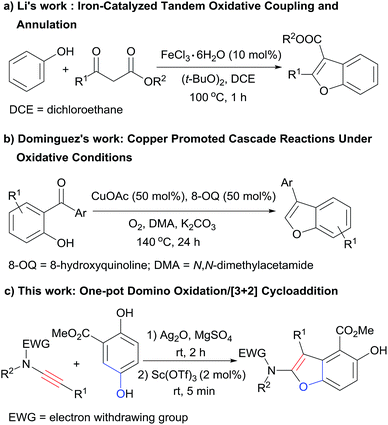
Benzofuran derivatives are valuable structural motifs that are often found in natural products and biologically active compounds.1 Various methods have been developed for the synthesis of those heterocyclic scaffolds,2,3 among which the intramolecular annulation of preformed ortho-alkynylated phenols catalyzed by transition metals is the most general way.2e–o Recently, Li and coworkers reported a novel iron-catalyzed tandem oxidative coupling and annulation process to access benzofurans from simple phenols and β-keto esters (Scheme 1a),3a and the group of Dominguez disclosed a one-pot approach to benzofurans from 2-hydroxybenzophenones and N,N-dimethylacetamide promoted by copper under oxidative conditions (Scheme 1b).3b Both of the two methods provided direct access to benzofuran derivatives from simple easy accessible starting materials under oxidative conditions, but all the reactions were performed at high temperature and with relatively high catalyst loading. Fast reactions under mild conditions are undoubtedly more desirable. In this context, we report the direct synthesis of densely substituted benzofurans from simple and inexpensive starting materials via the first one-pot domino oxidation/[3+2] cyclization of a hydroquinone derivative and ynamides under mild conditions (Scheme 1c).
Over the past decades, ynamides, as powerful synthons, have been involved in the transition-metal catalyzed cyclization reactions for the construction of diverse building blocks of functionalized molecules including important pharmacophores.4 Although various cyclic systems have been achieved via the reactions of ynamides,4a–q to the best of our knowledge, construction of amino-functionalized benzofurans from ynamides have not been reported. Therefore, we initiated our design to access benzofuran derivatives via the [3+2] cyclizations of ynamides with quinones. Quinones can be easily oxidized from hydroquinones, which provides the possibility for coupling the oxidation and cyclization into a one-pot domino process.5,6b Before coupling the two steps together, we first chose to optimize the cyclization step.
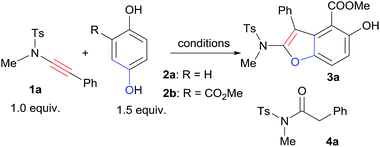
First, we started the investigation with ynamide 1a and quinone 2a. Lewis acids have been reported as good activators of quinones.6,7a However, by the use of Lewis acid Sc(OTf)3 as the catalyst, there was no desired benzofuran product formation at all while the ynamide hydrolysis byproduct 4a formed exclusively (Table 1, entry 1). Attempts by the addition of 4 Å molecular sieves and carrying out the reaction under inert atmosphere suppressed the hydrolysis somehow with still no product formation (Table 1, entry 2). To overcome the low reactivity problem of 2a and the hydrolysis problem of 1a, we pursued to improve the reactivity of quinone by installing an ester group on the quinone ring, which may enhance its electrophilicity.7 Quinone ester 2b was easily synthesized via oxidation of commercially available methyl 2,5-dihydroxybenzoate (5). Gratifyingly, the reaction of 1a with quinone ester 2b catalyzed by Sc(OTf)3 afforded the desired [3+2] cyclization product 3a in 89% yield within 5 min with only trace amount of hydrolysis product detected (Table 1, entry 3). By the use of Cu(OTf)2 as the catalyst, in 5 min the reaction of 1a and 2b afforded both 3a and 4a, in 61% and 18% yield respectively (Table 1, entry 4). Yb(OTf)3 also catalyzed the reaction efficiently with small amount of 4a (4%) formation (Table 1, entry 5). Other Lewis acids, such as AlCl3 could catalyze the reaction to afford 3a with no 4a formation though the efficiency is much lower than that of Sc(OTf)3 (Table 1, entry 6). Screening of a small set of solvents indicated that CH2Cl2 was the most suitable solvent for this [3+2] cyclization (Table 1, entries 7 and 8). Reducing the amount of 2b from 1.5 equiv. to 1.2 equiv. did not affect the reaction efficiency and product 3a was obtained in 90% yield (Table 1, entry 9). As expected, when excess amount of 1a was used, product 3a was formed along with small amount of 4a (Table 1, entry 10). The catalyst loading could be reduced to 5 mol%, even 2 mol% without significant negative effect for the efficiency of the [3+2] cyclization (Table 1, entries 11 and 12).
| Entry | Catalyst (10 mol%) | Solvent | R | Time | Yieldb (3a) [%] | Yieldb (4a) [%] |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, reactions were carried out using ynamide 1a (0.10 mmol), 2 (0.15 mmol) with catalyst (0.01 mmol) in solvent 2.0 mL at room temperature (25 °C) in air.b Isolated yields relative to 1a.c 100 mg 4 Å molecular sieves were added and under Ar atmosphere.d 2b (0.12 mmol) was used.e 1a (0.12 mmol), 2b (0.10 mmol) was used.f Yield relative to 2b.g Catalyst (0.005 mmol) was used.h Catalyst (0.002 mmol) was used.i Hydroquinone ester 5 (0.12 mmol), Ag2O (0.24 mmol), and MgSO4 (0.24 mmol) were mixed in CH2Cl2 (2.0 mL) and the mixture was stirred for 2 h, and then 1a (0.10 mmol) and Sc(OTf)3 (0.002 mmol) were added. | ||||||
| 1 | Sc(OTf)3 | CH2Cl2 | H | 5 min | — | 91 |
| 2c | Sc(OTf)3 | CH2Cl2 | H | 2 h | — | 33 |
| 3 | Sc(OTf)3 | CH2Cl2 | CO2Me | 5 min | 89 | Trace |
| 4 | Cu(OTf)2 | CH2Cl2 | CO2Me | 5 min | 61 | 18 |
| 5 | Yb(OTf)3 | CH2Cl2 | CO2Me | 5 min | 83 | 4 |
| 6 | AlCl3 | CH2Cl2 | CO2Me | 30 min | 33 | Trace |
| 7 | Sc(OTf)3 | THF | CO2Me | 5 min | 80 | 5 |
| 8 | Sc(OTf)3 | PhMe | CO2Me | 5 min | 86 | Trace |
| 9d | Sc(OTf)3 | CH2Cl2 | CO2Me | 5 min | 90 | Trace |
| 10e | Sc(OTf)3 | CH2Cl2 | CO2Me | 5 min | 88f | 9 |
| 11d,g | Sc(OTf)3 | CH2Cl2 | CO2Me | 5 min | 91 | Trace |
| 12d,h | Sc(OTf)3 | CH2Cl2 | CO2Me | 5 min | 88 | Trace |
| 13d,h,i | Sc(OTf)3 | CH2Cl2 | CO2Me | 5 min | 91 | Trace |
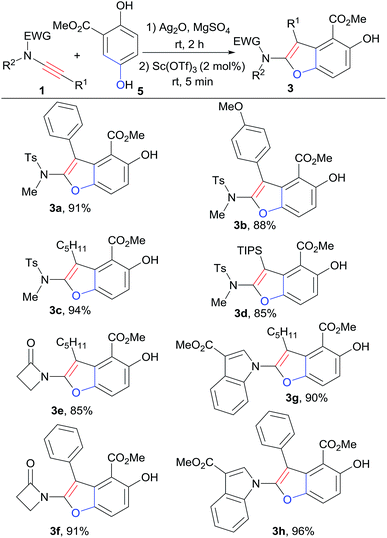
With the best cyclization reaction conditions in hand, next we tried to combine the oxidation step with the [3+2] cyclization into a one-pot domino process.5a Hydroquinone ester 5 was first mixed with oxidant Ag2O and MgSO4 in CH2Cl2 and the mixture was stirred for 2 h. Then 1a and Sc(OTf)3 were directly added to the above mixture.8,9 To our delight, the one-pot domino oxidation/[3+2] cyclization occurred efficiently, affording 3a in 91% yield (Table 1, entry 13). The one-pot process provided the densely substituted benzofuran (3a) in a step-economy manner. Next, for the substrate scope exploration, the one-pot domino process was employed (Scheme 2).
In general, the reactions of 5 with a series of ynamides in the one-pot system afforded poly-substituted benzofurans 3 in good to excellent yields (85–96%). Ynamides with both aromatic and alkyl groups at the terminal position could be tolerated, furnishing 3-aromatic or 3-alkyl substituted benzofurans with high yields (Scheme 2). The reactions of alkyl-terminated ynamides gave slightly higher yields than that of aromatic-terminated ones (3a, 3b vs. 3c; 3e vs. 3f; 3g vs. 3h). For ynamides with sulfonyl system, the triisopropylsilyl-terminated ynamide was successfully applied in the one-pot reaction, and the desired product 3d was obtained in 85% yield. The reactions of ynamides with propiolactam system also worked well to give 3e (85%) and 3f (91%). Ynamides derived from indole ester reacted well with 5 in this one-pot system, giving rise to the interesting 1-benzofuranyl indole derivatives (3g, 3h) in perfect yields, 90% and 96% respectively.
It is worth to mention that ynamides (1) used here were all easily prepared in one step via copper-catalyzed oxidative cross coupling of corresponding simple amides and terminal alkynes according to literature procedures,4q,10 which indicates that the complex poly-functionalized benzofurans (3) were able to be efficiently synthesized all from simple and inexpensive staring materials in only two steps (Scheme 3). A large scale reaction was performed to synthesize 1-benzofuranyl indole 3h. Starting from simple commercial available 1-heptyne, methyl indole-3-carboxylate, and methyl 2,5-dihydroxybenzoate (5), 1.64 g of 3h was obtained in two steps with high efficiency (Scheme 3b).
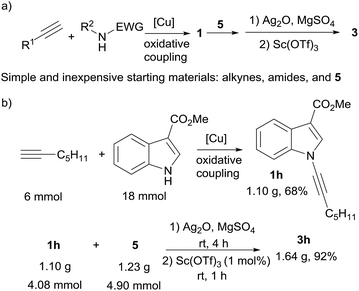 | ||
| Scheme 3 (a) Concise access to complex benzofurans from simple and inexpensive starting materials. (b) A large scale reaction for the synthesis of 3h. | ||
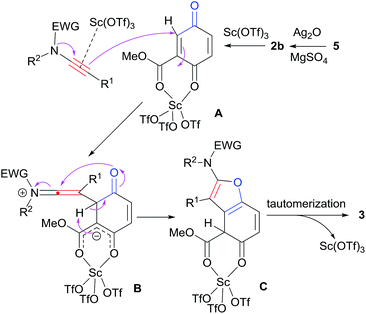
The postulated mechanisms resulting in the formation of densely substituted benzofurans 3 are proposed as shown in Scheme 4. Hydroquinone 5 is first oxidized by Ag2O to give quinone ester 2b, which is subsequently activated by Sc(OTf)3. According to Johnson et al.,4q ynamides may also be functioned by the scandium Lewis acid to increase the nucleophilicity. A undergoes nucleophilic attack by the ynamide to give keteniminium ion B. Then, 1,2-proton shift followed by the intramolecular cyclization of B furnishes intermediate C. Finally, after tautomerization and release of Sc(OTf)3, desired product 3 is formed.
In summary, we have developed a fast and step-economical one-pot domino oxidation/[3+2] cyclization reactions of a hydroquinone ester and ynamides. A series of densely functionalized benzofurans were concisely achieved in good to excellent yields from simple and inexpensive starting materials under mild conditions. A gram scale reaction proved that the one-pot reaction was able to be scaled up easily. Further studies on the expansion of the reaction scope and on the construction of other heterocycles based on the domino strategy used in this study are ongoing and will be reported in due course.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by Anhui Provincial Natural Science Foundation (No. 1908085MB33) and the Anhui University of Technology.Notes and references
- For recent reviews and examples: (a) K. Chand, Rajeshwari, A. Hiremathad, M. Singh, M. A. Santos and R. S. Keri, Pharmacol. Rep., 2017, 69, 281 CrossRef CAS PubMed; (b) A. Soleimani, J. Asadi, F. Rostami-Charati and R. Gharaei, Comb. Chem. High Throughput Screening, 2015, 18, 505 CrossRef CAS; (c) A. Radadiya and A. Shah, Eur. J. Med. Chem., 2015, 97, 356 CrossRef CAS PubMed; (d) R. Naik, D. S. Harmalkar, X. Xu, K. Jang and K. Lee, Eur. J. Med. Chem., 2015, 90, 379 CrossRef CAS PubMed; (e) H. Khanam and Shamsuzzaman, Eur. J. Med. Chem., 2015, 97, 483 CrossRef CAS PubMed; (f) M. Mostofi, G. Mohammadi Ziarani and N. Lashgari, Bioorg. Med. Chem., 2018, 26, 3076 CrossRef CAS PubMed; (g) B. Lu, S. Huang, J. Cao, Q. Hu, R. Shen, H. Wan, D. Wang, J. Yuan, L. Zhang, J. Zhang, M. Zhang, W. Tao and L. Zhang, Bioorg. Med. Chem., 2018, 26, 581 CrossRef CAS PubMed; (h) P. Kushwaha, A. K. Tripathi, S. Gupta, P. Kothari, A. Upadhyay, N. Ahmad, T. Sharma, M. I. Siddiqi, R. Trivedi and K. V. Sashidhara, Eur. J. Med. Chem., 2018, 156, 103 CrossRef CAS PubMed; (i) M. Thevenin, S. Thoret, P. Grellier and J. Dubois, Bioorg. Med. Chem., 2013, 21, 4885 CrossRef CAS PubMed; (j) J. J. La Clair, A. L. Rheingold and M. D. Burkart, J. Nat. Prod., 2011, 74, 2045 CrossRef CAS PubMed; (k) J. Sakurai, T. Oguchi, K. Watanabe, H. Abe, S. Kanno, M. Ishikawa and T. Katoh, Chem.–Eur. J., 2008, 14, 829 CrossRef CAS PubMed; (l) R. Romagnoli, P. G. Baraldi, M. D. Carrion, C. L. Cara, O. Cruz-Lopez, M. Tolomeo, S. Grimaudo, A. Di Cristina, M. R. Pipitone, J. Balzarini, N. Zonta, A. Brancale and E. Hamel, Bioorg. Med. Chem., 2009, 17, 6862–6871 CrossRef CAS PubMed.
- For representative reviews and examples: (a) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed; (b) A. R. Katritzky, C. N. Fali and J. Li, J. Org. Chem., 1997, 62, 8205 CrossRef CAS PubMed; (c) K. C. Nicolaou, S. A. Snyder, A. Bigot and J. A. Pfefferkorn, Angew. Chem., Int. Ed., 2000, 39, 1093 CrossRef CAS; (d) M. Rueping, M. Leiendecker, A. Das, T. Poisson and L. Bui, Chem. Commun., 2011, 47, 10629 RSC; (e) M. C. Willis, D. Taylor and A. T. Gillmore, Org. Lett., 2004, 6, 4755 CrossRef CAS PubMed; (f) H. Zhang, E. M. Ferreira and B. M. Stoltz, Angew. Chem., Int. Ed., 2004, 43, 6144 CrossRef CAS PubMed; (g) A. Furstner and P. W. Davies, J. Am. Chem. Soc., 2005, 127, 15024 CrossRef PubMed; (h) T. Ishikawa, T. Miyahara, M. Asakura, S. Higuchi, Y. Miyauchi and S. Saito, Org. Lett., 2005, 7, 1211 CrossRef CAS PubMed; (i) K. W. Anderson, T. Ikawa, R. E. Tundel and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 10694 CrossRef CAS PubMed; (j) M. A. Honey, A. J. Blake, I. B. Campbell, B. D. Judkins and C. J. Moody, Tetrahedron, 2009, 65, 8995 CrossRef CAS; (k) F. Q. Yuan and F. S. Han, Adv. Synth. Catal., 2013, 355, 537 CAS; (l) W. Hu, M. Li, G. Jiang, W. Wu and H. Jiang, Org. Lett., 2018, 20, 3500 CrossRef CAS PubMed; (m) Y. Shen, X. X. Wu, S. Chen, Y. Xia and Y. M. Liang, Chem. Commun., 2018, 54, 2256 RSC; (n) G. Deng, M. Li, K. Yu, C. Liu, Z. Liu, S. Duan, W. Chen, X. Yang, H. Zhang and P. J. Walsh, Angew. Chem., Int. Ed., 2019, 58, 2826 CrossRef CAS PubMed; (o) B. Kalvacherla, S. Batthula, S. Balasubramanian and R. K. Palakodety, Org. Lett., 2018, 20, 3824 CrossRef CAS PubMed.
- (a) X. Guo, R. Yu, H. Li and Z. Li, J. Am. Chem. Soc., 2009, 131, 17387 CrossRef CAS PubMed; (b) M. J. Moure, R. SanMartin and E. Dominguez, Angew. Chem., Int. Ed., 2012, 51, 3220 CrossRef CAS PubMed.
- For selected examples: (a) J. R. Dunetz and R. L. Danheiser, J. Am. Chem. Soc., 2005, 127, 5776 CrossRef CAS PubMed; (b) M. Movassaghi, M. D. Hill and O. K. Ahmad, J. Am. Chem. Soc., 2007, 129, 10096 CrossRef CAS PubMed; (c) P. Y. Yao, Y. Zhang, R. P. Hsung and K. Zhao, Org. Lett., 2008, 10, 4275 CrossRef CAS PubMed; (d) J. Cao, Y. Xu, Y. Kong, Y. Cui, Z. Hu, G. Wang, Y. Deng and G. Lai, Org. Lett., 2012, 14, 38 CrossRef CAS PubMed; (e) W. Gati, M. M. Rammah, M. B. Rammah, F. Couty and G. Evano, J. Am. Chem. Soc., 2012, 134, 9078 CrossRef CAS PubMed; (f) P. R. Walker, C. D. Campbell, A. Suleman, G. Carr and E. A. Anderson, Angew. Chem., Int. Ed., 2013, 52, 9139 CrossRef CAS PubMed; (g) L. Zhu, Y. Yu, Z. Mao and X. Huang, Org. Lett., 2015, 17, 30 CrossRef CAS PubMed; (h) H. Liu, Y. Yang, S. Wang, J. Wu, X. N. Wang and J. Chang, Org. Lett., 2015, 17, 4472 CrossRef CAS PubMed; (i) A. D. Gillie, R. J. Reddy and P. W. Davies, Adv. Synth. Catal., 2016, 358, 226 CrossRef CAS; (j) H. Liu, Y. Yang, J. Wu, X. N. Wang and J. Chang, Chem. Commun., 2016, 52, 6801 RSC; (k) R. N. Straker, Q. Peng, A. Mekareeya, R. S. Paton and E. A. Anderson, Nat. Commun., 2016, 7, 10109 CrossRef CAS PubMed; (l) J. Zhang, Q. Zhang, B. Xia, J. Wu, X. N. Wang and J. Chang, Org. Lett., 2016, 18, 3390 CrossRef CAS PubMed; (m) L.-G. Xie, S. Niyomchon, A. J. Mota, L. González and N. Maulide, Nat. Commun., 2016, 7, 10914 CrossRef PubMed; (n) Z. Zeng, H. Jin, X. Song, Q. Wang, M. Rudolph, F. Rominger and A. S. K. Hashmi, Chem. Commun., 2017, 53, 4304 RSC; (o) Z. Zeng, H. Jin, J. Xie, B. Tian, M. Rudolph, F. Rominger and A. S. K. Hashmi, Org. Lett., 2017, 19, 1020 CrossRef CAS PubMed; (p) K. H. Oh, J. G. Kim and J. K. Park, Org. Lett., 2017, 19, 3994 CrossRef CAS PubMed; (q) W. D. Mackay, M. Fistikci, R. M. Carris and J. S. Johnson, Org. Lett., 2014, 16, 1626 CrossRef CAS PubMedFor leading reviews: (r) K. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064 CrossRef CAS PubMed; (s) G. Evano, A. Coste and K. Jouvin, Angew. Chem., Int. Ed., 2010, 49, 2840 CrossRef CAS PubMed; (t) X. N. Wang, H. S. Yeom, L. C. Fang, S. He, Z. X. Ma, B. L. Kedrowski and R. P. Hsung, Acc. Chem. Res., 2014, 47, 560 CrossRef CAS PubMed.
- (a) X. L. Jiang, S. J. Liu, Y. Q. Gu, G. J. Mei and F. Shi, Adv. Synth. Catal., 2017, 359, 3341 CrossRef CAS; (b) B. Wu, X. Gao, Z. Yan, M. W. Chen and Y. G. Zhou, Org. Lett., 2015, 17, 6134 CrossRef CAS PubMed.
- (a) C. Shu, L. H. Liao, Y. J. Liao, X. Y. Hu, Y. H. Zhang, W. C. Yuan and X. M. Zhang, Eur. J. Org. Chem., 2014, 4467 CrossRef CAS; (b) L. F. Wang, Z. M. Han and R. H. Fan, Adv. Synth. Catal., 2010, 352, 3230 CrossRef CAS.
- (a) Q. J. Liu, J. Zhu, X. Y. Song, L. Wang, S. R. Wang and Y. Tang, Angew. Chem., Int. Ed., 2018, 57, 3810 CrossRef CAS PubMed; (b) Y. H. Chen, D. J. Cheng, J. Zhang, Y. Wang, X. Y. Liu and B. Tan, J. Am. Chem. Soc., 2015, 137, 15062 CrossRef CAS PubMed.
- When all the starting materials were added at the same time, the ynamide hydrolysis product 4a was detected and no desired product 3a formation.
- MgSO4 was used to absorb water generated from the oxidation of hydroquinone. And in the one-pot system, excess amount of MgSO4 may help to attenuate the hydrolysis of the ynamide.
- T. Hamada, X. Ye and S. S. Stahl, J. Am. Chem. Soc., 2008, 130, 833 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data of reactants and products, and copies of NMR spectroscopy. See DOI: 10.1039/c9ra02144b |
| This journal is © The Royal Society of Chemistry 2019 |